Background. Liver transplantation is often associated with metabolic derangements. Adipocyte fatty-acid-binding protein 4 (AFABP4) integrates inflammatory and metabolic responses. It has also been associated with metabolic syndrome in animal models and clinical studies in the general population.
Aim. To determine the role of AFABP4 in post-transplant metabolic syndrome.
Material and methods. Consecutive patients followed for at least 6 months after liver transplantation were tested for insulin resistance by homeostasis model assessment (HOMA). Serum levels of AFABP4 were tested by an enzyme-linked immunosorbent assay.
Results. The study group included 76 patients (64.5% male, mean age 56.3 ± 12.4 years). Hypertension was present in 56.5%, hyperlipidemia in 69.7%, diabetes mellitus in 23.6%. Half of the patients met at least 3 criteria for metabolic syndrome. Serum AFABP4 levels (p < 0.0001), HOMA index ≥ 2.5 vs. < 2.5 (p < 0.0002) and BMI ≥ 30 vs. < 30 (p < 0.0006) were significantly higher in patients with metabolic syndrome. Within the metabolic syndrome subgroup, AFABP4 levels significantly correlated with age, aspartate aminotransaminase level, waist circumference, and HOMA index. High AFABP4 significantly increased the odds of acquiring metabolic syndrome (OR 1.04, 95% CI 1.007-1.074, p = 0.017). On multiple logistic regression analysis, independent predictors of high AFABP4 were cryptogenic liver disease, steroid administration, high HOMA index, and a high degree of fatty infiltration.
Conclusion. Prevalence of metabolic syndrome is significantly higher in liver transplant recipients than in the general population. AFABP4 may serve as a circulating biomarker in the clinical prediction/diagnosis of metabolic syndrome in patients post-liver transplantation.
Metabolic derangements commonly occur in patients after liver transplantation. Reported rates for hypertension are 40-85%,1,2 13-61% for diabetes mellitus,2-4 40-66% for dyslipidemia (mainly hypertriglyceridemia),2,4,5 and 24-40% for obesity.2,4,6,7 Potential causative factors include immunosuppressive medications,8 return to normal daily life and free food intake.6 Another factor is the apparent direct effect of hepatitis C virus (HCV) infection on the insulin-signaling pathways, recognized in the non-transplant population and, according to cumulative data, in the transplant population as well.9,10
Metabolic derangements may also be due to the persistence of factors that led to the development of primary non-alcoholic fatty liver disease or their recurrence after transplantation in patients with nonalcoholic steatohepatitis (NASH)-related cirrhosis.11-13 Metabolic syndrome which includes obesity, hypertension, hyperglycemia, and dyslipidemia has been found to increase the risk of cardiovascular morbidity and mortality.14
We recently studied liver-transplant recipients and found that 51.1% had metabolic syndrome, twice the reported rate of the general population.15 Post-transplant metabolic syndrome was associated with cardiovascular morbidity but not mortality.
Adipocyte fatty-acid-binding protein 4 (AFABP4), a member of the lipid chaperone fatty-acid-binding protein family is produced by both adipocytes and macrophages. Its gene expression is several orders of magnitude higher in adipocytes.16,17 AFABP4 integrates inflammatory and metabolic responses, thereby affecting insulin sensitivity and lipid metabolism.18-20 AFABP4 has been linked to components of metabolic syndrome in both experimental and clinical studies of the general population.16 Serum AFABP4 levels increased in subjects with nonalcoholic fatty liver disease (NAFLD) and contributed to the development of metabolic syndrome.21
The aim of the present study was to determine if AFABP4 plays a role in post-transplant metabolic syndrome.
Material and MethodsPatientsThe study group consisted of consecutive patients who had undergone a cadaveric or living donor liver transplant at a major tertiary medical center from January 2000 to December 2007 and were followed (and survived) for at least 6 months. Follow-up visits were conducted on an outpatient basis. All patients received the same intraoperative and postoperative care. Nearly all patients received standard immunosuppression with a calcineurin inhibitor (tacrolimus 61.8%, cyclosporine 38.2%) and mycophenolate mofetil together with tapered corticosteroids during follow up. Follow-up monitoring included assessment of immunosuppressive therapy as well as clinical evaluation (vital signs, weight, current medications, adverse reactions, and new major complications) and laboratory tests (blood count, full chemistry including lipid profile, and blood level of immunosuppressive medications), as needed.
Data collectionThe following data were extracted from patients’ medical records: sex, age, cause of liver disease, date of transplantation, total length of follow-up, post-transplantation weight and height, post-transplantation waist circumference, presence of post-transplantation diabetes mellitus, hypertension, or hyperlipidemia and prescribed medications (immuno-suppressive, anti-hypertensive, hypoglycemic, and lipid-lowering drugs). In addition, we collected fasting venous blood samples at the last follow-up visit for same-day assay of the lipid profile (total cholesterol, low-density lipoprotein, high-density lipoprotein and triglycerides), fasting glucose, and levels of creatinine and hemoglobin. Serum samples were drawn once a month over a 4-5-month period, after last follow-up (i.e., in 2009) to measure glucose and insulin levels. The samples were then frozen at-20 °C. Insulin was measured with an enzyme-labeled chemiluminescent immunometric assay (Immulite 2000; Siemens). The frozen samples were used for measuring AFABP4 levels. Diabetic patients receiving insulin treatment were excluded from the glucose test since the presence of anti-insulin antibodies could interfere with the results.
Assessment of insulin resistanceInsulin resistance (IR) was estimated by the homeostasis model assessment (HOMA) index according to the following formula:22
HOMA-IR = fasting insulin (mU/mL) x fasting glucose (mmol/L), divided by 22.5.
This method has proved accurate in predicting insulin resistance in the normoglycemic population before the development of glucose intolerance or overt diabetes.23 The cutoff for diagnosis of insulin resistance was set at 2.5, as accepted.
Definition of the metabolic syndromeMetabolic syndrome was defined according to the 2001 guidelines of the National Cholesterol Education Program Adult Treatment Panel III (ATP III)24 and the 2004 revision by the National Heart, Lung and Blood Institute and the American Heart Association.25 Although body mass index (BMI) ≥ 30 kg/m2 has as yet not been adopted by the ATP III guidelines as a factor in defining metabolic syndrome, overweight clearly correlates with metabolic risk factors. Therefore, in the present study, metabolic syndrome was diagnosed when at least 3 of the following criteria were met:
- •
BMI ≥ 30 kg/m2 or waist circumference > 102 cm for men and 88 cm for women.
- •
Fasting plasma glucose ≥100 mg/dL.
- •
Blood pressure ≥ 130/85 mmHg.
- •
Triglycerides ≥ 150 mg/dL; and
- •
High-density lipoprotein (HDL) < 40 mg/dL in men and < 50 mg/dL in women.
If our patients were receiving hypoglycemic, anti-hypertensive agents or fibrates, they were considered as having abnormal blood glucose levels, high blood pressure, or high triglyceride levels.
Serum AFABP4 assaySerum AFABP4 level was measured by an enzyme-linked immunosorbent assay (BioVendor Research & Diagnostic Products, Candler, North Carolina, USA) according to the manufacturer’s instructions. Calibrations and quality control were performed.
Abdominal ultrasoundFatty liver was diagnosed by abdominal ultrasound. The presence of steatosis was recognized by a marked increase in hepatic echogenicity, categorized as mild, moderate or severe fatty infiltration. Livers with a homogenous texture exhibiting fine-level echoes or minimally hyperechoic or isoechoic compared with normal renal cortex were considered nonsteatotic.
Definition of major vascular eventsMajor vascular events were defined as a transient ischemic attack, cerebrovascular accident, acute coronary syndrome, and myocardial infarction. Coronary events were identified by coronary angiography or coronary revascularization. Most vascular events occurred in our institution; data on events occurring in other institutions were obtained from their medical records. Patients were not systematically screened for asymptomatic coronary events or cere-brovascular disease after transplantation.
Statistical analysisDescriptive statistics are given as mean ± standard deviation (SD) for continuous variables and frequency distribution for categorical variables. Chi-square or Fisher’s exact test was used for categorical variables; two-sample t-test compared groups for continuous variables. Data were fitted to a multiple logistic regression model identifying parameters independently associated with high serum AFABP4 levels. A Cox proportional hazards regression model was also applied. Results are presented as hazard ratios (HR) with their 95% confidence intervals. Statistical analyses were performed using SAS for Windows 9.2.
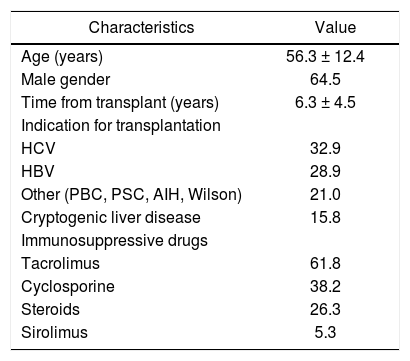
ResultsStudy patientsSeventy-six patients underwent liver transplantation at our center during the study period. Patient characteristics are summarized in table 1. Mean age was 56.3 ± 12.4 years; 64.5% were male. HCV infection indicated transplantation in 32.8% of patients, and cryptogenic liver disease in 15.79%. Patients who underwent transplantation because of liver disease of autoimmune origin (26.3%) were still receiving low-dose methylprednisolone (2.5 to 5 mg/day) at the time of the study. The mean duration of follow-up was 6.3 ± 4.5 years (range: 1.0-23.0 years).
Baseline characteristics of 76 liver-transplant recipients.
| Characteristics | Value |
|---|---|
| Age (years) | 56.3 ± 12.4 |
| Male gender | 64.5 |
| Time from transplant (years) | 6.3 ± 4.5 |
| Indication for transplantation | |
| HCV | 32.9 |
| HBV | 28.9 |
| Other (PBC, PSC, AIH, Wilson) | 21.0 |
| Cryptogenic liver disease | 15.8 |
| Immunosuppressive drugs | |
| Tacrolimus | 61.8 |
| Cyclosporine | 38.2 |
| Steroids | 26.3 |
| Sirolimus | 5.3 |
HCV: hepatitis C virus. HBV: hepatitis B virus. PBC: primary bi¬liary cirrhosis. PSC: primary sclerosing cholangitis. AIH: au¬toimmune hepatitis. Values given as percentage of total patients or mean ± SD.
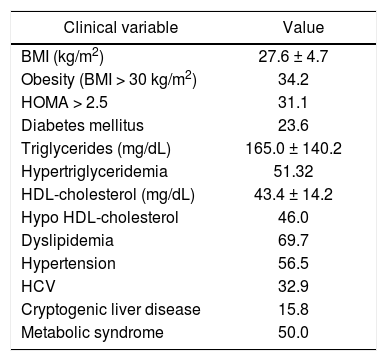
Table 2 shows the rates of metabolic syndrome constituents after transplantation. Overall, the post-transplant period was characterized by a high rate of obesity (BMI > 30 kg/m2) (34.2%), hypertriglyceridemia (>150 mg/dL) (51.3%), high HDL-cho-lesterol < 40 mg/dL (46.0%), dyslipidemia (69.7%), hypertension (56.5%), and diabetes mellitus (23.6%). Thirty-eight patients (50%) met at least 3 of the 5 criteria for a diagnosis of metabolic syndrome after transplantation. Cardiovascular disease developed in 11.8% of patients; 5.3% of the total cohort died during the study period.
Prevalence of the constituents of post-transplant metabolic syndrome in 76 patients.
| Clinical variable | Value |
|---|---|
| BMI (kg/m2) | 27.6 ± 4.7 |
| Obesity (BMI > 30 kg/m2) | 34.2 |
| HOMA > 2.5 | 31.1 |
| Diabetes mellitus | 23.6 |
| Triglycerides (mg/dL) | 165.0 ± 140.2 |
| Hypertriglyceridemia | 51.32 |
| HDL-cholesterol (mg/dL) | 43.4 ± 14.2 |
| Hypo HDL-cholesterol | 46.0 |
| Dyslipidemia | 69.7 |
| Hypertension | 56.5 |
| HCV | 32.9 |
| Cryptogenic liver disease | 15.8 |
| Metabolic syndrome | 50.0 |
BMI: body mass index. HOMA: hoemostasis model assessment. HDL: high density lipoprotein. HCV: hepatic C virus. Values given as percentage of pa¬tients or mean ± SD.
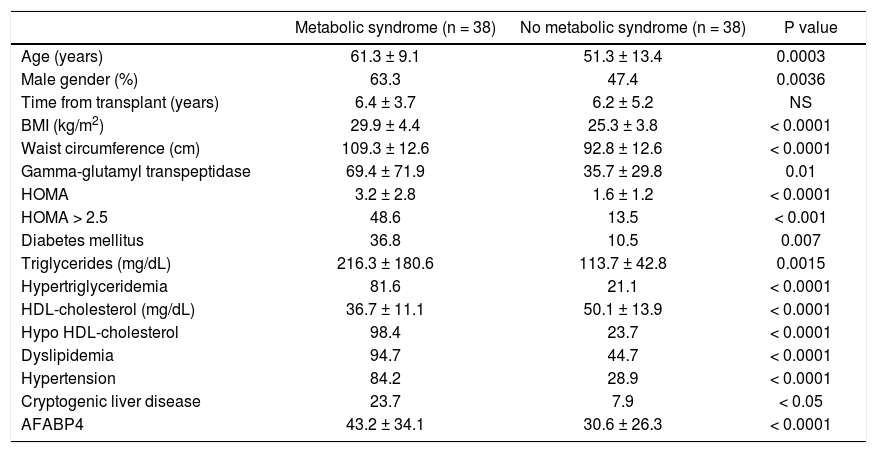
Table 3 compares the characteristics of the patients with and without post-transplant metabolic syndrome or its constituents. The metabolic syndrome group was characterized by a significantly older age (p = 0.0003) and male predominance (63.3 vs. 47.4%; p = 0.00036). This group also had a significantly higher rate of cryptogenic liver disease (23.7 vs. 7.9%, p < 0.05) in addition to significantly higher serum gamma-glutamyl transpeptidase (GGT), triglyceride levels, lower HDL-cholesterol levels, higher BMI and waist circumference, higher HOMA index, and higher rates of hypertension and diabetes mellitus (Table 4).
Characteristics of liver-transplant recipients with and without post transplant metabolic syndrome.
| Metabolic syndrome (n = 38) | No metabolic syndrome (n = 38) | P value | |
|---|---|---|---|
| Age (years) | 61.3 ± 9.1 | 51.3 ± 13.4 | 0.0003 |
| Male gender (%) | 63.3 | 47.4 | 0.0036 |
| Time from transplant (years) | 6.4 ± 3.7 | 6.2 ± 5.2 | NS |
| BMI (kg/m2) | 29.9 ± 4.4 | 25.3 ± 3.8 | < 0.0001 |
| Waist circumference (cm) | 109.3 ± 12.6 | 92.8 ± 12.6 | < 0.0001 |
| Gamma-glutamyl transpeptidase | 69.4 ± 71.9 | 35.7 ± 29.8 | 0.01 |
| HOMA | 3.2 ± 2.8 | 1.6 ± 1.2 | < 0.0001 |
| HOMA > 2.5 | 48.6 | 13.5 | < 0.001 |
| Diabetes mellitus | 36.8 | 10.5 | 0.007 |
| Triglycerides (mg/dL) | 216.3 ± 180.6 | 113.7 ± 42.8 | 0.0015 |
| Hypertriglyceridemia | 81.6 | 21.1 | < 0.0001 |
| HDL-cholesterol (mg/dL) | 36.7 ± 11.1 | 50.1 ± 13.9 | < 0.0001 |
| Hypo HDL-cholesterol | 98.4 | 23.7 | < 0.0001 |
| Dyslipidemia | 94.7 | 44.7 | < 0.0001 |
| Hypertension | 84.2 | 28.9 | < 0.0001 |
| Cryptogenic liver disease | 23.7 | 7.9 | < 0.05 |
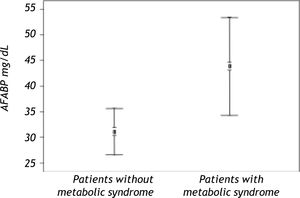
| AFABP4 | 43.2 ± 34.1 | 30.6 ± 26.3 | < 0.0001 |
BMI: body mass index. HOMA: homeostasis model assessment. AFABP4: adipocyte fatty-acid-binding protein 4.
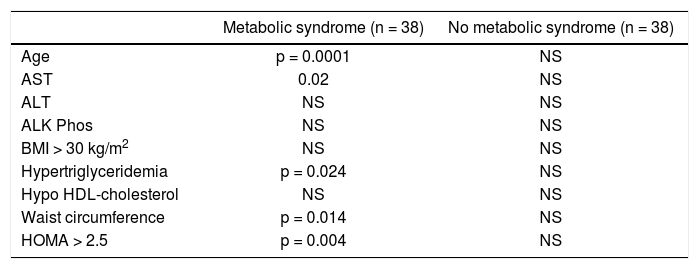
Correlation between serum levels of AFABP4 and clinical/biochemical parameters among patients with and without post-transplant metabolic syndrome (n = 76).
| Metabolic syndrome (n = 38) | No metabolic syndrome (n = 38) | |
|---|---|---|
| Age | p = 0.0001 | NS |
| AST | 0.02 | NS |
| ALT | NS | NS |
| ALK Phos | NS | NS |
| BMI > 30 kg/m2 | NS | NS |
| Hypertriglyceridemia | p = 0.024 | NS |
| Hypo HDL-cholesterol | NS | NS |
| Waist circumference | p = 0.014 | NS |
| HOMA > 2.5 | p = 0.004 | NS |
AFABP4: adipocyte fatty-acid-binding protein 4.
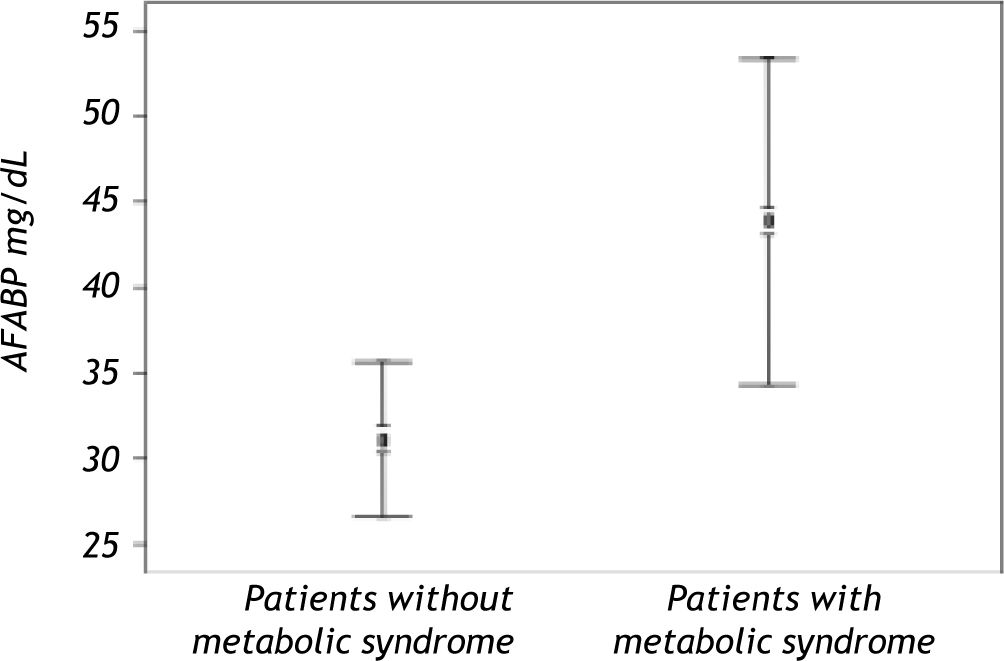
Serum AFABP4 level was significantly elevated in the liver transplant recipients with metabolic syndrome (p < 0.0001) (Figure 1). Insulin resistance assessed by the HOMA index was observed in 31.08% of the study group and was significantly more prevalent in patients with metabolic syndrome. Serum AFABP4 levels were significantly higher in patients with a HOMA index of ≥ 2.5 than in those with an index of < 2.5 (p < 0.0002) and in patients who were overweight/obese (BMI ≥ 30.0) than in those who were not (p < 0.0006). Serum AFABP4 rose significantly with an increase in the number of components of metabolic syndrome (p < 0.05). Levels were significantly lower in the liver transplant recipients receiving tacrolimus (Prograf®) (p < 0. 011) and elevated in the recipients receiving cyclosporine (p < 0.05) than in the patients receiving other immunosuppressive regimens.
A significant correlation of serum AFABP levels with age, aspartate aminotransaminase level, waist circumference, and HOMA index was found in the metabolic syndrome subgroup. None of these findings were found in the liver transplant recipients without metabolic syndrome.
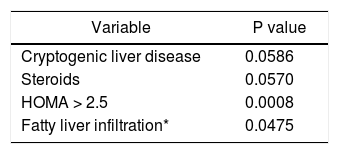
On multiple logistic regression analysis, high serum AFABP4 levels significantly increased the odds of acquiring metabolic syndrome (OR 1.04, 95% CI 1.007-1.074, p = 0.017). Specifically, the chances of having the syndrome rose by a relative 4% for each one unit increase in AFABP4. Predictors of high serum AFABP4 levels using multiple regression analysis (Table 5) were cryptogenic liver disease (the etiology of the liver disease), steroid use, HOMA > 2.5 and fatty liver infiltration on ultrasound.
DiscussionClinical findings of the present study confirm earlier reports of an association between liver transplantation and metabolic syndrome. The rate of metabolic syndrome in our cohort was more than twice the estimated age-adjusted prevalence reported in the general Western population (23.7%).26 Rates of hypertension (56.5%), hyperlipidemia (69.7%), and diabetes mellitus (23.6%) were comparable to those reported in previous studies.27 Importantly, our study supports the view that AFABP4, a regulator of systemic insulin sensitivity, is a central player in the post-transplantation development of key pathologies associated with metabolic syndrome.
Serum AFABP4 levels were significantly higher in patients who met the criteria for post-transplant metabolic syndrome than in those who did not. High serum AFABF4 levels after transplantation significantly increased the odds of acquiring metabolic syndrome.
High levels of AFABP4 have been associated with features of metabolic syndrome in the general popula-tion.2,16,28,29 In animal studies, pharmacological blockade of AFABP4 prevented development of dyslipidemia, atherosclerosis, insulin resistance and fatty liver.18 Accordingly, AFAPB-null mice were found to exhibit a striking phenotype of almost complete protection against diet-induced obesity, insulin resistance, dyslipidemia, type 2 diabetes mellitus, and fatty liver disease.30 Together, these findings suggest that AFABP4 is an important factor linking obesity and various features of metabolic syndrome.30,31
The present study demonstrates a similar close positive association between circulating concentrations of AFABP4, HOMA index and BMI in liver transplant recipients. Thus, AFABP might serve as an independent contributor to glucose intolerance in humans.
The strong positive association between serum AFABP4 concentrations and indicators of adiposity (BMI and waist circumference) further suggest that adipose tissue, composed of adipocytes and macrophages, accounts for the major proportion of AFABP4 secreted into the circulation.
NAFLD is considered a representative metabolic disorder. Insulin resistance syndrome has been identified as a crucial pathophysiological factor in NAFLD.32 Milner, et al. reported increased serum AFABP4 levels in subjects with NAFLD which apparently contributed to the development of metabolic syndrome.21 In the present study, the two independent factors predicting high serum AFABP4 levels were the degree of fatty liver infiltration on ultrasound and an idiopathic cause of the liver disease (“cryptogenic” cirrhosis). The latter finding is in line with the reported high rate of metabolic syndrome or some of its components in patients with crypto-genic cirrhosis, suggesting that in these cases, “cryptogenic” cirrhosis may be unrecognized NASH.33 Accordingly, Malik, et al.34 observed that compared to controls, patients undergoing liver transplantation for NASH cirrhosis had a higher BMI and were probably diabetic and hypertensive.
Immunosuppressive medications play an important role in the pathogenesis of metabolic derangements after liver transplantation. Calcineurin inhibitors such as cyclosporine, tacrolimus, and steroids have been found to be associated with hypertension, hyperglycemia, and dyslipidemia.8 In our cohort, serum AFABP4 levels were significantly lower in the liver transplant recipients receiving tacrolimus and significantly higher in the transplant recipients receiving cyclosporine (p < 0.05). Previous studies found that post-transplant metabolic syndrome was associated with cyclosporine use (p = 0.01) but not tacrolimus.35,36
ConclusionsPrevalence of metabolic syndrome is significantly higher in liver transplant recipients than in the general population. AFABP4 is a circulating biomarker closely associated with post-transplant metabolic syndrome. Routine measurement of serum AFABP4 in liver transplant recipients might be useful in clinically diagnosing metabolic syndrome in this high-risk population; high serum AFABP4 levels increase the probability of developing post-transplant metabolic syndrome.
Abbreviations- •
AFABP4. Adipocyte fatty-acid-binding protein 4.
- •
HOMA. Homeostasis model assessment.
- •
HCV. Hepatitis C virus.
- •
NASH. Nonalcoholic steato-hepatitis.
- •
NAFLD. Nonalcoholic fatty liver disease.
- •
IR. Insulin resistance.
- •
ATP III. National Cholesterol Education Program Adult Treatment Panel III.
- •
BMI. Body mass index.
The authors would like to thank Mrs. Phyllis Curchack Kornspan for her editorial assistance.
There are no grants or other financial support.