Chronic hepatitis C (CHC) is one of the most important causes of chronic liver disease in the world, potentially resulting in cirrhosis, hepatocellular carcinoma, and the need for liver transplantation. Liver biopsy is currently performed before therapy indication. Although, it is the golden standard there are many reasons to avoid or delay the procedure. APRI Score is an easy, low cost and practice alternative method which was described as an alternative for assessing structural changes in chronic hepatitis C (CHC). The rationale of this study was to observe the accuracy of APRI Score in comparison to liver biopsy in 400 patients divided into two groups of 200 carriers (Validation and Experimental groups respectively) selected at random or according to liver fibrosis staging (METAVIR). The ROC curves showed a concordance among these two methods of 92% and 88.5% when 1.05 was the cut off (F3 and F4), and 87% and 83%, on 0.75 cut offs (F2-F4). The discordance in advanced fibrosis staging (F3 and F4) was only 16 (8%) and 22 (11%) out of 200 patients in the experimental and validation groups, respectively. In 26 (13%) out of 200 patients in the experimental group and 34 (17%) out of 200 patients in the validation group, there was discordance between APRI Score and liver biopsy in moderate and advanced fibrosis (F2-F4). In conclusion APRI is a serological marker that has satisfactory sensitivity and specificity together with a high predictive value and it can be useful either in the absence of a biopsy or to reduce the frequency with which biopsies need to be carried out to monitor the evolution of chronic hepatitis C and the right moment for treatment indication.
Abbreviations:
HCV – Hepatitis C virus
CHC – Chronic hepatitis C
APRI Score - AST platelet ratio index
ROC – Receiver operative curve
NPN – Negative predictive value
PPV – Positive predictive value
AST – Aspartate amine transferase
IntroductionThe high prevalence of Chronic hepatitis C (CHC) and its impact on the development of cirrhosis and hepatocellular carcinoma warrant, for all patients with vire-mia, a therapeutic decision-making based on histopatho-logical results.
Liver biopsy is currently the gold standard for assessing structural changes in chronic hepatitis C (CHC). However, certain clinical conditions, such as thrombocy-topenia and increased prothrombin time, often prevent a biopsy being performed.1,2 Furthermore, the procedure is a costly and invasive one, its results are observer depen-dent,3–5 the patient’s authorization to carry out the biopsy may not be obtained and sampling error can also occur. The search for a practical, noninvasive method that could be used as an alternative to liver biopsy has been the subject of many studies.
In 2003 Wai et al6 published a study in which they validated the index known as APRI Score that establishes the relationship between serum aspartate aminotrans-ferase levels and platelet count. The parameters are simple, inexpensive and available in the remotest locations. It was proposed as an alternative method to liver biopsy with satisfactory sensitivity and specificity.
Patients and methodsPatients in the Experimental Group. Between May and December 2005, 200 patients were selected from a total of 553 chronic hepatitis C carriers who had been followed up for a minimum of 6 months at the Liver Outpatient Department in the Gastroenterology Service of the Francisco Morato de Oliveira State Hospital for Public Employees (HSPE). The starting point for patients selection was liver biopsy. Patients who met the inclusion criteria were chosen consecutively until achieving the necessary number of patients in each group. Five subgroups of 40 patients with METAVIR7 histological staging F0, F1, F2, F3 and F4 were established. When the number of patients in a subgroup had reached the maximum, the next patient chosen was excluded. This group of ideal distribution of fibrosis staging consisted of 107 male (53.5%) and 93 female (46.5%) patients, with an average age of 51 ± 11.6 years (21 to 84 years). The genotype 1b predominated in this group (54.5%), followed by the genotype 3a (23%).
Patients in the Validation Group. Between October and December 2007, a total of 200 patients were chosen at random (sealed envelopes) from the patient population; also randomly selected for fibrosis staging to assess the true accuracy of APRI in this population. This group of real life distribution consisted of 122 female (61%) and 78 male (49%) patients, with an average age of 50 ± 10 years (33 to 68 years). The genotype 1b predominated again in this group.
Patient Selection. The inclusion criteria were: positive serological tests; proven viremia; genotype determination; and a liver biopsy with a minimum of 10 portal spaces. The exclusion criteria were: alcohol intake • • 40 g/day for men or • • 20 g/day for women; a diagnosis of HIV or hepatitis B; and a liver biopsy with less than 10 portal spaces.
Serological tests. Third generation ELISA (MEIA Abbott Diag. USA) was used to detect HCV antibodies, and positive samples were confirmed by an Immunoblot test (RIBA®, Cambridge Biotech, USA).
Molecular tests. HCV RNA was detected by PCR, which was carried out at the Paulista School of Medicine or the Albert Einstein Jewish Hospital using a COBAS Amplicor® HVC kit (Roche Diagnostic Systems, USA). Genotypes were determined by Line Immunoassay® (In-noLia, Innogenetics, Belgium).
Histological Criteria. Assessment of histological staging followed the Metavir classification,7 according to which stage 0 is characterized by normal lobular architecture; stage 1 is defined by fibrous portal expansion; stage 2 is characterized by fibrous portal expansion with portal-portal septa; in stage 3 the lobular architecture is only partially preserved, with portal-portal and portal-central septa and outlines of nodules visible; and stage 4 is characterized by cirrhosis, which is clearly identifiable at biopsy, or a predominance of nodular areas (F0, F1, F2, F3 and F4). Liver biopsies were read by a single hepato-pathologist, who was blind regarding the APRI Score. Determination of AST and platelet levels. Serum AST levels were measured using an Advia 1650 autoanalyzer (Bayer Health Care), and platelet count was performed using an ADVIA hematology analyzer (Bayer Health Care) in accordance with the manufacturer’s instructions.
Calculation of APRI. To calculate APRI, serum AST activity and platelet count were used, both of which were obtained during the 15 days prior to the liver biopsy. APRI was calculated according to the formula proposed by Wai et al. in 2003,6 namely, [(AST of the sample/ reference AST) x 100] / platelets -3. The reference value for AST was considered to be 40 IU, which is the upper normal limit in our laboratory. The results obtained were used to plot two ROC (Receiver Operating Characteristic) curves to determine the best cutoff points for advanced fibrosis (F3 and F4). A second point on the curve was established for moderate and advanced fibrosis (Stages 2, 3 and 4), which we refer to as the treatment curve, as these patients should receive medication in accordance with the international consensus opinions on CHC.8–10 A significance level of 0.05 was used.
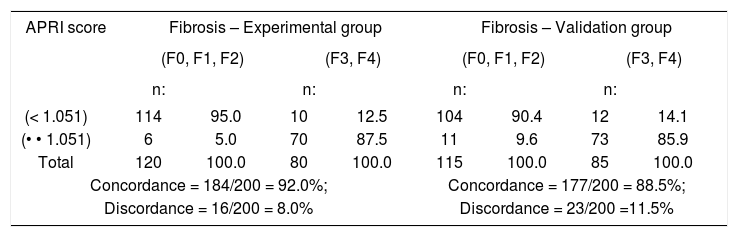
ResultsThe concordance and discordance between APRI Score and liver biopsy for a cutoff value of 1.05 are shown in table I. When the APRI Score was lower than 1.05, liver biopsy diagnosed advanced fibrosis staging (F3 and F4) in only 16 (8%) and 23 (11.5%) out of 200 patients in the experimental and validation groups, respectively. The concordance between these two methods for the patients in these groups with advanced fibrosis staging was 92% and 88.5%, respectively.
Concordance and discordance between APRI Score and Liver Biopsy when a cutoff of 1.05 was used for the APRI score. Experimental Group, consisting of 200 patients classified according to their staging, and Validation Group, consisting of 200 patients distributed at random according to their staging.
| APRI score | Fibrosis – Experimental group | Fibrosis – Validation group | ||||||
|---|---|---|---|---|---|---|---|---|
| (F0, F1, F2) | (F3, F4) | (F0, F1, F2) | (F3, F4) | |||||
| n: | n: | n: | n: | |||||
| (< 1.051) | 114 | 95.0 | 10 | 12.5 | 104 | 90.4 | 12 | 14.1 |
| (• • 1.051) | 6 | 5.0 | 70 | 87.5 | 11 | 9.6 | 73 | 85.9 |
| Total | 120 | 100.0 | 80 | 100.0 | 115 | 100.0 | 85 | 100.0 |
| Concordance = 184/200 = 92.0%; | Concordance = 177/200 = 88.5%; | |||||||
| Discordance = 16/200 = 8.0% | Discordance = 23/200 =11.5% | |||||||
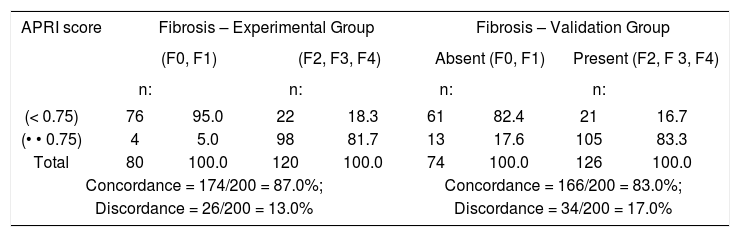
Table II shows the concordance and discordance between APRI Score and liver biopsy for a cutoff value of 0.75 for moderate and advanced fibrosis (F2 – F4). In 26 (13%) out of 200 patients in the experimental group and 34 (17%) out of 200 patients in the validation group, there was discordance between APRI Score and liver biopsy. The concordance between these two methods for patients in the two groups with moderate and advanced fibrosis staging was 87% and 83%, respectively.
Concordance and discordance between APRI Score and Liver Biopsy when a cutoff of 0.75 was used for the APRI score. Experimental Group, consisting of 200 patients classified according to their staging, and Validation Group, consisting of 200 patients distributed at random according to their staging.
| APRI score | Fibrosis – Experimental Group | Fibrosis – Validation Group | ||||||
|---|---|---|---|---|---|---|---|---|
| (F0, F1) | (F2, F3, F4) | Absent (F0, F1) | Present (F2, F 3, F4) | |||||
| n: | n: | n: | n: | |||||
| (< 0.75) | 76 | 95.0 | 22 | 18.3 | 61 | 82.4 | 21 | 16.7 |
| (• • 0.75) | 4 | 5.0 | 98 | 81.7 | 13 | 17.6 | 105 | 83.3 |
| Total | 80 | 100.0 | 120 | 100.0 | 74 | 100.0 | 126 | 100.0 |
| Concordance = 174/200 = 87.0%; | Concordance = 166/200 = 83.0%; | |||||||
| Discordance = 26/200 = 13.0% | Discordance = 34/200 = 17.0% | |||||||
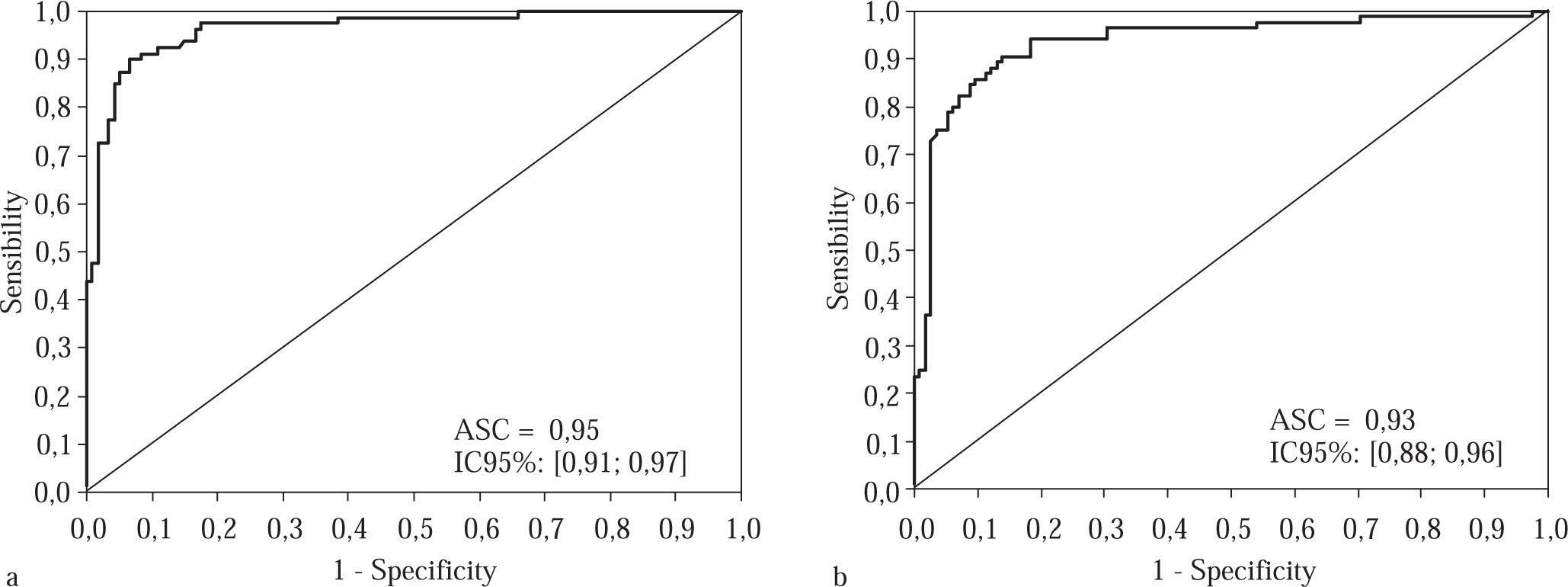
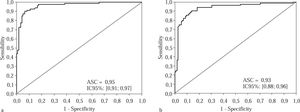
The results of ROC curve are presented in Curve 1, which represents the accuracy of this method for patients with advanced fibrosis (F3 and F4) based on a cutoff val-ue of 1.05, was plotted for both groups (Figure 1a and 1b). In the experimental group, the area under the curve was 0.96, CI 95% [0.93; 0.98], p < 0.001; we chose specificity 95% and found that the sensitivity at this point was 87.5%. The positive predictive value (PPV) found was 92.1%, and the negative predictive value (NPV) 91.9%, with an accuracy of 92.0%.
Curve for advanced fibrosis in the experimental group, consisting of 200 patients classified according to their staging, when a cutoff of 1.05 was used for the APRI scores. Figure 1b Curve for advanced fibrosis in 200 patients in the validation group selected randomly in terms of their staging, using a cutoff of 1.05 for the APRI scores.
In the validation group, with the same cutoff point of 1.05, the area under the curve was 0.93, CI 95% [0.88; 0.96], the specificity 90%, and the sensitivity 85.9%. The PPV was 86.9%, and the NPV 89.7%, with an accuracy of 88.5%.
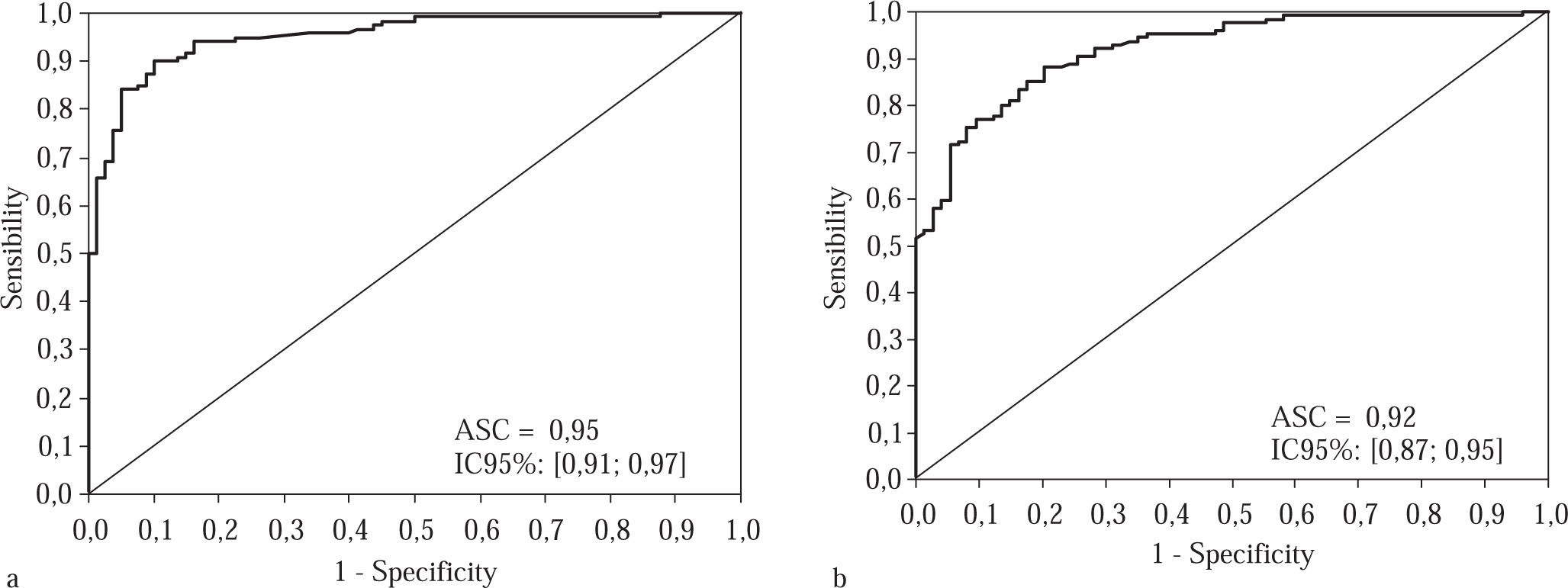
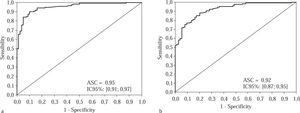
Curve 2, which we refer to as the «Treatment Curve», was plotted for moderate and advanced stages of fibrosis (F2, F3 and F4); the cutoff point chosen was 0.75 and the specificity 95%. In the experimental group, the area under the curve was 0.95, CI 95% [0.91; 0.97], p < 0.001 and the sensitivity was 81.7 %. The PPV was 96.1% and the NPV 77.6%, with an accuracy of 87.0% and a kappa of 83 (p < 0.001).
In the validation group, with the same cutoff point of 0.75 and an area under the curve of 0.92, CI 92% [0.87; 0.95], (Figure 2) the specificity fell to 82.4% and the sensitivity was 83.3%; the PPV was 89.0% and the NPV 74.4%, with an accuracy of 83.0% and kappa of 0.64 (p < 0.001).
Treatment curve for the experimental group, consisting of 200 patients classified according to their staging, when a cutoff of 0.75 was used for the APRI scores. Figure 2b Treatment curve for the validation group, which consisted of patients distributed randomly in terms of their structural changes, with a cutoff of 0.75
Liver biopsy has been an indispensable reference method for therapeutic decisions regarding Chronic Hepatitis C (CHC), as treatment indication is based on histo-logical findings (F2, F3, F4) and/or periportal inflammatory activity greater than or equal to grade two, whether associated with each other or not.8–11 Liver fibrosis staging is one of the main factors that influences the decision to indicate therapy for chronic hepatitis C carriers. According to current protocols and guidelines for treating hepatitis C, which reflect the fact that liver disease evolves more slowly in patients who do not have fibrosis or who have minimal fibrosis, treatment does not necessarily need to be started for these patients. In contrast, treatment should be indicated for patients with moderate or more severe fibrosis (F • •2) because of the risk of evolution to cirrhosis and its associated complications.8–11 However, biopsy is an invasive method, can be subject to error3–5 and is not free from complications such as bleeding, perforations and even death.13–16 In addition, some authors suggest that liver biopsies may yield false negative results, as only 1/50,000th of the parenchyma is represented in a sample.2–4 Subjective variations in the interpretation of biopsies occur in 10% to 20%3 of cases, and sample error can also occur. These limitations have generated doubts about the role of biopsies in the management of CHC patients.3,15 Alternative methods for assessing fibrosis have a number of advantages over histology, including low cost, their noninvasive nature and the absence of contraindications, such as severely altered pro-thrombin activity or thrombocytopenia. Researchers have been trying to find alternative methods for diagnosing fi-brosis, and APRI is considered one of the simplest and least expensive. In this study we used this method in a group stratified into the five stages of fibrosis and found that it had a higher diagnostic performance than that described in most reports in the literature.18–21 These results encouraged us to evaluate APRI in another group of the same size but distributed in random in terms of fibrosis staging (the validation group) in order to assess the true performance of this diagnostic test when used in the population receiving treatment in various hepatology departments.
The results for the validation group agree with those obtained in an earlier study by our group involving 50 patients distributed randomly in terms of fibrosis staging.23 The high specificity found in these 50 patients was probably due to the marked predominance of patients with stage 3 or stage 4 fibrosis. The results obtained for this group were also better than those obtained by other authors.6,20 The higher sensitivity found in the present study may be due to the use of a cutoff value of 1.05, which is lower than that chosen by Wai et al.6 These authors have used four cut off points in order to evaluate different stages of liver fi-brosis. Other authors have used two cut off points with good results,22,23 similar to ours. In our study aiming specifically the diagnosis of moderate to advanced fibrosis, a single cut off point was used based on high percentage of specificity in the ROC curve.
The use of APRI as a screening test requires that definite cutoff points be established.
In the population that we studied, cutoff values of 0.75 and 1.05 proved to be the most suitable reference values for defining the need for treatment and diagnosing advanced fibrosis, respectively. The choice of cutoff values was based on a specificity of 95% and 90% for advanced or moderate fibrosis respectively to reduce the likelihood of false negative results and thus improve the positive predictive value of the method.
According to the data we obtained using a cutoff point of 1.05, the accuracy of this method allows it to be used as a parameter for treatment indication. However, the accuracy achieved with a cutoff value of 0.75 shows that this method needs to be used in conjunction with other alternatives to a liver biopsy for assessing fibrosis. In 26 of the 200 patients in the experimental group and 34 of the 200 patients in the validation group with staging • • 2, there was discordance between the APRI score and the liver biopsy. In all of these patients, serum AST was either less than twice the upper normal limit or very high (more than five times the upper normal limit). No other data that appeared to interfere with the calculation of APRI were found. A possible explanation for this finding is that in the presence of flare, or increased immuno-pathological activity, the increase in AST may reflect a necroinflammatory lesion observed in the biopsy, i.e., it may represent altered periportal activity rather than pro-gression of the fibrosis. These findings suggest that calculation of APRI should be avoided during periods of intense inflammatory activity, as this could lead to false positive results.
Alternative methods for assessing fibrosis can be divided into nonspecific methods and those that reflect the extracellular matrix (ECM) metabolism.24–27 The latter include procollagen, hyaluronic acid, laminin and YKL-40, as well as metalloproteins and their inhibitors.27 Nonspecific methods include simple biochemical tests and he-matological parameters, such as the aspartate aminotrans-ferase (AST) to alanine aminotransferase (ALT) ratio,28,29 APRI,6 Fibrotest,30,33 Forns score20 and platelet count.31,32 One imaging method that is being used is liver elastogra-phy34 or FibroScan, which appears to have the best accuracy of the tests mentioned above, although it is more expensive and not yet routinely used in Brazil. Among extracellular matrix (ECM) markers, higher levels of hyaluronic acid are found in CHC carriers who develop cirrhosis, in whom the levels appear to be directly proportional to the clinical severity of the disease.25–27 However, this test is also complicated to carry out and is more expensive. These considerations, and the frequent need for a more practical, less onerous alternative method, have motivated a better assessment of APRI in developing countries as an alternative to liver biopsy for evaluating structural alterations and deciding whether to indicate treatment. Such an alternative would be of great value, particularly in those patients in whom a liver biopsy cannot be carried out. In practice, APRI is already being used by a number of authors18,19 in some patients for whom an invasive procedure is absolutely contraindicated.
These alternative methods may also be indicated for the assessment of fibrosis progression in CHC carriers who have already had a liver biopsy and for whom treatment was not indicated at the time, although prospective evaluations are needed to confirm this assumption. These patients are currently advised to repeat the histopathologi-cal examination every 3 to 5 years so that the progress of the disease can be reassessed.21,36 They are therefore exposed to new biopsies several times during clinical follow-up. It is worth remembering that many of these patients do not have any significantly increased transami-nase levels and may progress silently to cirrhosis.21
According to current consensus opinions and guidelines for treatment of hepatitis C, therapy is indicated in patients with structural alterations corresponding to stage two or higher.8,9 Compared with the gold standard, the performance of APRI in our patients was reasonable in terms of sensitivity, with 17% of the patients for whom treatment would be indicated not being detected.
In light of the above, we conclude that APRI is a sero-logical marker that has satisfactory sensitivity and specificity together with a high predictive value and that it can be useful either in the absence of a biopsy or to reduce the frequency with which biopsies need to be carried out to monitor the evolution of chronic hepatitis C.
When used with other alternatives to liver biopsy, APRI appears to have an accuracy comparable to that of a biopsy and can replace histopathological analysis for therapeutic indications.37