Pegylated interferon (Peg-IFN) in combination with ribavirin is the standard of care in the treatment of chronic hepatitis C (HCV). Peg-IFN is known to have a number of side effects but severe respiratory complications are uncommon. We report two cases, one of Peg-IFN induced interstitial pneumonitis (IP) and the other of bronchiolitis obliterans organising pneumonia (BOOP) in patients with chronic hepatitis C infection. In general, respiratory complications of Peg-IFN are mild and resolve with withdrawal of Peg-IFN. However, as illustrated in our first case fatal interstitial pneumonitis can occur. We present a review of the available literature on Peg-IFN induced lung toxicity. In conclusion, pulmonary toxicity with Peg-IFN is rare but fatality can occur. We highlight the importance of maintaining a high index of suspicion for early diagnosis and prompt treatment, which includes withdrawal of Peg-IFN and consideration of corticosteroid treatment.
Approximately 200 million people worldwide are infected with the hepatitis C virus (HCV) with chronic infection in up to 85 percent, resulting in significant morbidity and mortality. Interferon based regimes have been the mainstay treatment of HCV for many years with pegylated interferon alpha (Peg IFN-α) replacing standard interferon in recent times. Pulmonary side effects of standard interferon are well known but rarely documented in the case of Peg IFN-α.
We present two cases of patients with chronic HCV commenced on anti-viral therapy for HCV who developed pulmonary toxicity. In case 1, the patient developed severe interstitial pneumonitis with subsequent acute respiratory distress syndrome (ARDS), resulting in death and in case 2 the patient presented with bronchiolitis obliterans organising pneumonia that responded to treatment.
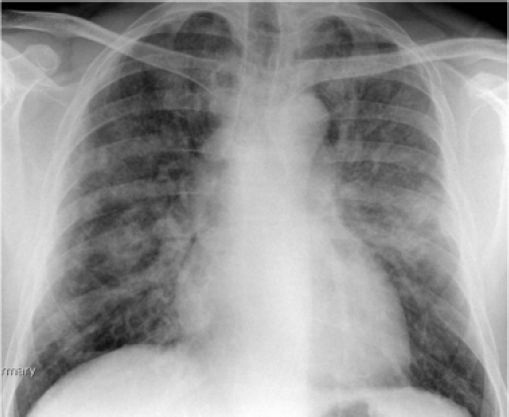
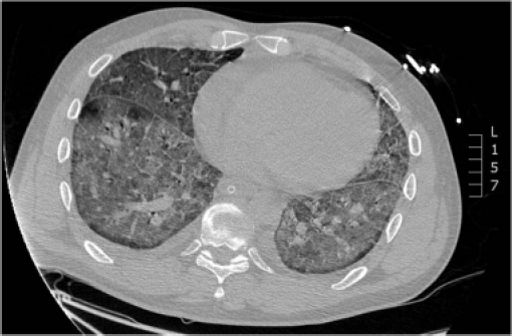
Case ReportsCase 1A 45-year-old Caucasian male was referred to the outpatient hepatology clinic for management of HCV genotype 1. He had a smoking history of 25 pack years and was thought to have acquired HCV through previous intravenous drug use. Clinical examination was unremarkable with no stigmata of chronic liver disease. Biochemical investigations showed normal liver function tests (LFT), and HCV PCR viral load of 4.8 × 102 IU/mL [Roche COBAS® TaqMan® HCV Test v2.0]. Liver biopsy revealed Ishak stage 3 fibrosis. He was initiated on combination treatment of once weekly subcutaneous injection of Peg IFN-α2b 1.5 µg/kg [Viraferon Peg®, Schering-Plough] and twice-daily oral ribavirin [Rebetol®, Schering-Plough]. He was monitored on a weekly basis in the specialist nurse led clinic. On the 9th week of treatment, he presented to the emergency department with sudden onset severe dyspnoea, cough and fever. Clinical and biochemical investigations showed that he was in acute type 2 respiratory failure, respiratory acidosis and hemodynamic instability. He was intubated, ventilated and transferred to the intensive care unit for cardio-respiratory support. Chest X ray (Figure 1) and a high resolution computed tomography (HRCT) scan (Figure 2) showed features of diffuse extensive bilateral ground glass opacification suggestive of interstitial pneumonitis. Peg IFN-α and ribavirin were discontinued and he was treated with intravenous hydrocortisone. A battery of investigations was negative including a septic screen (blood, urine and sputum culture), urinary legionella antigen, PCR of broncheo-alveolar lavage for respiratory viruses and serum mycoplasma immunoglobulin M antibody test. The patient continued to deteriorate and developed multisystem failure and died on the 16th day of hospitalisation. A post mortem examination concluded that the patient died from severe bilateral interstitial pneumonitis caused by Peg IFN-α2b, resulting in ARDS and subsequent multiorgan failure.
Case 2A 62-year-old Asian man was seen in the outpatient hepatology clinic for co-infection with chronic hepatitis B virus (HBV) and HCV genotype 3. There was no other significant medical history. Biochemical and viral studies showed ALT 105 iu/L (< 40 iu/L), HBV DNA PCR < 20 IU/mL [Roche COBAS® TaqMan® HBV Test v2.0] and HCV PCR of 3.1 × 106 IU/mL. Percutaneous liver biopsy revealed Ishak stage 6 fibrosis(cirrhosis). He was initiated on combination treatment with once weekly subcutaneous injection of Peg IFN-α2a 180 µg/week [Pegasys®, Roche] and twice-daily oral ribavirin [Copegus®, Roche]. He tolerated the treatment well and developed only minimal side effects of headache and lethargy.
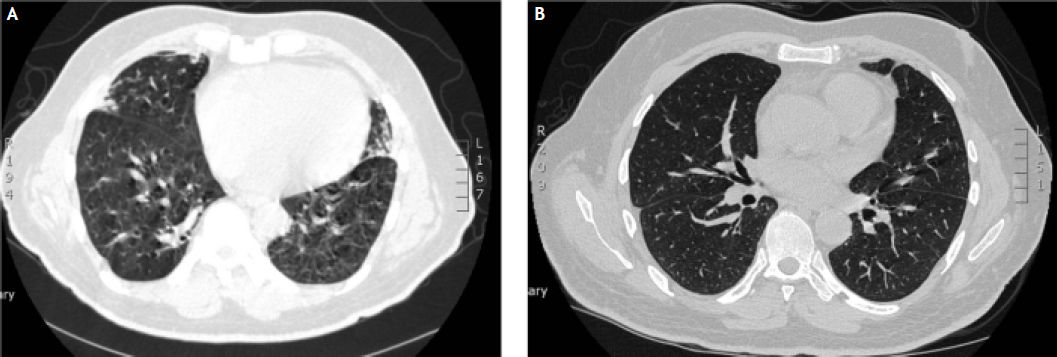
During the 15th week of his treatment, he complained of gradual onset dry cough, wheeze and exertional dyspnoea and clinical examination confirmed the presence of bilateral rhonchi. A subsequent chest X-ray was unremarkable. Despite treatment with salbutamol inhalers for two weeks his symptoms persisted with increasing severity of dyspnoea. In view of his progressive symptoms, he underwent a HRCT scan (Figure 3A). Based on the typical features of bronchial wall thickening and mosaic pattern of attenuation seen on the HRCT images, a diagnosis of bronichiolitis obliterans organising pneumonia (BOOP) was established and treatment with oral corticosteroids, prednisolone 30 mg once daily, was commenced. Over the next few weeks, the patient’s symptoms improved dramatically and a tapering dose of prednisolone was continued for a total of 20 weeks. He was able to complete the full 48 weeks of treatment with Peg IFN-α and ribavirin, and achieved sustained virological response (SVR). A repeat HRCT scan 1 year later confirmed complete resolution of the previously noted abnormalities (Figure 3B).
DiscussionHepatitis C (HCV) is a significant health problem worldwide with the burden of the disease predicted to increase in the next two decades. In England alone, approximately 6,400 new patients with chronic HCV are diagnosed annually and approximately 2,900 patients are commenced on anti-HCV treatment.1 With the availability of new generation protease inhibitors (PI), the number of patients qualifying for treatment is expected to increase. Growing number of patients are receiving either dual therapy (Peg-IFN plus ribavirin) or triple therapy (Peg-IFN plus ribavirin plus PI) and it is therefore imperative to be aware of the spectrum of possible complications.
Interferon is a naturally occurring glycoprotein that has antiproliferative, anti-inflammatory and immunomodulatory properties. Peg IFN-α has increased half-life compared to standard interferon, which allows for once weekly administration. The combination of Peg IFN-α and ribavirin is the approved and well-accepted standard of care (SoC) for chronic hepatitis C.2 Currently there are two Peg IFN-α molecules in use, Peg IFN-α2a and Peg IFN-α2b.The pharmacokinetics of these compounds differ but there is no conclusive evidence that one Peg IFN-α should be preferred to the other.2 Sustained virological response (SVR), the current measure of successful therapy, is defined as an undetectable serum HCV RNA at 24 weeks after cessation of treatment. With the current SoC, SVR can be achieved in up to 80% of patients with genotype 2 & 3 HCV and up to 50 % in genotype 1 HCV. The most common side effects of Peg IFN-α are headache, nausea, influenza-like symptoms, gastrointestinal upset, lethargy, anorexia and neuropsychiatric problems. Rare and severe complications include cardiac arrhythmias, cardiomyopathy and pancreatitis.
Pulmonary toxicity, although well described in the literature with standard interferon appears rare with Peg IFN-α. The spectrum of pulmonary complications associated with Peg IFN-α includes interstitial pneumonitis (IP), bronchiolitis obliterans organising pneumonia (BOOP), sarcoidosis, pleural effusion, exacerbation of asthma and acute respiratory distress syndrome. The mechanism of interferon-α induced pulmonary toxicity has not been elucidated, although two possible mechanisms postulated include direct toxicity to the pulmonary organ and indirect toxicity via immunological pathways.3
The overall incidence of pulmonary complications with Peg IFN-α is < 1%.4 Currently available information related to pulmonary complications with peg IFN therapy comes from case reports and the true incidences of IP and BOOP are not known. Though physicians treating HCV should be aware of these complications, it may be debatable whether patients should be consented for the possibility of pulmonary complications occurring at any stage of the HCV therapy. However, it is now our practice to inform our patients of these complications.
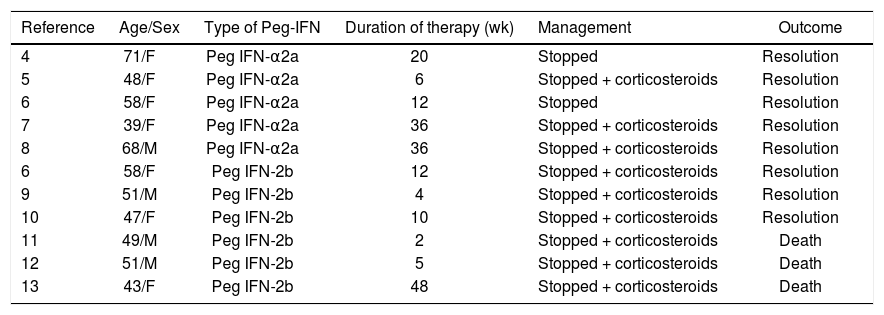
There are eleven reported cases in the literature of interstitial pneumonitis (IP) in association with Peg IFN.4–13 Patients were treated with Peg IFN-α2a in five cases4–8 and Peg IFN-α2b in six cases.9–13 All five reported cases of Peg IFN-α2a had favourable outcome following discontinuation of the drug with or without steroid therapy. Of the six reported cases of IP associated with Peg IFN-α2b, despite discontinuation of the Peg IFN and steroid therapy, three had fatal outcomes, i.e. IP associated with mortality was exclusively seen with Peg IFN-α2b. Table 1 summarises the characteristics of the reported cases of IP in association with Peg IFN. In general, IP tends to develop early on in the treatment cycle, usually between week 2 and week 16 treatment.8 The mean treatment time with Peg IFN prior to the diagnosis of IP was less than 12 weeks in 73% cases.14 Exceptionally it can occur later in the course of the treatment. Common presenting symptoms of IP are dyspnoea, dry cough and fever; less common symptoms include fatigue, arthralgia, myalgia and anorexia.14 HRCT is the most important non-invasive tool for in diagnosis of IP with ground glass and reticular opacities as the most common radiographic features.15 Treatment strategy includes discontinuation of Peg IFN-α with or without corticosteroid therapy. There are currently no guidelines recommending dosage and length of corticosteroid therapy.
Characteristics of case reports of interstitial pneumonitis (IP) associated with pegylated interferon therapy alpha 2a (Peg IFN-α2a) and alpha (Peg IFN-α2b) for hepatitis C virus (HCV).
| Reference | Age/Sex | Type of Peg-IFN | Duration of therapy (wk) | Management | Outcome |
|---|---|---|---|---|---|
| 4 | 71/F | Peg IFN-α2a | 20 | Stopped | Resolution |
| 5 | 48/F | Peg IFN-α2a | 6 | Stopped + corticosteroids | Resolution |
| 6 | 58/F | Peg IFN-α2a | 12 | Stopped | Resolution |
| 7 | 39/F | Peg IFN-α2a | 36 | Stopped + corticosteroids | Resolution |
| 8 | 68/M | Peg IFN-α2a | 36 | Stopped + corticosteroids | Resolution |
| 6 | 58/F | Peg IFN-2b | 12 | Stopped + corticosteroids | Resolution |
| 9 | 51/M | Peg IFN-2b | 4 | Stopped + corticosteroids | Resolution |
| 10 | 47/F | Peg IFN-2b | 10 | Stopped + corticosteroids | Resolution |
| 11 | 49/M | Peg IFN-2b | 2 | Stopped + corticosteroids | Death |
| 12 | 51/M | Peg IFN-2b | 5 | Stopped + corticosteroids | Death |
| 13 | 43/F | Peg IFN-2b | 48 | Stopped + corticosteroids | Death |
Bronchiolitis obliterans organising pneumonia (BOOP) caused by Peg IFN-α appears to be even rarer with only two previously documented cases.5,16 In these cases, infiltrates resolved with discontinuation of Peg IFN-α and corticosteroids treatment. Features of BOOP on HRCT scans are expiratory air trapping, mosaic pattern of attenuation and bronchial wall thickening.17
Interstitial lung diseases have also been reported with Albinterferon-a 2b (albIFN; Zalbin/Joulferon, Human Genome Sciences, Rockville, MD/Novartis, Basel, Switzerland), a novel, longer acting interferon formulation. AlbIFN had held promise with safety and efficacy in phase II trials. However, in phase III trials, increased pulmonary adverse events were seen with 1,200 µg of albIFN.18,19 The pulmonary complications associated with the higher doses of albIFN, including two deaths from interstitial lung disease and bacterial pneumonia19 caused significant concerns to the drug regulating agencies in the US and EU, resulting in discontinuation of the development of albIFN.20–22
In conclusion, pulmonary toxicity with pegylated interferon alpha is rare, but fatal pneumonitis can occur during the treatment of chronic hepatitis C. Mortality has been seen exclusively with Peg IFN-α2b. Physicians involved in the treatment of hepatitis C should be aware of pulmonary complications and need to maintain a high index of suspicion in patients presenting with respiratory symptoms. Prompt withdrawal of the therapy is the mainstay of treatment but steroid treatment may be essential.
Abbreviations- •
ARDS: acute respiratory distress syndrome.
- •
BOOP: bronchiolitis obliterans organising pneumonia.
- •
HBV: hepatitis B virus.
- •
HCV: hepatitis C virus.
- •
HRCT: high resolution computed tomography.
- •
LFT: liver function tests.
- •
PCR: polymerase chain reaction.
- •
Peg IFN-α: pegylated interferon alpha.
- •
SoC: standard of care.
- •
SVR: sustained virological response.