Background & aims. Some phytochemicals present in coffee have a potential antioxidant role which seems to protect the human body against cardiovascular diseases, liver disease and malignancies. Nonalcoholic fatty liver disease is a common disease with limited therapeutic options. This study investigated the antioxidant effect of coffee by measuring antioxidant enzymes and lipid peroxidation markers in patients with nonalcoholic fatty liver disease.
Material and methods. We performed a case-control study at the University Hospital, Mexico City. Anthropometric, metabolic, dietary and biochemical variables of all patients were determined and compared. The presence of nonalcoholic fatty liver disease was established by ultrasonography. All patients completed a dietary questionnaire in order to determine their of coffee consumption. Catalase, superoxide dismutase and thiobarbituric acid reactive substances were measured in all of the patients.
Results. Seventy-three subjects with and 57 without nonalcoholic fatty liver disease were included. Patients with nonalcoholic fatty liver disease had significantly higher body mass index, blood glucose, homeostasis model of assessment-insulin resistance and insulin values in comparison to patients without nonalcoholic fatty liver disease. On the one hand, there was a significant difference in coffee intake between the groups (p < 0.05, for all comparisons). There was no significant difference between groups in catalase (0.39 ± 0.74 vs. 0.28 ± 0.69 nM/min/mL), superoxide dismutase (5.4 ± 3.45 vs. 4.7 ± 2.1 U/mL) or thiobarbituric acid-reactive substances (4.05 ± 1.87 vs. 3.94 ± 1.59 μM/mL).
Conclusions. A high intake of coffee has a protective effect against nonalcoholic fatty liver disease however there was no significant difference in the antioxidant variables analyzed.
Coffee is one of the most frequently consumed beverages in the world, and it is known to be a psychoactive beverage with stimulating effects on the central nervous system. Caffeine, one of the main constituents of coffee, has been shown to have a wide spectrum of activities in several biological systems including glucose metabolism and smooth muscle. In addition to caffeine, coffee contains chlorogenic acid, which has antioxidant, antimutation, anticarcinogenic, antibiotic, antihypercholesterolemic, antihypertensive and anti-inflammatory actions.1
In 1992, Klatsky & Armstrong reported an inverse relation between coffee drinking and the risk of liver cirrhosis in a 10-year cohort follow-up. In that study, they reported that coffee drinking appeared to protect against cirrhosis attributed to alcoholic liver disease but not to nonalcoholic disease.2 Recently, high-level coffee consumption has been associated with reduced progression of preexisting liver disease and lower risk of hepatocellular carcinoma.3 It has also been related to a better response to hepatitis C treatment4 and suggested as a potential treatment for metabolic syndrome. Polyphenols, organic compounds present in several plants and fruits including coffee seeds and green tea, have been studied because they might act as potential treatments for a wide spectrum of features of metabolic syndrome.5
Nonalcoholic fatty liver disease (NAFLD) is a common disease worldwide, and is considered the most frequent chronic liver disease. Public health measures have focused on prevention.6 This is most important considering the limited therapeutic options7 and the expected increase in this common liver disease.8
The potential role of coffee as a treatment or preventive for hepatic diseases has been widely discussed; however, as far as we know there are no studies of the probable antioxidant effect of coffee as a factor in preventing liver disease progression in populations with a high prevalence of NAFLD. In this study, we investigate the potential antioxidant role of coffee in the Mexican population and its association with NAFLD by measuring the level of the an-tioxidant enzymes superoxide dismutase (SOD) and catalase (CAT) and lipoperoxidation activity by measuring thiobarbituric acid-reactive species (T-BARS).
Material and MethodsPatient populationWe conducted a cross-sectional study in the checkup unit of the Diagnostic Clinic at the Medica Sur Clinic & Foundation between February 2010 and December 2010. This hospital provides care for mainly middle-and high-income individuals from Mexico City and surrounding metropolitan areas.
Our sample population was selected from a consecutive series of asymptomatic subjects who were referred to the checkup unit by their companies as an annual employment requirement, not for symptomatic disease, and who had no knowledge of having a chronic disease. Exclusion criteria were an alcohol intake of > 20 g/d, known liver disease or current use of medication. For liver disease, subjects who tested positive for hepatitis B antigen or hepatitis C antibody and those who reported a history of known liver disease, including viral, genetic, autoimmune or drug-induced liver disease, were also excluded. The study comprised 130 patients, 73 with NAFLD and 57 without NAFLD, categorized according to the degree of steatosis measured by ultrasonography.
The study was approved by the Human Subjects Committee at the Medica Sur Clinic & Foundation and conformed to the ethical guidelines of the 1983 Declaration of Helsinki. Written informed consent was obtained from all participants before entry into the study.
NAFLD diagnosisThe diagnosis of NAFLD was based on the presence of a bright liver at ultrasound scanning. Realtime ultrasonographic studies were performed while the subjects were fasting. A 3.5 MHz transducer (Elegra; Siemens Medical Systems, Mountain Grove, CA) was used to obtain the following images: sagittal view of the right lobe of the liver and right kidney, transverse view of the left lateral segment of the liver and spleen, transverse view of the liver and pancreas, and any focal areas of altered echotexture.
The severity of echogenicity was graded as follows:9
- •
Grade 0. Normal echogenicity.
- •
Grade 1. A slight, diffuse increase in fine echoes in liver parenchyma with normal visualization of the diaphragm and intrahepatic vessel borders.
- •
Grade 2. A moderate, diffuse increase in fine echoes with slightly impaired visualization of intrahepatic vessels and the diaphragm; and
- •
Grade 3. A marked increase in fine echoes with poor or no visualization of the intrahepatic vessel borders, diaphragm and posterior right lobe of the liver.
Sonographic patterns were classed as:
- •
0, homogeneous, normal.
- •
1, hyperechoic nodules.
- •
2, multiple, confluent hyperechoic lesions.
- •
3, hypoechoic skip nodules.
- •
4, irregular hyperechoic and hypoechoic areas.
- •
5, diffuse involvement.
Participants completed a food frequency questionnaire in which commonly used portions were defined. The questionnaire included questions on the brands of multivitamin and individual vitamin supplements used and the frequency with which they were consumed. Daily intakes of caffeine, energy, protein, carbohydrate, total fat, saturated fat, polyunsaturated fat, monounsaturated fat, vitamins, minerals and antioxidants were determined using SNUT software, a program developed by the Public Health National Institute, Mexico City, which is appropriate for the Mexican population. The SNUT software combines the food consumption results from the questionnaire with known nutrient contents of the foods to estimate daily nutrient intakes.10
Analytical proceduresInsulin concentrations were measured using an immunoenzymometric assay (MEIA; Abbott Diagnostics. Illinois, USA), with inter-and intra-assay coefficients of variation < 3%. Fasting plasma glucose was measured in duplicate with an automated analyzer. The coefficient of variation for a single determination was 1.5%. Total cholesterol, high-density lipoprotein (HDL-C) and triglyceride concentrations were measured by enzymatic colori-metric methods, using CHOL, HDL-C plus (second generation) and TG assays (Roche Diagnostics Co., Indianapolis, IN), respectively. Low-density lipoprotein cholesterol (LDL-C) concentrations were calculated using the Friedewald formula. Assessment of insulin resistance was made using the homeostasis model assessment (HOMA-IR) originally described by Matthews, et al.:11
HOMA-IR = [(fasting insulin (U/L) x fasting glucose (mmol/L))/22.5].
Determination of antioxidant enzymes and lipid peroxidation in serum was determined in duplicate. Commercial kits from Cayman Chemical (Michigan, USA), which use a colorimetric reaction detected by spectrophotometry, were used as outlined below.
- •
SOD detection. Cayman’s SOD assay kit (No. 706002) utilizes a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical. The SOD assay measures all three types of SOD (Cu/Zn, Mn and FeSOD). To SOD standard and sample wells were added 200 μL of radical detector and 10 μL of standard or sample. Reactions were initiated by adding 20 μL xanthine oxidase and carefully shaking the 96-well plate for a few seconds to mix. Plates were covered and incubated on a shaker for 20 min at room temperature and the absorbance was read at 460 nm.
- •
CAT detection. Cayman’s CAT assay kit (No. 707002) utilizes the peroxidase function of CAT to determine enzyme activity. The method is based on the reaction of the enzyme with methanol in the presence of an optimal concentration of H2O2. The formaldehyde produced is measured colorimetrically with 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (Purpald) as the chromogen. Purpal specifically forms a bicyclic heterocycle with aldehydes, which upon oxidation changes from colorless to a purple color. The assay can be used to measure CAT activity in serum. To duplicate sample and standard wells was added 100 μL of assay buffer, 30 μL of methanol and 20 μL of standard or sample. The reaction was initiated by adding 20 μL of hydrogen peroxide to all wells, and then the plate was covered and incubated on a shaker for 20 min at room temperature. Potassium hydroxide (30 μL) was added to each well to terminate the reaction, followed by 30 μL of Purpal (chromogen). The plate was covered and incubated for 10 min at room temperature on a shaker. Finally, 10 μL of potassium periodate was added to each well, and the plate was covered and incubated for 5 min at room temperature before the absorbance was read at 540 nm.
- •
T-BARS detection. Cayman’s T-BARS assay kit (No. 10009055), for assaying lipid peroxidation in serum, measures colorimetrically at 540 nm the malondialdehyde (MDA)- thiobarbituric acid (TBA) adduct formed by the reaction of MDA and TBA under acidic conditions at high temperature (90-100 °C). One hundred microliters of sample or standard was added to appropriately labeled 5 mL vials, to which 100 μL of sodium dodecyl sulfate solution was added and swirled to mix, before the forceful addition of 4 mL of color reagent down the side of each vial. Vials were capped and placed in a holder to keep them upright during boiling, and then placed in vigorously boiling water for 1 h. After 1 h, vials were removed and placed in an ice bath to stop the reaction, incubated on ice for 10 min, then centrifuged for 10 min at 1,600 x g at 4 °C. After warming the vials to room temperature, duplicate 150 μL aliquots from each vial were added to a clear plate and the absorbance read at 540 nm.
Mean values and their standard deviations (SD) were used to summarize the distribution of continuous variables, comparing patients with and without NAFLD. Because caffeine intake did not have a normal distribution, it was transformed as a logarithmic variable. Unconditional univariate logistic regression analysis was conducted to estimate the probability of NAFLD being associated with the level of caffeine intake. All analyses were carried out with SPSS/PC v 16.0 (Chicago IL). Differences were considered significant with P values of < 0.05.
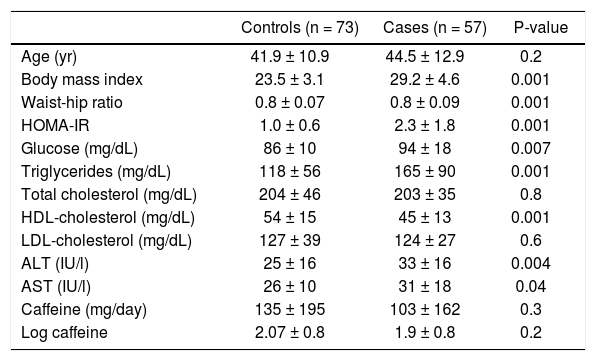
ResultsOf the 130 patients included, 57 had NAFLD and 73 did not. There were no differences in age (41.9 ± 10 vs. 44.6 ± 12.9 years, P = 0.2), total cholesterol (204 ± 46 vs. 203 ± 35 mg/dL, P = 0.8) or LDL-C (127 ± 39 vs. 124 ± 27 mg/dL, P = 0.6). Neither caffeine intake (135 ± 195 vs. 103 ± 162 mg/day, P = 0.3) nor log caffeine intake (2.07 ± 0.8 vs. 1.9 ± 0.8, P = 0.2) differed between the groups. Other characteristics associated with obesity and altered liver function were higher in those with NAFLD (Table 1).
General characteristics of the participants divided according to the presence of NAFLD.
| Controls (n = 73) | Cases (n = 57) | P-value | |
|---|---|---|---|
| Age (yr) | 41.9 ± 10.9 | 44.5 ± 12.9 | 0.2 |
| Body mass index | 23.5 ± 3.1 | 29.2 ± 4.6 | 0.001 |
| Waist-hip ratio | 0.8 ± 0.07 | 0.8 ± 0.09 | 0.001 |
| HOMA-IR | 1.0 ± 0.6 | 2.3 ± 1.8 | 0.001 |
| Glucose (mg/dL) | 86 ± 10 | 94 ± 18 | 0.007 |
| Triglycerides (mg/dL) | 118 ± 56 | 165 ± 90 | 0.001 |
| Total cholesterol (mg/dL) | 204 ± 46 | 203 ± 35 | 0.8 |
| HDL-cholesterol (mg/dL) | 54 ± 15 | 45 ± 13 | 0.001 |
| LDL-cholesterol (mg/dL) | 127 ± 39 | 124 ± 27 | 0.6 |
| ALT (IU/l) | 25 ± 16 | 33 ± 16 | 0.004 |
| AST (IU/l) | 26 ± 10 | 31 ± 18 | 0.04 |
| Caffeine (mg/day) | 135 ± 195 | 103 ± 162 | 0.3 |
| Log caffeine | 2.07 ± 0.8 | 1.9 ± 0.8 | 0.2 |
HOMA-IR: homeostasis model assessment of insulin resistance. HDL: high-density lipoprotein. LDL: low-density lipoprotein. ALT: alanine aminotransferase. AST: aspartate aminotransferase. Data expressed as mean ± standard deviation
The severity of steatosis was assessed against caffeine consumption. Caffeine intake in those with severe steatosis (log caffeine 0.15 ± 0.05) differed from intakes in patients with moderate steatosis (log caffeine 1.58 ± 0.72, P = 0.05), mild steatosis (log caffeine 1.61 ± 0.79, P = 0.04) and without steatosis (log caffeine 1.75 ± 0.70, P = 0.01).
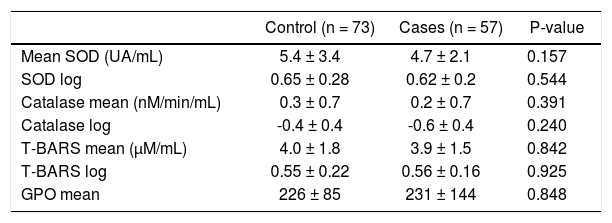
There were no significant differences between patients with and without NAFLD in the antioxidant variables assessed by SOD and CAT and the lipoperoxidation activity measured by T-BARS (Table 2). A subanalysis was performed based on steatosis severity and sex, but it showed no significant differences (data not shown).
Antioxidant characteristics according to the presence of NAFLD.
| Control (n = 73) | Cases (n = 57) | P-value | |
|---|---|---|---|
| Mean SOD (UA/mL) | 5.4 ± 3.4 | 4.7 ± 2.1 | 0.157 |
| SOD log | 0.65 ± 0.28 | 0.62 ± 0.2 | 0.544 |
| Catalase mean (nM/min/mL) | 0.3 ± 0.7 | 0.2 ± 0.7 | 0.391 |
| Catalase log | -0.4 ± 0.4 | -0.6 ± 0.4 | 0.240 |
| T-BARS mean (μM/mL) | 4.0 ± 1.8 | 3.9 ± 1.5 | 0.842 |
| T-BARS log | 0.55 ± 0.22 | 0.56 ± 0.16 | 0.925 |
| GPO mean | 226 ± 85 | 231 ± 144 | 0.848 |
SOD: superoxide dismutase. T-BARS: thiobarbituric acid-reactive species. GPO: data expressed as mean ± standard deviation.
The caffeine consumption and oxidative stress markers was analyzed according the presence of overweight and obesity, and not differences across the groups were founded (data not shown).
DiscussionThe consumption of coffee is more than a cultural issue. Caffeine is a purine alkaloid, acting through the antagonism of adenosine receptors A1 and A2A, with its main effect being observed at therapeutic concentrations of 10-100 μM. However many other potential effects have been described.12 Coffee contains numerous substances, including caffeine, chlorogenic acid, quinides, trigonelline and lignan, that have been shown to affect glucose metabolism in animals or metabolic studies.13
In this study, we analyzed the effects of caffeine consumption on the prevalence and severity of NAFLD in the general population. Focusing on the antioxidant capacity of coffee, we observed a dose-dependent reduction in the consumption of caffeine with increasing severity of steatosis. This effect has been observed in animal models of fatty liver, in which caffeine intake improves insulin resistance and reduces inflammatory cytokine production. In addition, the weight of the animals and the intrahepatic levels of glucose were reduced with coffee consumption.13 Similar findings were obtained in a human case-control study in which coffee consumption was an independent protective risk factor against the severity of fatty liver disease, although no correlation between coffee consumption and insulin resistance was observed.14 This lack of association with insulin resistance markers was also observed in patients with biopsy-proven nonalcoholic steatohepatitis (NASH); there was a significant association with the probability of fibrosis in patients with NASH, but not with the presence of NASH itself.14
These findings are consistent with those of our study. We were interested in the hypothesis that coffee has antioxidant properties. The antioxidant activity of coffee is generally attributed to Maillard reaction products formed during roasting, in addition to certain natural phenolic compounds such as chlorogenic acid, caffeic acid, ferulic acid and pcoumaric acid.15 In our work, the antioxidant activity was assessed in a case-control study by measuring the end products of oxidant activity. This could partially explain the lack of differences between the groups, because it is not clear how the acute consumption of coffee can affect a quick process such as antioxidant activity. However, the dose-dependent effect observed on the severity of fatty liver, together with the previously mentioned studies, suggests an important role of coffee consumption as a protective risk factor against not only steatosis but also fibrosis associated with NASH. In experimental studies, this protective effect was also demonstrated to participate in the regulation of genes involved in the fibrogenic res-ponse.16 This antifibrogenic effect was also described in other chronic liver diseases including hepatitis C virus infection17 and cirrhosis resulting from alcohol abuse.18 In addition, the general risk of liver cirrhosis is diminished in people who drink coffee;19 this could in part be explained by its protective effect in NAFLD.
This study provides epidemiological evidence of the protective effects of coffee consumption. However, the association of these effects with the antioxidant activity of coffee is not clear and requires further research.
ConclusionIn conclusion, this study demonstrates a reduction in the severity of NAFLD in those subjects with a higher consumption of coffee, but it does not show an association between NAFLD and the antioxidant properties of coffee. More studies are necessary to define the mechanisms involved in such protective effects.
What is Current KnowledgeThere is negative association between coffee intake and severity of non-alcoholic fatty liver disease.
The anti-oxidant properties are the putative mechanism involved in the protective effect of coffee.
What is New HereWe observe protective effect in patients with high intake of caffeine.
There is no association of this protective effect with markers of oxidative stress.
Conflict of InterestNone.
Financial SupportNone.
Abbreviations- •
NAFLD: non-alcoholic fatty liver disease.
- •
SOD: superoxide dismutase.
- •
CAT: catalase.
- •
T-BARS: thiobarbituric acid-reactive species.
- •
HDL-C: high-density lipoprotein cholesterol.
- •
LDL: low-density lipoprotein.
- •
HOMA-IR: homeostasis model assessment for insulin resistance.
- •
MDA: malondialdehyde.
- •
TBA: thiobarbituric acid.
- •
SD: standard deviation.
- •
NASH: non-alcoholic steatohepatitis.