Background. Pro-inflammatory cytokine production is directly inhibited by acetylcholine (ACh), and a relationship between total circulating ACh hydrolytic capacity and inflammatory reactions has been previously reported. Butyrylcholinesterase (BChE) is the major ACh hydrolyzing enzyme in plasma, and the aim of our study was to evaluate its association with low-grade systemic inflammation.
Material and methods. A total of 4,077 patients clinically managed in the Cardiology, Hypertension, and Digestive Medicine Units were included in our study. Three subclinical chronic inflammatory degrees were established in accordance with the high-sensitivity C-reactive protein (hsCRP) concentrations proposed, for low (< 1 mg/L), average (1-3 mg/L), and high (> 3-10 mg/L) cardiovascular disease risk estimation.
Results. In male patients with subclinical chronic inflammation and hsCRP concentrations < 1 mg/L, a significant positive correlation was observed between BChE and hsCRP (p < 0.02); however, for hsCRP concentrations > 3 mg/L, the correlation between these variables in both sexes becomes significantly negative (p < 0.001), as in patients with acute inflammation (hsCRP > 10 mg/L). In all cases significant positive correlations were obtained between the BChE activities and albumin concentrations (p < 0.001).
Conclusions. The liver production of BChE and albumin occurs in a coupled fashion, and these biochemical variables may be considered as negative inflammatory reactants, whose serum levels are inversely associated with the increasing degree of subclinical inflammation.
The enzyme family of cholinesterases includes the acetylcholinesterase (AChE, EC 3.1.1.7), also known as true, specific, or type I cholinesterase, and the butyrylcholinesterase (BChE, EC 3.1.1.8), also known as plasma, nonspecific, or type II cholinesterase.1 Several functions have been suggested for BChE in lipoprotein metabolism, myelin maintenance, cellular adhesion, hydrolysis of acetylcholine (ACh) and other choline esters, and also hydrolysis of non-choline esters such as a number of local anesthetics, muscle relaxants, aspirin, and cocaine.1 BChE is synthesized and secreted into blood by the liver, although this enzyme is also found in the lungs, heart, brain, small intestine, and adipose tissue, and its serum/plasma activity has been primarily used in clinical biochemistry to test diminished protein-synthesizing capacity of the liver and orga-nophosphorus insecticide poisoning.2,3
The cholinergic anti-inflammatory pathway mediated by the neurotransmitter ACh exerts a direct inhibitory effect on pro-inflammatory cytokine pro-duction.4 Increased activities of AChE and BChE may lead to a greater hydrolytic destruction and diminished concentrations of ACh, and this fact could trigger and perpetuate systemic inflammation.5–8 Several studies have addressed the association of chronic low-grade inflammation, as defined by serum C-reactive protein levels,9–11 and BChE activity,12–14 with obesity, metabolic syndrome, insulin resistance, and cardiovascular risk. Consequently, it has been suggested that increased plasma and tissue activities of BChE seen in various clinical conditions could serve as a marker of low-grade systemic inflammation.5,6 If this assumption is true, the metabolism of some drugs and xenobiotics would be enhanced in these inflammatory conditions.
The aim of our study was to investigate the association of serum BChE activity with different degrees of subclinical chronic inflammation and acute inflammation in a large number of patients. The inflammatory status was evaluated in function of the levels of C-reactive protein, considered the marker of inflammation of choice for clinical practice, and which interesting in vitro may bind Ach, inhibiting its enzymatic breakdown.15 In some cases the concentrations of procalcitonin were also determined.
Material and MethodsThe 4,077 subjects (2,518 males and 1,559 females) included in this study, with a mean age (± SEM) of 64.0 ± 0.26 years (range 15-101 years), were recruited from patients clinically managed in the Cardiology, Hypertension, and Digestive Medicine Units, and for whom high-sensitivity C-reactive protein (hsCRP) determination was demanded for their inflammatory status evaluation. The blood samples were drawn before breakfast, and after a fasting period of at least 10 h. The study was made in accordance with the good practice rules for investigation in humans of the Conselleria de Sanidade (Regional Ministry of Health) of the Xunta de Galicia, Spain.
Serum activities of butyrylcholinesterase (BChE), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), and alkaline phosphatase (ALP), and concentrations of albumin, glucose, cholesterol, and triglycerides, were determined in an Advia 2400 Chemistry System (Siemens Health Care Diagnostics Inc., Newark, USA). The platelet count was measured in an Advia 2120 Hematology System (Siemens Health Care Diagnostics Inc.). The AST to platelet ratio liver fibrosis index (APRI) was calculated in accordance with Wai, et al.16:
APRI = (AST:URL/platelet count (109/L) x 100,
Where URL corresponds to the AST upper reference limits for men a women. Using this index, a score < 0.5 exclude the presence of liver fibrosis with a high level of confidence, and a score > 1.5 is suggestive of significant liver fibrosis.16,17 Determination of hsCRP was made in a BN II nephelometer (Siemens Health Care Diagnostics Inc.). A concentration of hsCRP > 10 mg/L suggest the presence of an acute inflammatory process,9,18 and for subclinical chronic inflammation three inflammatory degrees were established according with the hsCRP ranges proposed for low (< 1 mg/L), average (1-3 mg/L), and high (> 3-10 mg/L) cardiovascular disease risk estimation.9,18 The determination of procalcitonin (PCT) was made using the B.R.A.H.M.S PCT Sensitive Kryptor from Thermo Fisher Scientific (Hennigsdorf, Germany).
Statistical analysis of data was performed using the StatGraphics package, and Kolmogorov-Smirnov test was applied to check for normality. The Pearson’s correlation coefficient and Student test were used when the data had a Gaussian distribution; otherwise, Spearman’s correlation coefficient and Mann-Whitney U were used. The results are expressed as mean ± SEM (median).
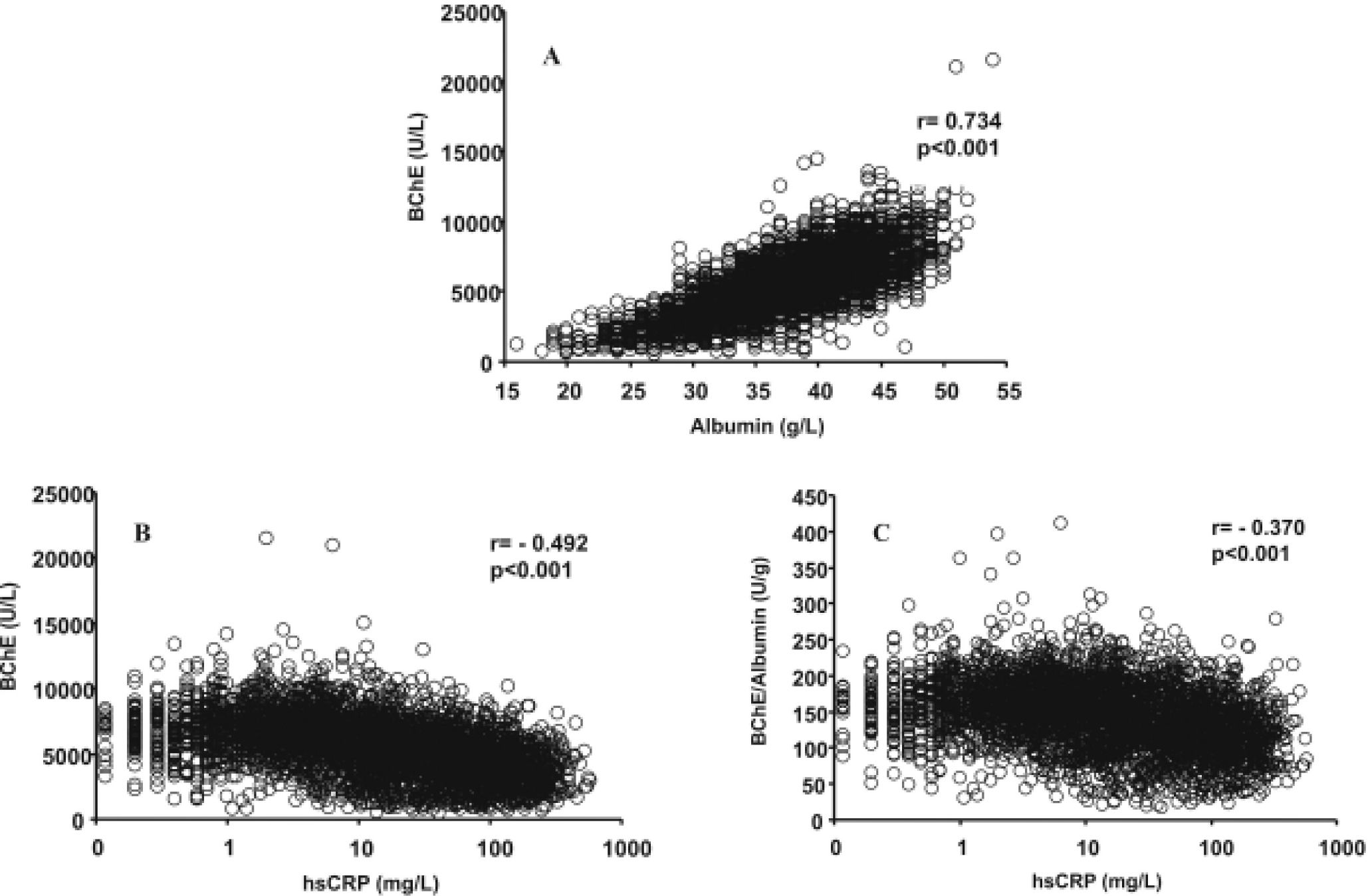
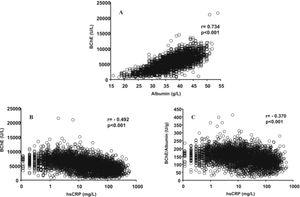
ResultsIn the total number of patients studied, a significant positive correlation between serum BChE activities and albumin concentrations was obtained (Figure 1A). Negative correlations were obtained between the hsCRP and BChE activity (Figure 1B) and BChE/albumin ratio (Figure 1C), which has recently been proposed19 as an index of adverse effects of anticholinesterase agents (cholinergic crisis). The coefficients of determination (r2) obtained shown that the BChE/albumin ratio is more dependent on the BChE activity (r2 = 0.889) than on the albumin concentration (r2 = 0.241). The inter-individual variations for BChE activity, albumin concentration and BChE/albumin ratio were respectively 39.9%, 15.6%, and 32.1%.
Relationship between serum albumin and BChE (A), hsCRP and BChE (B), and hsCRP and BChE/albumin ratio (C) in the studied patients with subclinical chronic inflammation (hsCRP ≤ 10 mg/L) and acute inflammation (hsCRP > 10 mg/L). Butyrylcho-linesterase (BChE), high-sensitivity C-reactive protein (hsCRP).
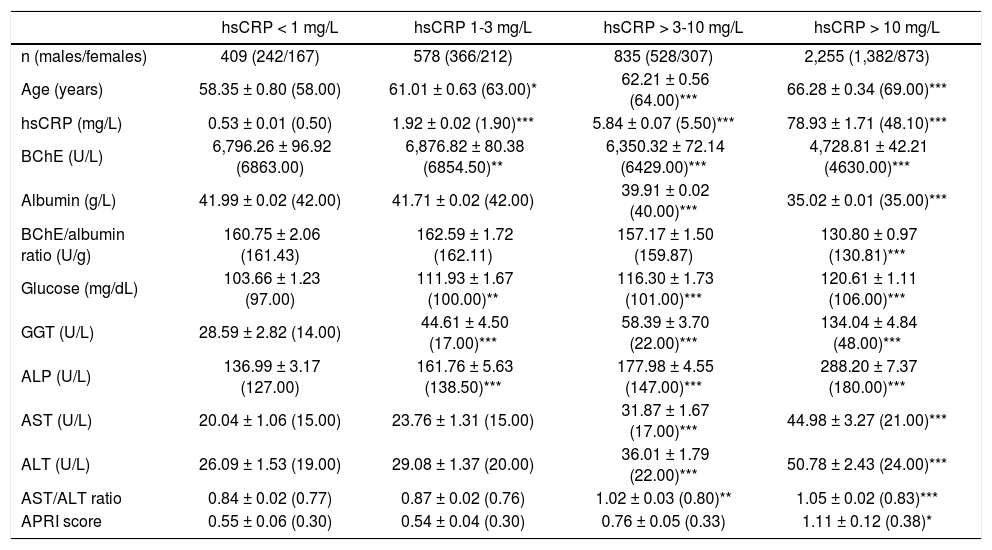
The results obtained for the biochemical variables assayed in function of the different degrees of subclinical chronic inflammation or acute inflammation conditions are indicated in table 1. The levels of BChE and albumin decrease, and the levels of GGT and ALP increase, with the degree of inflammation. For the BChE/albumin ratio a significant decrease was only obtained in the group of patients with acute inflammation. In the different groups of patients with subclinical chronic inflammation, the mean (median) hsCRP levels were analogous in male and female patients; however, the mean (median) BChE activities were greater in males, although the statistical significance for the difference was only achieved in the group of patients with hsCPR < 1 mg/L (p < 0.005). In the patients with acute inflammation, the mean (median) levels of BChE and hsCRP were analogous in males and females.
Serum BChE and other biochemical variables according to hsCRP concentrations in the studied patients.
| hsCRP < 1 mg/L | hsCRP 1-3 mg/L | hsCRP > 3-10 mg/L | hsCRP > 10 mg/L | |
|---|---|---|---|---|
| n (males/females) | 409 (242/167) | 578 (366/212) | 835 (528/307) | 2,255 (1,382/873) |
| Age (years) | 58.35 ± 0.80 (58.00) | 61.01 ± 0.63 (63.00)* | 62.21 ± 0.56 (64.00)*** | 66.28 ± 0.34 (69.00)*** |
| hsCRP (mg/L) | 0.53 ± 0.01 (0.50) | 1.92 ± 0.02 (1.90)*** | 5.84 ± 0.07 (5.50)*** | 78.93 ± 1.71 (48.10)*** |
| BChE (U/L) | 6,796.26 ± 96.92 (6863.00) | 6,876.82 ± 80.38 (6854.50)** | 6,350.32 ± 72.14 (6429.00)*** | 4,728.81 ± 42.21 (4630.00)*** |
| Albumin (g/L) | 41.99 ± 0.02 (42.00) | 41.71 ± 0.02 (42.00) | 39.91 ± 0.02 (40.00)*** | 35.02 ± 0.01 (35.00)*** |
| BChE/albumin ratio (U/g) | 160.75 ± 2.06 (161.43) | 162.59 ± 1.72 (162.11) | 157.17 ± 1.50 (159.87) | 130.80 ± 0.97 (130.81)*** |
| Glucose (mg/dL) | 103.66 ± 1.23 (97.00) | 111.93 ± 1.67 (100.00)** | 116.30 ± 1.73 (101.00)*** | 120.61 ± 1.11 (106.00)*** |
| GGT (U/L) | 28.59 ± 2.82 (14.00) | 44.61 ± 4.50 (17.00)*** | 58.39 ± 3.70 (22.00)*** | 134.04 ± 4.84 (48.00)*** |
| ALP (U/L) | 136.99 ± 3.17 (127.00) | 161.76 ± 5.63 (138.50)*** | 177.98 ± 4.55 (147.00)*** | 288.20 ± 7.37 (180.00)*** |
| AST (U/L) | 20.04 ± 1.06 (15.00) | 23.76 ± 1.31 (15.00) | 31.87 ± 1.67 (17.00)*** | 44.98 ± 3.27 (21.00)*** |
| ALT (U/L) | 26.09 ± 1.53 (19.00) | 29.08 ± 1.37 (20.00) | 36.01 ± 1.79 (22.00)*** | 50.78 ± 2.43 (24.00)*** |
| AST/ALT ratio | 0.84 ± 0.02 (0.77) | 0.87 ± 0.02 (0.76) | 1.02 ± 0.03 (0.80)** | 1.05 ± 0.02 (0.83)*** |
| APRI score | 0.55 ± 0.06 (0.30) | 0.54 ± 0.04 (0.30) | 0.76 ± 0.05 (0.33) | 1.11 ± 0.12 (0.38)* |
Statistical significance against to the group with hsCRP levels < 1 mg/L: Reference ranges: BChE (3,500-10,500 U/L), GGT (5-38 U/L), FAL (65-195 U/L), AST (0-25 U/L), ALT (0-29 U/L).
* p < 0.05 p < 0.01 p < 0.001.
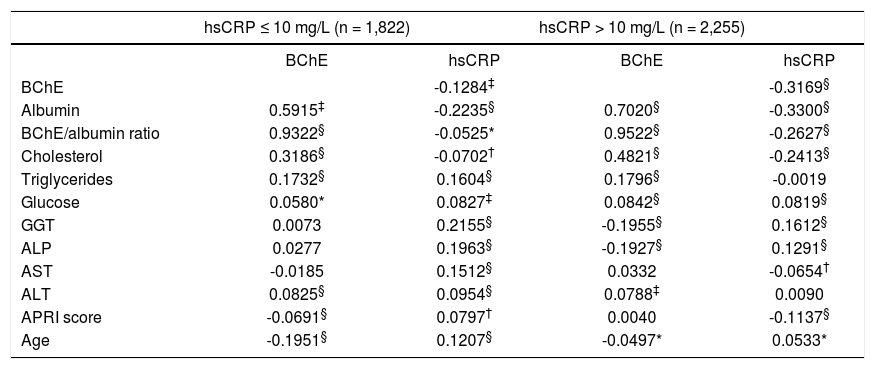
The negative correlation coefficients obtained between serum BChE activities and hsCRP concentrations were statistically significant both in the groups of patients with subclinical chronic inflammation or with acute inflammation (Table 2). A dichotomy of the patients in function of sex did not lead to results with an additional interest, except for the fact that significant negative correlations of BChE activity with the age was obtained in males with subclinical chronic inflammation (r = -0.283, p < 0.001) or acute inflammation (r = -0.112, p < 0.001), but not in female patients. For the first-order partial correlations between BChE and hsCRP, maintaining the age as a constant, analogous correlation coefficients and significances to those indicated in table 2 were obtained.
Correlation between the serum levels of BChE, hsCRP and other biochemical variables in the patients with subclinical chronic (hsCRP ≤ 10 mg/L) and acute (hsCRP > 10 mg/L) inflammation.
| hsCRP ≤ 10 mg/L (n = 1,822) | hsCRP > 10 mg/L (n = 2,255) | |||
|---|---|---|---|---|
| BChE | hsCRP | BChE | hsCRP | |
| BChE | -0.1284‡ | -0.3169§ | ||
| Albumin | 0.5915‡ | -0.2235§ | 0.7020§ | -0.3300§ |
| BChE/albumin ratio | 0.9322§ | -0.0525* | 0.9522§ | -0.2627§ |
| Cholesterol | 0.3186§ | -0.0702† | 0.4821§ | -0.2413§ |
| Triglycerides | 0.1732§ | 0.1604§ | 0.1796§ | -0.0019 |
| Glucose | 0.0580* | 0.0827‡ | 0.0842§ | 0.0819§ |
| GGT | 0.0073 | 0.2155§ | -0.1955§ | 0.1612§ |
| ALP | 0.0277 | 0.1963§ | -0.1927§ | 0.1291§ |
| AST | -0.0185 | 0.1512§ | 0.0332 | -0.0654† |
| ALT | 0.0825§ | 0.0954§ | 0.0788‡ | 0.0090 |
| APRI score | -0.0691§ | 0.0797† | 0.0040 | -0.1137§ |
| Age | -0.1951§ | 0.1207§ | -0.0497* | 0.0533* |
Statistical significance:
* p < 0.5 p < 0.05 p < 0.001 p < 0.0001.
The PCT concentration was determined in 1,897 patients, obtaining significant negative correlations of this inflammatory marker with BChE activities for the 493 cases with subclinical inflammation (r = -0.411, p < 0.001) and 1,404 cases with acute inflammation (r = -0.266, p < 0.001). Excluding 395 cases with serum PCT concentrations > 0.5 ng/mL, which would be due to systemic bacterial infection,20 lead to obtaining analogous correlation coefficients and significances between BChE and PCT levels, both in the patients with subclinical chronic (r = -0.3935, p < 0.001) or acute (r = -0.2416, p < 0.001) inflammatory conditions. Significant negative correlations were also obtained in these patients with subclinical chronic or acute inflammation, between albumin and PCT concentrations (r =-0.395, p < 0.001 and r = -0.348, p < 0.001) respectively.
In the group of 409 patients with hsCRP concentration < 1 mg/L, a significant positive correlation was obtained between the levels of this inflammatory marker and BChE activities (r = 0.113, p < 0.02). This fact was due to the significant correlation in males (n = 242, r = 0.148, p < 0.02), as in the female patients the correlation was not signifi-cant (n = 167, r = 0.010). Analogous correlation coefficients and significances were obtained for the first-order partial correlations between hsCRP and BChE maintaining the age as a constant. In the 578 patients with hsCRP levels of 1-3 mg/L the correlation between hsCRP and BChE was not significant (r = -0.0023); however, in the 835 patients with hsCRP concentrations > 3-10 mg/L, a significant negative correlation was obtained between these two variables (r = -0.162, p < 0.001).
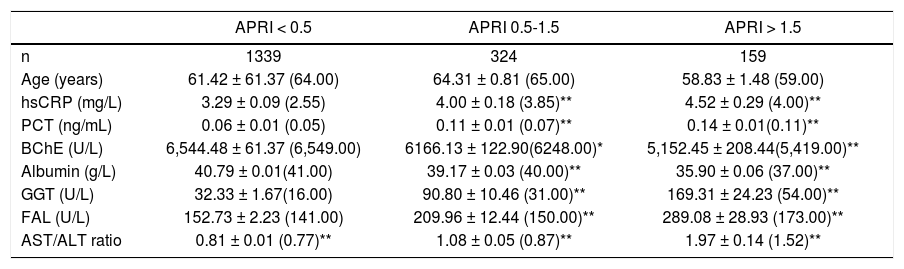
The APRI score was significantly increased in the groups of patients with subclinical chronic inflammation and hsCRP levels > 3-10 mg/L and with acute inflammation (Table 1). In the group of patients with hsCPR levels < 1 mg/L, the 79.3% present an APRI score < 0.5, 14.2% a score 0.5-1.5, and 6.5% a score > 1.5%. In the patients with hsCRP levels 1-3 mg/L, the 79.2% present a score < 0.5, 14.2% a score 0.5-1.5, and 6.6% a score > 1.5. In the patients with hsCPR levels > 3-10 mg/L, the 67.6% present a score < 0.5, 21.5% a score 0.5-1.5, and 10.8% a score > 1.5. In the group of patients with acute inflammation (hsCPR > 10 mg/L), the 59.2% present a score < 0.5, 26.6% a score 0.5-1.5, and 14.1% a score > 1.5. A weak, although statistically significant negative correlation was obtained between the BChE activities and APRI score in the 1,822 patients with subclinical chronic inflammation (r = -0.069, p < 0.005), but not in the patients with acute inflammation (r = 0.004). In table 3 are shown the results obtained for serum BChE, hsCRP, albumin, and other biochemical variables in function of the APRI score in the patients with subclinical chronic inflammation. BChE and albumin levels were significantly lower, and the hsCRP and PCT concentrations were significantly higher, in the groups of patients with APRI score 0.5-1.5 and > 1.5, than in the group with a score < 0.5.
BChE and other biochemical variables in function of the APRI score in the patients with subclinical chronic inflammation (hsCRP < 10 mg/L).
| APRI < 0.5 | APRI 0.5-1.5 | APRI > 1.5 | |
|---|---|---|---|
| n | 1339 | 324 | 159 |
| Age (years) | 61.42 ± 61.37 (64.00) | 64.31 ± 0.81 (65.00) | 58.83 ± 1.48 (59.00) |
| hsCRP (mg/L) | 3.29 ± 0.09 (2.55) | 4.00 ± 0.18 (3.85)** | 4.52 ± 0.29 (4.00)** |
| PCT (ng/mL) | 0.06 ± 0.01 (0.05) | 0.11 ± 0.01 (0.07)** | 0.14 ± 0.01(0.11)** |
| BChE (U/L) | 6,544.48 ± 61.37 (6,549.00) | 6166.13 ± 122.90(6248.00)* | 5,152.45 ± 208.44(5,419.00)** |
| Albumin (g/L) | 40.79 ± 0.01(41.00) | 39.17 ± 0.03 (40.00)** | 35.90 ± 0.06 (37.00)** |
| GGT (U/L) | 32.33 ± 1.67(16.00) | 90.80 ± 10.46 (31.00)** | 169.31 ± 24.23 (54.00)** |
| FAL (U/L) | 152.73 ± 2.23 (141.00) | 209.96 ± 12.44 (150.00)** | 289.08 ± 28.93 (173.00)** |
| AST/ALT ratio | 0.81 ± 0.01 (0.77)** | 1.08 ± 0.05 (0.87)** | 1.97 ± 0.14 (1.52)** |
Statistical significance against to the group with APRI score < 0.5:
* p < 0.05 p < 0.001.
A parallel increase of GGT, ALP, and glucose levels with the inflammatory degree of the patients was observed (Table 1), presenting GGT and ALP activities positive significant correlations with hs-CRP concentrations, both in the patients with chronic subclinical inflammation as in the patients with acute inflammation (Table 2). The AST and ALT activities and AST/ALT ratio were significantly increased in the groups of patients with hsCRP concentrations > 3 mg/L (Table 1).
DiscussionIn human plasma only four esterases have been found: AChE, although in negligible amounts, BChE, paraoxanase, and albumin esterase.21 Albumin is considered an inert protein; however, although the enzymatic activity of a single molecule is low, as its concentration is very high, albumin makes a significant contribution to the plasma esterase activity.21 In accordance with previously published results,14,22,23 a highly significant correlation was observed between the serum levels of BChE and albumin in the total number of patients studied (Figure 1A). Although several mechanisms may be involved,14,22,23 the more plausible explanation for this association is that the serum levels of BChE and albumin are determined by its rates of production in the liver, which occurs in a coupled fashion.2,24
The cholinergic anti-inflammatory pathway may be mitigated by AChE and BChE, both of which hydrolyze and inactivate AChE, antagonizing vagal cholinergic signalling at macrophage level, with the release of pro-inflammatory cytokines and promoting the systemic inflammatory response.4,25,26 BChE is the main ACh hydrolyzing enzyme in plasma, and an interrelationship has been reported between inflammatory reactions and the total circulating capacity for ACh hydrolysis.25,26 Recently, it has been described significant increases of plasma BChE activity, correlated with different inflammatory markers, such as fibrinogen, interleukin-6, and CRP, during the acute phase in stroke patients.26 Similarly, increased BChE activities have been described in several pathophysiological conditions such as obesity, hyperlipidemia, hypertension, insulin resistance, metabolic syndrome, liver steatosis, diabetes mellitus or Alzheimer disease,5–7,12–14,23,27,28 in which low-grade systemic inflammation may play a role in its development.5–7,12,14,29 Consequently, increased plasma and tissues BChE activity has been proposed as a marker of low-grade systemic inflammation.5,6
In the total group of patients we studied, significant negative correlations were obtained between the hsCRP and albumin (r = -0.579, p < 0.001), BChE (Figure 1B), and BChE/albumin ratio (Figure 1C). In accordance with the results of Kariyone, et al.,20 the BChE/albumin ratio may be a better indicator of cholinergic status with less inter-individual variation than the BChE activity. In our study, the inter-individual variations of the BChE/albumin ratio in the different groups of patients were only moderately lower than those obtained for BChE activity (Table 1). When the patients were grouped in function of their subclinical chronic (hsCRP ≤ 10 mg/L) or acute (hsCRP > 10 mg/L) inflammatory status, significant negative correlations were also obtained between hsCRP levels and BChE activities, albumin concentrations, and BChE/albumin ratio (Table 2). Concordant results were obtained for the relationship between BChE and PCT levels. A significant positive correlation between hsCRP and BChE was only obtained in male patients with a very low inflammation degree and hsCRP concentration < 1 mg/L (r = 0.148, p < 0.001).
Recently Stojanov, et al.21 reported that lowered levels of BChE and albumin are related with greater mortality in hemodialysis patients. Similarly, in accordance with Ben Assayag, et al.26 lower BChE activities are associated with an adverse outcome in patients suffering from acute ischaemic stroke, suggesting that higher BChE activities reflected a better recovery process. Calderon-Margalit, et al.22 found an association between reduced BChE activity and higher cardiovascular disease mortality risk, with similar sensitivities of low BChE and albumin levels as predictors of death. Consequently, although BChE activity may be positively associated with cardiovascular disease risk factors, as serum lipids, obesity, hypertension, and insulin resistance,12–14,22,25 its inverse relationship with the cardiovascular mortality, suggest that this later association is not mediated trough BChE relationship with these cardiovascular risk factors.22
In frail older patients, lower BChE and other esterase activities associated with higher inflammatory markers have been reported, suggesting that inflammation may be mediating the effects of frailty on drug metabolism.30 In our patients with subclinical chronic inflammation, a positive significant correlation was only obtained between serum BChE and hsCRP levels in males with a very low-grade inflammation and hsCRP < 1.0 mg/L (low cardiovascular disease risk). For hsCRP concentrations of 1-3 mg/L (average cardiovascular disease risk), the correlation between BChE and hsCRP was not significant; however, for hsCRP concentrations > 3-10 mg/L (high cardiovascular disease risk), the association between these two biochemical variables becomes significantly negative as in the patients with acute inflammation.
Increased serum BChE activity is considered a marker of liver steatosis,27,31s and associated with obesity, lipid profile, insulin resistance, hypertension,6,7,12–14,23,28,32 and fatty liver infiltration degree,33 and these clinical features may have pathophysiological relevance to the hepatic manifestation of metabolic syndrome such as non-alcoholic fatty liver disease. However, non-alcoholic fatty liver disease may ranges from simple liver steatosis to steatohepatitis with necro-inflammation and liver fibrosis. In our patients with subclinical chronic inflammation, an inverse association of the BChE and albumin levels with the APRI index was observed. In the cases with an APRI score < 0.5, excluding the presence of liver fibrosis with a high confidence level,16,17 the serum BChE and albumin levels were significantly higher, and hsCPR concentrations lower, than in cases with APRI score 0.5-1.5 or > 1.5, in some of which significant liver fibrosis may be supposed. On the contrary, a significant positive relationship was observed between the APRI score and hsCRP concentrations with the GGT and ALP activities, enzymes which have been previously associated with metabolic syndrome, cardiovascular disease, and inflammation.34–39
In the present study, a positive association between serum BChE activity and low-grade inflammation in accordance with the expectancy of Dass, et al.,5,6 was only observed for male patients with hsCRP levels < 1 mg/L; however, even in patients with subclinical chronic inflammation, for concen-trations of hsCRP > 3 mg/L, this association becomes negative as in the cases with acute inflammation. The negative association between BChE activity and inflammatory markers may have a causal basis, comparable to the inflammatory suppression of cytochrome P450 with decreased expression of mRNA and cytochrome protein synthesis.40 BChE and albumin may be considered negative inflammatory reactants, whose serum levels tend to diminish, on the contrary to positive inflammatory reactants, with increasing grades of chronic or acute inflammation. The previously described association between low BChE activities and higher mortality risk by cardiovascular disease,22 long-term dialysis,21 or acute stroke,26 would be mediated by an inverse relationship between the BChE activity and the grade of inflammation.
We have recently reported41 a significant positive correlation between BChE and hsCRP levels (p < 0.001) in a group of valproic acid-treated epileptic patients presenting subclinical chronic inflammation (hsCRP ≤ 10 mg/L); however, in the patients with hsCRP concentrations > 10 mg/L the correlation between these two variables becomes negative.41 The possible effect of the anticonvulsant drugs, particularly valproic acid, on the hsCRP concentrations has barely been studied,42 and this is an interesting subject that warrants studying in greater detail.