We report a case of symptomatic massive liver echinococcosis due to Echinococcus granulosus, unexpectedly found in a 34 year old woman living in Apulia, Italy. Based on size (max diameter 18 cm), clinical presentation, geographical area, and natural history of echinococcosis, we estimate that the initial infection should have occurred 9-20 yrs before. Presenting symptoms were those of typical mass effect with RUQ pain, pruritus, malaise, and recent weight loss. Abdominal ultrasound diagnosis of probable echinococcal cyst was subsequentely confirmed by positive serology and further detailed by radiological imaging. The cyst was massively occupying subdiaphragmatic liver segments and extending to the omentum and the stomach. The characteristics of the lesion were compatible with the WHO 2003 classification type CE2l, indicating a large active fertile cyst with daughter cysts. The cyst was successfully treated with medical therapy followed by surgery. The prevalence, diagnostic workup, management, and costs of echinococcosis are discussed in this case presentation.
Echinococcosis results from an infection with the metacestode stage of the tapeworm Echinococcus, a member of the Taeniidae family. Four species are involved in human disease: the most common forms are Echinococcus granulosus (EG) and Echinococcus multilocularis (EM) responsible for cystic echinococcosis and alveolar echinococcosis, respectively. Two other forms, namely E. oligarthrus and E. vogeli cause polycystic echinococcosis. Two new species, E. felidis and E. shiquicus may also contribute to human infection, although little is known.1
Both EG and EM may cause serious diseases, with high fatality rate and poor prognosis. EG represents a re-emerging infection with worldwide distribution, which differs by country and region, and is endemic in South and Central America, North and East Africa, Australia, Middle Est, and China.2,3 However, prevalence data are likely underestimated because of lack of systematic surveillance programs. EG is responsible for cystic hydatid disease in intermediate hosts, such as sheep by ingestion of shed eggs, while humans are incidental intermediate hosts. Molecular studies have identified 10 genetic types (G1-G10), which differ for geographic distributions, morphology, molecular characteristics, and host specificity.4,5 Cystic echinococcosis localizes to the liver, lungs and central nervous system. By imaging, hydatid cyst appears as unilocular fluid-filled structures with a bilaminated wall. The inner lamina is derived from EG and is the germinative layer which secretes hydatid fluid and generates brood capsules. If the inner layer and the brood capsules split up, daughter cysts can result. The outer lamina is a fibrous capsule resulting from a host granulomatous reaction. The initial immune response during the cyst development is cell-mediated with neutrophils, eosinophils and macrophages. After one week, an increase of IgE, IgG2 and IgG4 occurs, leading in some patients to allergic manifestations such as hives, itching and anaphylactic shock. Subsequently the infection stimulates T-helper TH1 and TH2 lymphocyte response.6–8
Subjects are often asymptomatic until the cyst compresses or ruptures into adjacent structures. When cysts enlarge, hepatomegaly, right upper hypocondrium or epigastric pain, vomiting and nausea may occur. Mass effects due to cystic compression are venous obstruction, portal hypertension, cholestasis, or the Budd-Chiari syndrome. Less commonly, if the cyst ruptures, the release of cystic fluid may lead to bacterial superinfection or anaphylaxis. According to the localization of cyst rupture, the following conditions may develop: biliary colic, cholangitis, obstructive jaundice, or pancreatitis, peritonitis, bronchial fistula or pulmonary hydatidosis. Moreover liver abscesses can represent secondary cystic bacterial infection.9,10
Serology and imaging exams are useful to diagnose and characterize EG cystic lesions. Routine laboratory tests may reveal mild eosinophilia, nonspecific leukopenia or thrombocytopenia, and nonspecific liver function abnormalities, however they are not diagnostic. Hydatid cysts can be discovered and characterized by computed tomography (CT) scanning, magnetic resonance imaging (MRI), and ultrasound. When diagnosis of hydatidosis is complete, the treatment of cystic echinococcus (CE) has two principal goals: elimination of EG and prevention of recurrence. The appropriate management is guided by cyst number, dimension, location, and presence of complications. Nowdays, several approaches ranging from chemotherapy, to surgery, and to percutaneous treatments are available according to the CE stage.11
Here, we report the case of a young women presenting with a large liver mass requiring surgery and we discuss the management of EG.
Clinical CaseA 34-years old woman was admitted to the emergency department because of intense right upper quadrant abdominal pain (visual analogue scale = 100 mm/100 mm) and pruritus.
The patient had been well until 6 months earlier, when she began to experience generalized pruritus, which was temporarily attributed to unspecified allergy. At observation she had malaise, weakness during minimal exercise, dyspeptic symptoms (postprandial fullness, slow digestion) with decreased appetite, and intermittent episodes of RUQ abdominal pain. She did not have diarrhea and the remaining medical history was unremarkable.
Three years earlier, the patient had given birth to a healthy child by cesarean surgery after an uncomplicated, full-term gestation. Prenatal testing for hepatitis B and C viruses (HBV and HCV) were negative, and the patient had normal liver function test. The patient smoked 15 cigarettes/day, and denied alcohol consumption. Because of pain and pruritus, she had used aspirin, nonsteroidal antiinflammatory drugs (NSAIDs) and antihistamines, with poor control.
Abdominal examination revealed RUQ tenderness with a negative Murphy’s sign, mild hepatomegaly (3 cm) but no splenomegaly or masses. The vital signs were normal, with no jaundice. The remainder of the examination was otherwise normal. Blood analyses showed a mild increase of eosinophils (4.0%, normal range 2-3%) and lipase of 300 U/L (normal range 114.0-286.0 U/L). The results of other liver and renal function tests, glucose, creatinine and electrolytes were normal. Abdominal ultra-sonography revealed a large (155 × 102 mm) anechoic multilocular fluid filled space with multiple membranes and characteristic internal septations with daughter cysts (with posterior acoustic enhancement) in the II, IV, V, VIII liver segments. The enzyme linked immunosorbent assay (Virion/Serion) to detect anti-Echinococcal IgG antibodies was positive. The patient confirmed that she had been in contact with dogs, cats, and horses throughout her life.
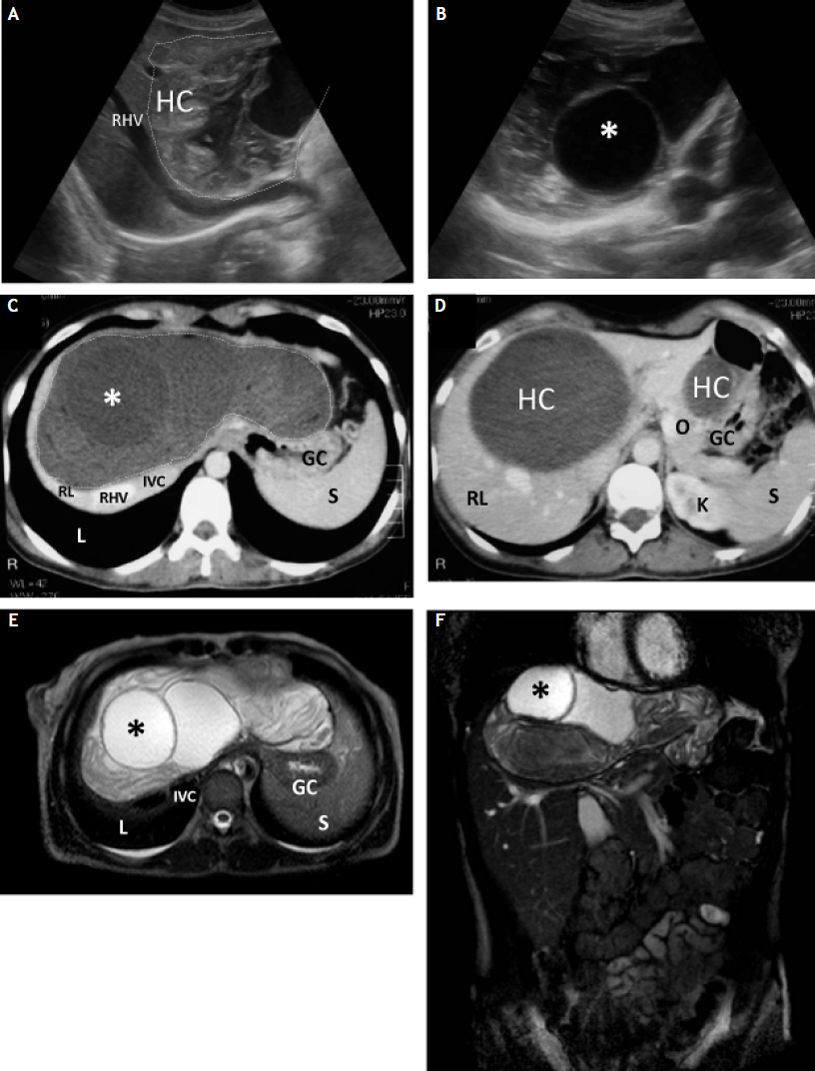
High resolution computed tomography (HR-CT) scanning showed a low-density cystic mass (largest diameter of 18 cm) occupying all liver segments. The mass extended to the left hypocondrium towards the greater omentum and stomach, with dislocation of portal vessels which were patent but congested (Figures 1A-1B). The intrahepatic vena cava was compressed. Perihilar biliary and intrahepatics ducts were dilated (Figures 1C, 1D). No other CE lesion was found in pelvis and lungs. A brain CT scan was also normal. A subsequent abdominal NMR was performed to better delineate the cyst capsule and to rule out complications (Figures 1E-1F).
Ultrasonographic (A, B), high-resolution CT scan (C, D), and NMR (E, F) images depicting the hydatic cyst in the liver. Ultrasound imaging of the liver shows A. Part of the large and multilocular hydatic cyst (dashed line) occupying segments II, IV, V and VIII, and containing fine debris and mixed hyper-iso- hypoechoic and anechoic (fluid) areas. The capsulated mass is 15.5 × 10.2 cm, and displaces and compresses the right hepatic vein. B. A large cystic image is shown, with a diameter of 6 cm (asterisk). According to WHO classification, the cyst is of the CE2l type (see table 1 for details). The CT scan confirms (C) the presence of the large hypodense cystic mass (18 cm max length, dashed line) extending to the left lobe of the liver within the greater omentum causing dislocation of portal vessels which are patent but congested. The left edge of the mass causes compression of the anterior wall of the stomach. D. CT cut showing the hypodense hydatic cyst extending in the lower part of the right hepatic lobe (RL) and omentum (O). The NMR obtained with contrast medium (gadolinium) comfirms the morphology of the large hyperintense multilocular cyst (E) 18 cm length with dislodgment of portal branches, inferior vena cava and perihilary bile ducts (mildly diilated). F. The complex architecture and intensity of the liver mass is shown on the frontal plane. The largest cyst is shown (astericks). GC: gastric corpus. HC: hepatic cyst. IVC: inferior vena cava. K: kidney. L: lung. O: omentum. RHV: right hepatic vein. RL: right lobe of liver. S: spleen.
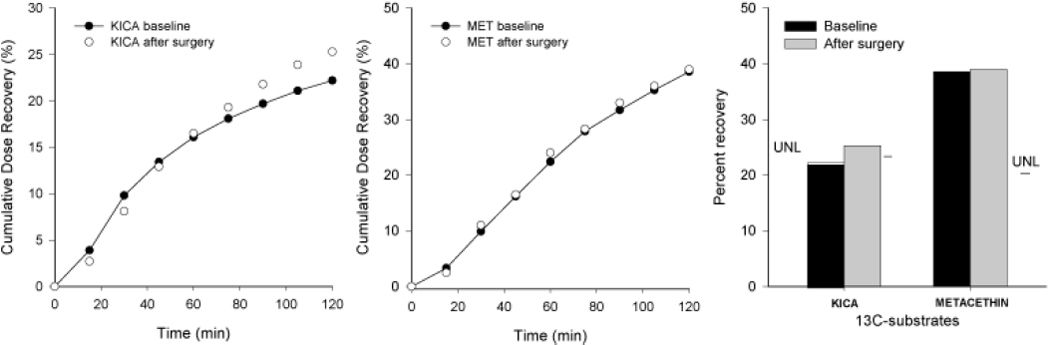
Because of the large involvement of liver parenchyma, and to assess the residual liver mass “function”, breath tests with two different substrates marked with the stable isotope 13C were used. 13C-methacetin was employed to assess residual cytosolic liver function, while 13C-Ketoisocaproic acid (KICA) was used to assess residual mitochondrial liver function. Procedures were performed according to established guidelines used at our unit.12,13 The results of 13C-KICA showed very slight mitochondrial malfunction confirmed by a lower Percentage of Cumulative Dose Recovery (CPDR) of 22% (normal: CPDR within 120 min ≥ 23% normal), while 13C-methacetin breath test was normal (Figure 2).
Results of the stable isotope (13C) breath test for functional analysis of residual liver mass by 13C-ketoisocaproic acid (KICA), and 13C-methacetin at baseline and 12 months after surgery. A. Time-dependent changes of Cumulative Dose Recovery (%) for KICA before and after surgery during 120 min, as a marker of mitochondrial liver function. Decreased metabolism of KICA is evident at baseline, indicating impaired mitochondrial function. B. 13C-methacetin, as marker of cytosolic liver function, did not shown any difference at baseline and after surgery. C. Maximal percent recovery of KICA and methacetin at baseline and after surgery, compared to upper-normal limit (UNL) for each substrate. For KICA the grey bar indicates recovery of residual liver mass function following surgery.
The patient was started on oral albendazole 400 mg b.i.d. for three cycles of 28 days with an interval of 14 days without treatment between the three cycles. Buprenorphine was used to reduce pain. No side effect was recorded while on therapy. Four months later, a subtotal pericystectomy was performed with limited resection of segment 3, cholecystectomy, and transcystic biliary drainage. The early post-operative course was complicated by local venous bleeding from the small gastric curvature where collateral shunts had developed due to initial portal hypertension secondary to previous CE compression. The patient was reoperated on at postoperative day 1 and the following course was uneventful. The patient underwent ultrasound, magnetic resonance and bioumoral exams follow-up at 3, 6, 12, and 24 months without recurrence of disease, and 13C-breath tests, including normal KICA 12 mo after.
DiscussionHydatidosis is a zoonosis due to EG. The patient lived in Southern Italy, a region where historically EG had a high prevalence. In Southern Italy livestock represents yet an important economic resource. Moreover, major factors closely linked to livestock are responsible for high prevalence of EG: farm dogs closely living with infected intermediate hosts (e.g. sheeps), poor veterinary supervision on farm animals and clandestine slaughter, and insufficient measures for the hygienic training of the livestock breeders, the hunters, the dog breeders and the members of their families.14
According to Grosso, et al.,15 the national incidence of hydatidosis in Italy is 1.3 cases per 100,000 inhabitants, with a maximum of 4-8 cases per 100,000 inhabitants in Sardinia. In particular, the incidence rate of echinococcosis is higher in Sardinia and Sicily, medium in Central-Southern Italy, and low (i.e., < 1%) in Northen Italy.15 Approximately 1,000 patients require surgery each year, with an annual mean incidence rate of surgery of 2.33 per 100,000 inhabitants in Apulia.16 Across Italy, incidence rates of surgical cases are 6.6-10.6 per 100,000 inhabitants in Sardinia, 1.57-5.6 in Emilia Romagna, 1.22 in Lombardia, 2.30 in Sicily, 1.76 in Basilicata, and 0.46 in Campania.
Our patient -who spent part of her childhood in the countryside- was likely infected by dogs, which are definitive hosts of EG. Indeed, the adult EG lives in the small bowel of dogs or other canids; gravid proglottids shed eggs in the feces and a suitable intermediate host (i.e., sheep, goat, swine, cattle, horses) ingests the eggs, which hatche in the small bowel with release of an oncosphere. The oncosphere can migrate through the intestinal circulatory system reaching different organs (especially liver and lungs) where the cycle continues with the cystic form that grows progressively. This step leads to the production of protoscoleces and daughter cysts. When the definitive host ingests organs of the infected intermediate host, the adult stage of EG is completed. Periodically, adult forms release eggs or gravid proglottids in dog feces, which can be ingested by humans (by consuming contaminated food or water).1 After ingestion, the protoscoleces evaginate, attach to the human intestinal mucosa, and develop into adult form. Localization of EG in target organs yelds to development of CE. In the liver, the right lobe is more frequently involved (60-85%). Cysts grow approximately from 1 to 5 mm in diameter per year and growth rates are usually related to the compliance of surrounding tissues.1 Thus, it is likely that the patient was infected over a period of 9-20 years, possibly with early involvement of the right lobe and subsequent involvement of additional segments. The case report illustrates the behavior of long-term asymptomatic hydatidosis followed by eventual development of pruritus and abdominal pain due to mass effect. The patient reported that she had undergone several extensive gynecological examinations (including ultrasound) while pregnant, all reported as normal. Likely, the liver cyst would have been discovered by performing an abdominal ultrasound focusing on the liver, but this was not the case. Routine laboratory tests revealed only a mild eosinophilia which was initially attributed to unspecified allergy.
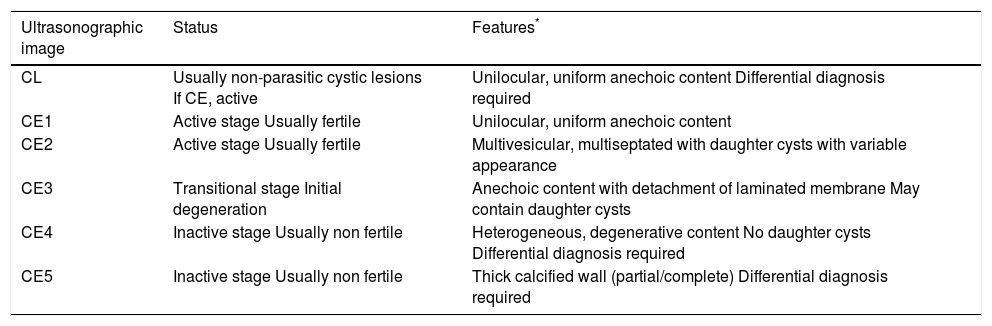
Abdominal ultrasonography, is useful to evaluate EG cysts for number, size, location, and life-cycle progression.17 Following the early classification by Gharbi, et al.,18 the World Health Organization (WHO) introduced a new standardized ultrasonographic classification, useful for the follow-up of patients after medical treatment. According to WHO, the score of hepatic cysts ranges from CL (s) (cystic lesion, “small”), to echinococcal CE1 to CE5. Clinically, cysts are divided into three main groups: active, transitional, and inactive (Table 1).19 We report here a case of CE2l cyst (i.e., > 10 cm large size), meaning an active (fertile) cyst in which US features are rather pathognomonic (Figure 1).
Type of cystic lesions and cystic echynococcosis.
| Ultrasonographic image | Status | Features* |
|---|---|---|
| CL | Usually non-parasitic cystic lesions If CE, active | Unilocular, uniform anechoic content Differential diagnosis required |
| CE1 | Active stage Usually fertile | Unilocular, uniform anechoic content |
| CE2 | Active stage Usually fertile | Multivesicular, multiseptated with daughter cysts with variable appearance |
| CE3 | Transitional stage Initial degeneration | Anechoic content with detachment of laminated membrane May contain daughter cysts |
| CE4 | Inactive stage Usually non fertile | Heterogeneous, degenerative content No daughter cysts Differential diagnosis required |
| CE5 | Inactive stage Usually non fertile | Thick calcified wall (partial/complete) Differential diagnosis required |
Adapted from WHO Informal Working Group 19. CE: cystic echynococcosis. CL: cystic lesion.
For each stage, size is expressed as “s” (small) (< 5.0 cm), “m” (medium) (5-10 cm), “l” (large) (> 10 cm).19
As compared to ultrasonography, CT and MRI may be more informative with respect to number of cysts, daughter cysts, rupture, calcification and exact location.20 MRI represents an even better diagnostic tool than CT, concerning cyst fluid within the matrix, and pre-surgical evaluation.20 A complete diagnostic workup is essential to choose the optimal management, and this was the case with the patient, due to the complex structure and size of the EG cyst.
Immunodiagnosis of EG by EG enzyme-linked immunosorbent assay (ELISA) and/or immunoblotting is useful to confirm the diagnosis; hydatid cyst fluid antigen B (AgB) and antigen 5 (Ag5) from EG are the most specific native antigens for the immunodiagnosis of CE.21,22 After surgery, the inactivity of EG was confirmed by the negative immunodiagnosis. Immunodiagnosis, however, lacks sensitivity and specificity, because cross-reactivity with antigens by other parasitic diseases can be present.23–25
One novel diagnostic aspect in this case was the use of stable isotope breath tests with two different substrates. To our knowledge, this is the first time that 13C-breath tests have been employed in liver echinococcosis. The functional targets in this case have been liver mithocondria and cytosol. Of note, mitochondrial function was impaired but recovered after surgery. Whether the liver mitochondrial malfunction was due to the mass effect per se or to impaired liver vascular supply or to production of local/systemic toxic factors by EG cyst or host reaction, requires further and prospective studies. 13C-breath tests have been proposed as fast, non-invasive, repeatable, reproducible, and simple functional methods in health and disease with special emphasis on liver function. Our group has provided evidences that especially KICA can yeld information about liver mithocondrial dysfunction in patients with steatohepathitis12,26 and hepatocarcinoma.27 Such breath tests might be used in a number of other conditions as well, although this might require a degree of expertise in special centers.13,26,28
The treatment of EG differs depending on overall evaluation of clinical, imaging, and immunodiagnosis findings. Current guidelines suggest a wait and see approach if serological and imaging data are concordant and hepatic cysts are small (diameter < 5 cm) and without complications (CE4-CE5 types), because these findings suggest inactivity. However, if serological and imaging data are not concordant or when CE-related complications are present, surgery is the first choice.29 Surgery becomes mandatory if one of the following is present: large CE with multiple daughter cysts, single CE with high risk of rupture, infected CE, CE that communicate with biliary tree or that exerts compression to vital organs.30,31 The major complication during the surgical maneuvers is CE break leading to spillage and widespread dissemination.30,31 The surgical procedures with the best results are total pericystectomy and liver resection in selected patients. Subtotal pericys-tectomy is a good surgical compromise, given the benign nature of this disease, especially in p atients whose cysts are close to vital structures, such as the hepatic hilum or the vena cava, as in our case.
A valid alternative to surgery in selected cases (CE1)29 is ultrasound-guided percutaneous treatment by PAIR (Puncture of the cyst, Aspiration of cyst fluid, Injection of a scolicidal agent, and Reaspiration of the cyst content). PAIR is the only method that offers a direct diagnosis of the parasitic nature of the cysts, and is useful to kill the germinal layers with scolicidal agents and to evacuate endocyst.32 However, anaphylactic shock, secondary echinococcosis, and chemical cholangitis have to be taken into account as complications. Lastly, radiofrequency thermal ablation represents another approach to destroy the germinal layer,33,34 but this procedure has not been adequately investigated.
The patient described here underwent surgery because of large cyst size, compression of adjacent anatomic structures, high risk of cyst rupture, and major abdominal pain with diminished quality of life. Before and after surgery, chemotherapy with albendazole was given to reduce infective risk. Treatment with mebendazole or albendazole (benzoimidazole carbamates) is used with increasing frequency for management of EC.30,35,36 Both drugs show similar effects leading to volumetric reduction and/or morphological changes (EG solidification, detaching of membranes, and calcification) until to CE death. Drugs are well tolerated, with occasional transient hypertransaminasemia.30,37
Prevention programs, albeit advisable, have been poorly investigated so far. A practical, recombinant vaccine (EG95) against EG has been proposed in sheep as intermediate host.38 Pilot vaccine/challenge experiments in dogs immunized with recombinant EG protein and live attenuated Salmonella, resulted in lower egg production.39,40 Such approaches represent potential public health measures to limit echinococcosis burden in high risk areas.
ConclusionsWe present the case of a massive echinococcal cyst of the liver causing abdominal pain and pruritis. Evaluation revealed normal liver function tests despite the volume of the cyst. The diagnosis of EC should be considered in areas with high prevalence of hydatidosis in farm animals and dogs, an early abdominal ultrasonography would have established the diagnosis of EG at an earlier stage where medical therapy would have been successful, avoiding later complications and need for surgery. Infection with E. granulosus should be seen as a re-emerging health problem in various countries of the world. Poor control programmes of echinococcal disease in animals may be responsible for this trend.
Abbrevations- •
Ag: antigen.
- •
CE: cystic echinococcus.
- •
CL: cystic lesions.
- •
CPDR: percentage of cumulative dose recovery.
- •
CT: computerized tomography.
- •
EG: echinococcus granulosus.
- •
ELISA: enzyme-linked immunosorbent assay.
- •
EM:Echinoccus multilocularis.
- •
HBV: hepatitis B virus.
- •
HCV: hepatitis C virus.
- •
HR-CT: high resolution computed tomography.
- •
KICA: ketoisocaproic acid.
- •
NMR: nuclear magnetic resonance imaging.
- •
NSAIDs: nonsteroidal antiinflammatory drugs.
- •
PAIR: puncture, aspiration, injection, reaspiration.
- •
RUQ: right upper quadrant.
- •
TH: T-helper cells.
- •
UNL: upper-normal limit.
- •
WHO: World Health Organization.
- •
L. Bonfrate: collected all the details of the case, followed the patient and wrote the first draft of the paper.
- •
F. Giuliante: followed the surgical part of the case and provided comments on the paper.
- •
G. Palasciano provided ultrasonography assessment of the case.
- •
J.T. LaMont commented on the clinical follow-up of the patient and revised the paper.
- •
P. Portincasa: collected and provided imaging photos, performed and interpreted the breath tests, revised the paper and is responsible for the integrity of the work as a whole.