Background and aim: Primary biliary cirrhosis (PBC) is a chronic cholestatic disease of autoimmune origin and has a genetic component. Although it has been reported that the prevalence of the HLA-DRB1*08 allele is high in various populations, the prevalence of HLA alleles in Mexican PBC patients has not been described. The aim of this study was to quantify the prevalence of HLA-B/-DR alleles in Mexican PBC patients.
Materials and methods: A case-control transversal study was performed during January and July 2008 with adult patients diagnosed with PBC. Cases were defined as subjects with PBC and controls were obtained from healthy subjects evaluated for bone marrow transplantation. Laboratory was performed at the moment of diagnosis. HLA-B/-DR allele frequency was obtained by gene counting and allele presence was determined by PCR-SSP procedure. Descriptive statistics between groups was evaluated by Chi-square with Yates correction.
Results: Nine patients (seven females and two males, mean ± SD age = 57.5 ± 10.5 years) were studied. The most prevalent alleles were HLA-DRB1*01 (n = 4), DRB1*04 (n = 4), B*39 (n = 34), B*14 (n = 3), and B*51 (n = 2). Linkage disequilibrium was detected for alleles HLA-B*39/DRB1*04 (n = 3), HLA-B*14/HLA-DRB1*01 (n = 2), and B*51-DRB1*04 (n = 1).
In conclusion:Mexican PBC patients express genes of native and Mediterranean origin.
Manuscript disclosure Authors do not have any financial or other relationship to disclose.
Grants This study was supported by Medica Sur Clinic & Foundation.
IntroductionPrimary biliary cirrhosis (PBC) is a chronic nonsuppurative cholestatic liver disease with a genetic component and is induced by ambient factors that are not well de-fined.1 First described by Ahrens et al,2 it is characterized by immunologically mediated biliary duct destruction that causes liver fibrosis and, eventually, cirrhosis. The disease usually affects Caucasian women aged 40-60 years3 and has an incidence of 2.7 cases per 100,000 person years.4 Most patients are asymptomatic at the early stage of the disease.5,6 About 20% of cases develop fatigue, jaundice, and pruritus.6–8 Patients with PBC present with antimitochondrial antibodies (AMAs) and antinuclear antibodies (95% and 70% of cases, respectively), which can be detected years before the onset of symp-toms.9 PBC is also associated with autoimmune diseases such as rheumatoid arthritis,10 autoimmune thyroiditis,11 Sjogren syndrome,12 scleroderma, and CREST syn-drome.13 Although its pathophysiology has not been fully elucidated, PBC is a chronic autoimmune disease in which certain ambient14–17 and infectious agents17–19 disrupt immune responses and perpetuate autoimmunity by molecular mimetism.20 PBC appears to have a genetic component because there is a 6% incidence of PBC among first-line relatives of index patients18,21 and a high concordance rate between monozygotic and dizygotic twins.22 As PBC is associated with other diseases of the immune system,23 the genes responsible for PBC may be located in the class II major histocompatibility complex (MHC), a very polymorphic and dense region. This region occurs on chromosome 6p21, which also contains other genes that affect the immune response. It’s believed that CD4+ and CD8+ T cell receptors are involved in certain autoimmune diseases and that they react specifically with pyruvate dehydrogenase complex E2 (PDC-E2).24–26 As CD4+ and CD8+ T cell receptors are present in the portal triads of patients with PBC and infiltrate the necrotic biliary ducts,27 the PDC-E2 reaction probably also occurs in PBC. PDC-E2 is normally present inside the mitochondrial membrane. It has been proposed that it is aberrantly expressed in the biliary epithelium of PBC patients via molecular mimetism.28 Autoimmunity is thus caused by an immune response triggered by monoclonal antibodies against the mitochondrial lipoyl domain of PDC-E2.
PBC is genetically complex and is characterized by incomplete penetrance, low Mendelian heritability, and a variable phenotype.29 Although there is evidence for a genetic component to the pathophysiology of PBC, its role has not been fully determined. As the male to female ratio among PBC patients is 8-10:118,30 and the frequency of X monosomy in the peripheral leukocytes of women with PBC is high,18 it is believed that female sex and genetics play important roles in the pathophysiology of PBC. The genes involved occur on chromosome 2q and include the cytotoxic lymphocytic antigen 4 (CTL 4) gene, the aggregating interleukin 1 gene, the caspase 8 gene, and natural macrophage associated antigen 1.29,31 Evidence of autoimmune phenomena, familial aggregation, female predominance, and monozygotic twin concordance in PBC patients indicates that the MHC and its immune regulatory genes, the human leukocyte antigens (HLAs), play important roles.
Of the frequencies of HLA alleles in PBC, only that of the HLA-DR8 (DRB1*08) allele family has been confirmed in cohort studies conducted in the United States (14.9% vs 6.5%, OR = 3.3, p < 0.01; 19.4% vs 8.7%, OR = 2.55, p < 0.01),32–34 Japan (35.5% vs 7.4%, OR = 6.84,p < 0.0001),35 Sweden (29.3% vs 11.4%, OR = 3.22, p < 0.001),36 and Italy and the United Kingdom (18% vs 6%, OR = 3.15, p < 0.02 and 12% vs 4%, OR = 3.05, p < 0.0008, respectively).31 Information on the frequencies of other alleles is scarce. An association between HLA-DR2 and PBC has been reported for Japan (68% vs 30%, OR = 5.0, p < 0.007)37,38 and associations between PBC and HLA-DRB1*0701 and HLA-DRB1*03 have been reported for China (29.2% vs 13.9%, OR = 2.55, p < 0.05 and 18.4% vs 7.2%, p < 0.05, respectively).39 A study conducted among Brazilians did not observe any association between PBC and the HLA-DR or HLA-DQ al-lele loci, which suggests that the background factors responsible for PBC in this mestizo population (Cau-casoid, Black, and Amerindian) differ from those in other populations.40 Although a study performed in Italy found that the DRB1*11 (10.7% vs 27.6%, OR = 0.03, p < 0.05)41 and DRB1*1342 alleles were protective against PBC, a study conducted in the United Kingdom failed to reproduce these findings.41 Furthermore, a study conducted in the United States suggested that diminution of the DRB1*1501-DQA1*0102-DQB1*0602 and DRB1*1302-DQA1*0102-DQB1*0604 alleles protects against PBC.34
The Mexican cirrhosis mortality rate is one of the highest in the world and constitutes the third highest cause of death among the general population of Mexico (27,566 deaths in 2005) and the second highest cause of death among adults aged between 15 and 64 years (17,872 deaths in 2005).43,44 The number of cirrhosis cases in Mexico is predicted to increase until the year 2050.45 Although PBC is the fourth highest cause of cirrhosis in Mexico,46 the genetic background of this disease has not been studied among the Mexican population, which characterized by a mestizo ethnicity.47
Material and methodsPatientsAdult subjects (> 18 years of age) with clinical, laboratory and biopsy criteria of PBC3 without other autoimmune diseases were included and designated as cases. All patients were born in Mexico and their ancestry was obtained. Controls were obtained from an study in healthy Mexican population evaluated for bone marrow transplantation performed by Barquera et at.41 The study protocol was approved by the Ethics Committee of Medica Sur Clinic & Foundation, and all patients gave informed consent before participating in the study.
Laboratory analysesSerum concentrations of albumin, bilirubin (total, direct, and indirect), alkaline phosphatase (AlkP) and gam-maglutamyl transpeptidase (GGT) were determined. We also determined immunological profiles by measuring levels of antinuclear antibodies (ANA), antimitochondri-al antibodies (AMA), anti-smooth muscle antibodies, and liver/kidney antimitochondrial antibodies.
HLA typingSamples of peripheral blood (4.5 mL) were obtained for DNA isolation.48 HLA class I (HLA-B) and HLA class II (HLA-DRB1) were then typed using a PCR-SSP procedure (Pel-Freez HLA-A/B/DR/DQ SSP Unitray®, Brown Deer, Wisconsin, USA). A chemiluminescence method was used to detect hybridization.49
Statistical analysisHLA allele and haplotype frequencies were determined by gene counting. The haplotype frequency for the control was obtained from historical records of 381 healthy Mexican subjects enrolled in the study by Barquera et al.47 Patient characteristics were expressed as means and standard deviations. Comparisons of allele frequencies between patients and controls were analyzed using the Chi-square method with Yates’ correction and significance was declared at p < 0.05. ORs and 95% CIs were determined to establish associations between variables. Statistical analyses were performed using SPSS PC/ v16.0 software (SPSS, Chicago, Illinois, USA).
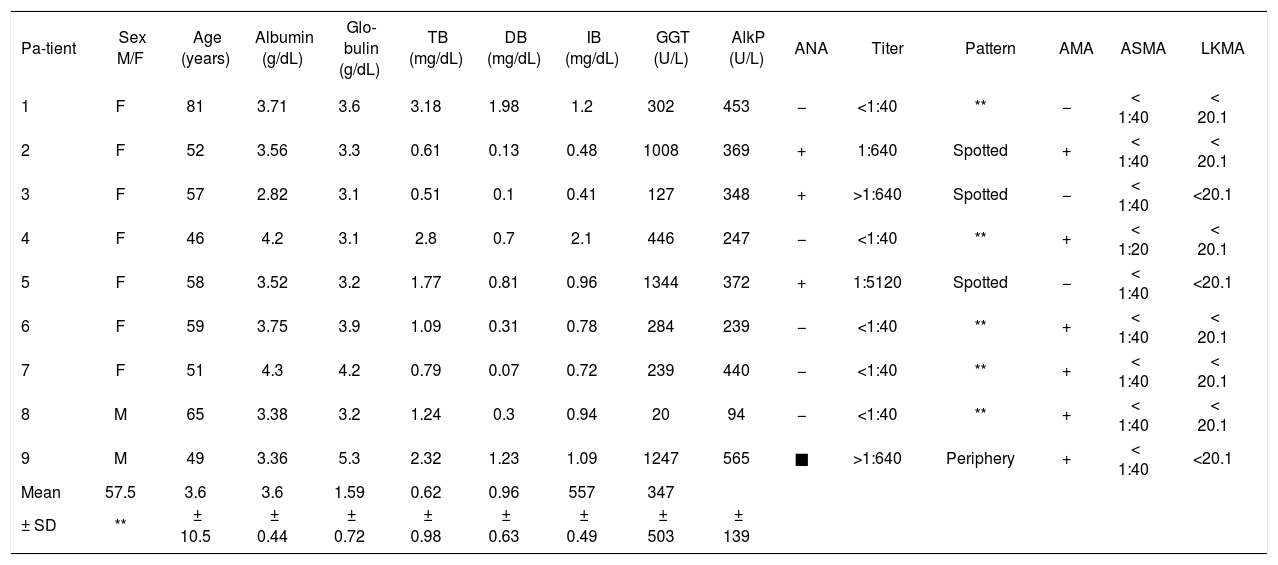
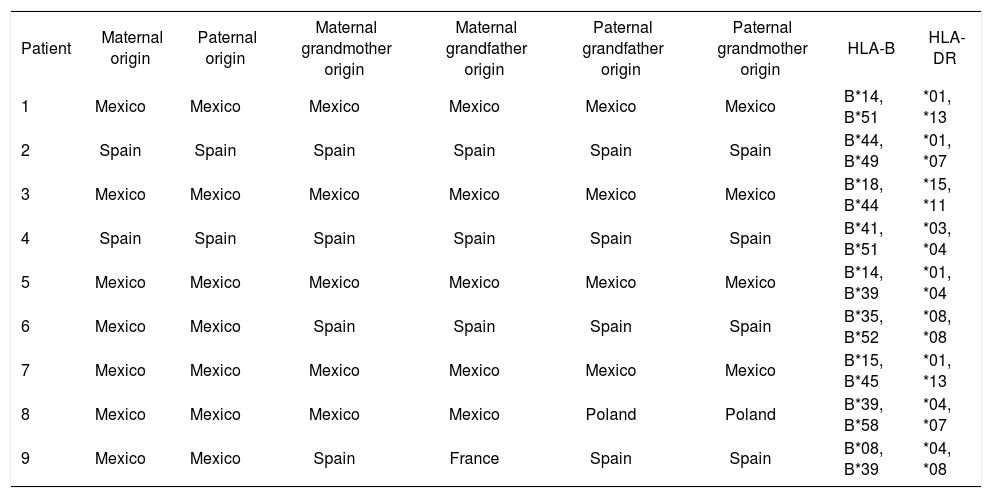
ResultsNine patients were enrolled in the study (seven women and two men, mean age = 57.5 ± 10.5 years). The results of laboratory and immunological analyses are shown in table I. Table II shows the ancestry and HLA al-leles of the patients. The haplotype frequencies of patients are presented in table III and alleles in linkage disequilibrium are presented in table IV.
Blood metabolite levels and HLA alleles for PBC patients.
| Pa-tient | Sex M/F | Age (years) | Albumin (g/dL) | Glo-bulin (g/dL) | TB (mg/dL) | DB (mg/dL) | IB (mg/dL) | GGT (U/L) | AlkP (U/L) | ANA | Titer | Pattern | AMA | ASMA | LKMA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 81 | 3.71 | 3.6 | 3.18 | 1.98 | 1.2 | 302 | 453 | − | <1:40 | ** | − | < 1:40 | < 20.1 |
| 2 | F | 52 | 3.56 | 3.3 | 0.61 | 0.13 | 0.48 | 1008 | 369 | + | 1:640 | Spotted | + | < 1:40 | < 20.1 |
| 3 | F | 57 | 2.82 | 3.1 | 0.51 | 0.1 | 0.41 | 127 | 348 | + | >1:640 | Spotted | − | < 1:40 | <20.1 |
| 4 | F | 46 | 4.2 | 3.1 | 2.8 | 0.7 | 2.1 | 446 | 247 | − | <1:40 | ** | + | < 1:20 | < 20.1 |
| 5 | F | 58 | 3.52 | 3.2 | 1.77 | 0.81 | 0.96 | 1344 | 372 | + | 1:5120 | Spotted | − | < 1:40 | <20.1 |
| 6 | F | 59 | 3.75 | 3.9 | 1.09 | 0.31 | 0.78 | 284 | 239 | − | <1:40 | ** | + | < 1:40 | < 20.1 |
| 7 | F | 51 | 4.3 | 4.2 | 0.79 | 0.07 | 0.72 | 239 | 440 | − | <1:40 | ** | + | < 1:40 | < 20.1 |
| 8 | M | 65 | 3.38 | 3.2 | 1.24 | 0.3 | 0.94 | 20 | 94 | − | <1:40 | ** | + | < 1:40 | < 20.1 |
| 9 | M | 49 | 3.36 | 5.3 | 2.32 | 1.23 | 1.09 | 1247 | 565 | ■ | >1:640 | Periphery | + | < 1:40 | <20.1 |
| Mean | 57.5 | 3.6 | 3.6 | 1.59 | 0.62 | 0.96 | 557 | 347 | |||||||
| ± SD | ** | ± 10.5 | ± 0.44 | ± 0.72 | ± 0.98 | ± 0.63 | ± 0.49 | ± 503 | ± 139 |
M – male, F - female, TB - total bilirubin, DB – direct bilirubin, IB – indirect bilirubin, AlkP – alkaline phosphatase, GGT – gammaglutamyl transpeptidase, ANA - antinuclear antibody, AMA – antimitochondrial antibody, ASMA – anti-smooth muscle antibody, LKMA - liver/kidney microsomal antibody, SD - standard deviation.
Ancestry and HLA alleles of patients.
| Patient | Maternal origin | Paternal origin | Maternal grandmother origin | Maternal grandfather origin | Paternal grandfather origin | Paternal grandmother origin | HLA-B | HLA-DR |
|---|---|---|---|---|---|---|---|---|
| 1 | Mexico | Mexico | Mexico | Mexico | Mexico | Mexico | B*14, B*51 | *01, *13 |
| 2 | Spain | Spain | Spain | Spain | Spain | Spain | B*44, B*49 | *01, *07 |
| 3 | Mexico | Mexico | Mexico | Mexico | Mexico | Mexico | B*18, B*44 | *15, *11 |
| 4 | Spain | Spain | Spain | Spain | Spain | Spain | B*41, B*51 | *03, *04 |
| 5 | Mexico | Mexico | Mexico | Mexico | Mexico | Mexico | B*14, B*39 | *01, *04 |
| 6 | Mexico | Mexico | Spain | Spain | Spain | Spain | B*35, B*52 | *08, *08 |
| 7 | Mexico | Mexico | Mexico | Mexico | Mexico | Mexico | B*15, B*45 | *01, *13 |
| 8 | Mexico | Mexico | Mexico | Mexico | Poland | Poland | B*39, B*58 | *04, *07 |
| 9 | Mexico | Mexico | Spain | France | Spain | Spain | B*08, B*39 | *04, *08 |
HLA – human leukocyte antigen.
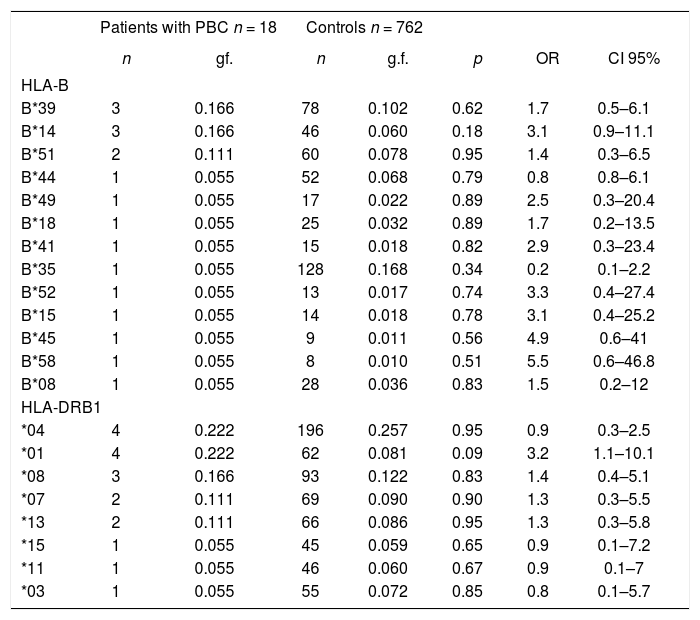
HLA-B and DRB1 frequencies in PBC patients and controls.
| Patients with PBC n = 18 | Controls n = 762 | ||||||
|---|---|---|---|---|---|---|---|
| n | gf. | n | g.f. | p | OR | CI 95% | |
| HLA-B | |||||||
| B*39 | 3 | 0.166 | 78 | 0.102 | 0.62 | 1.7 | 0.5–6.1 |
| B*14 | 3 | 0.166 | 46 | 0.060 | 0.18 | 3.1 | 0.9–11.1 |
| B*51 | 2 | 0.111 | 60 | 0.078 | 0.95 | 1.4 | 0.3–6.5 |
| B*44 | 1 | 0.055 | 52 | 0.068 | 0.79 | 0.8 | 0.8–6.1 |
| B*49 | 1 | 0.055 | 17 | 0.022 | 0.89 | 2.5 | 0.3–20.4 |
| B*18 | 1 | 0.055 | 25 | 0.032 | 0.89 | 1.7 | 0.2–13.5 |
| B*41 | 1 | 0.055 | 15 | 0.018 | 0.82 | 2.9 | 0.3–23.4 |
| B*35 | 1 | 0.055 | 128 | 0.168 | 0.34 | 0.2 | 0.1–2.2 |
| B*52 | 1 | 0.055 | 13 | 0.017 | 0.74 | 3.3 | 0.4–27.4 |
| B*15 | 1 | 0.055 | 14 | 0.018 | 0.78 | 3.1 | 0.4–25.2 |
| B*45 | 1 | 0.055 | 9 | 0.011 | 0.56 | 4.9 | 0.6–41 |
| B*58 | 1 | 0.055 | 8 | 0.010 | 0.51 | 5.5 | 0.6–46.8 |
| B*08 | 1 | 0.055 | 28 | 0.036 | 0.83 | 1.5 | 0.2–12 |
| HLA-DRB1 | |||||||
| *04 | 4 | 0.222 | 196 | 0.257 | 0.95 | 0.9 | 0.3–2.5 |
| *01 | 4 | 0.222 | 62 | 0.081 | 0.09 | 3.2 | 1.1–10.1 |
| *08 | 3 | 0.166 | 93 | 0.122 | 0.83 | 1.4 | 0.4–5.1 |
| *07 | 2 | 0.111 | 69 | 0.090 | 0.90 | 1.3 | 0.3–5.5 |
| *13 | 2 | 0.111 | 66 | 0.086 | 0.95 | 1.3 | 0.3–5.8 |
| *15 | 1 | 0.055 | 45 | 0.059 | 0.65 | 0.9 | 0.1–7.2 |
| *11 | 1 | 0.055 | 46 | 0.060 | 0.67 | 0.9 | 0.1–7 |
| *03 | 1 | 0.055 | 55 | 0.072 | 0.85 | 0.8 | 0.1–5.7 |
g.f. – genetic frequency.
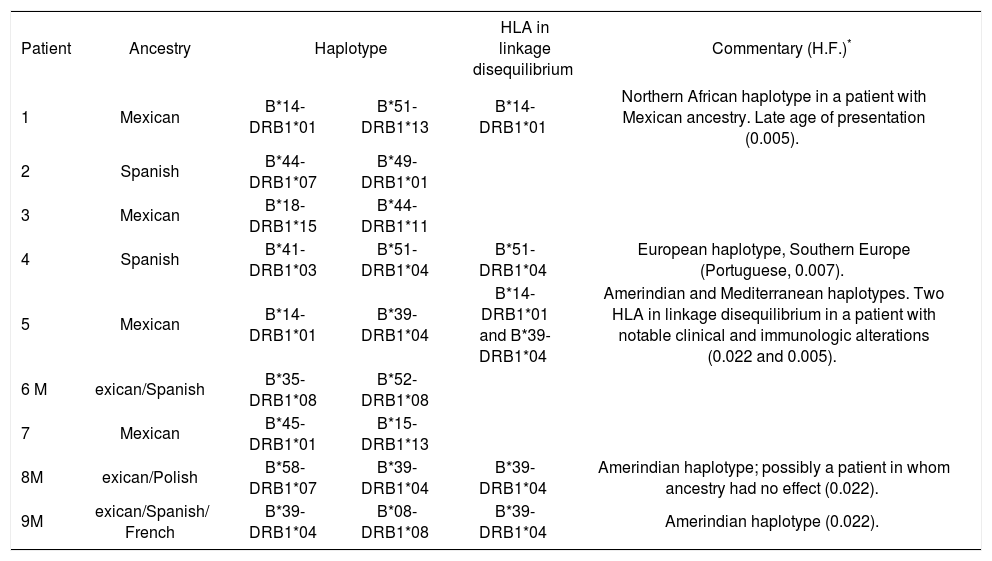
Haplotypes in linkage disequilibrium among PBC patients.
| Patient | Ancestry | Haplotype | HLA in linkage disequilibrium | Commentary (H.F.)* | |
|---|---|---|---|---|---|
| 1 | Mexican | B*14-DRB1*01 | B*51-DRB1*13 | B*14-DRB1*01 | Northern African haplotype in a patient with Mexican ancestry. Late age of presentation (0.005). |
| 2 | Spanish | B*44-DRB1*07 | B*49-DRB1*01 | ||
| 3 | Mexican | B*18-DRB1*15 | B*44-DRB1*11 | ||
| 4 | Spanish | B*41-DRB1*03 | B*51-DRB1*04 | B*51-DRB1*04 | European haplotype, Southern Europe (Portuguese, 0.007). |
| 5 | Mexican | B*14-DRB1*01 | B*39-DRB1*04 | B*14-DRB1*01 and B*39-DRB1*04 | Amerindian and Mediterranean haplotypes. Two HLA in linkage disequilibrium in a patient with notable clinical and immunologic alterations (0.022 and 0.005). |
| 6 M | exican/Spanish | B*35-DRB1*08 | B*52-DRB1*08 | ||
| 7 | Mexican | B*45-DRB1*01 | B*15-DRB1*13 | ||
| 8M | exican/Polish | B*58-DRB1*07 | B*39-DRB1*04 | B*39-DRB1*04 | Amerindian haplotype; possibly a patient in whom ancestry had no effect (0.022). |
| 9M | exican/Spanish/ French | B*39-DRB1*04 | B*08-DRB1*08 | B*39-DRB1*04 | Amerindian haplotype (0.022). |
Comparison of the HLA haplotypes of nine Mexican mestizo PBC cases with those of healthy controls showed that the PBC patients had haplotypes of Caucasian ori-gin, particularly Mediterranean, and of Amerindian and Black origin. The most frequent alleles of the HLA-B locus were HLA-B*39, HLA-B*14, and HLA-B*51 and the most frequent alleles of the HLA-DR locus were HLA-DRB1*04 and HLA-DRB1*01. Linkage disequilibrium of HLA-B/-DRB1 was detected for B*14/DRB1*01, B*51/DRB1*04, and B*39/DRB1*04, which occurs in populations in Southern Europe, Northern Africa, and Central and South America.
These results indicate that the HLA-B*14/DRB1*01 haplotype is a marker for genetic susceptibility to PBC and that its origin is probably Mediterranean. In contrast, other studies have reported that haplotypes associated with PBC belong to the HLA-DRB1*08 allele family.31,33,35,36,41 It is noteworthy that this allele and those found in the Mexican population have similar geographical origins (Southern Europe). This suggests that the genetic background of PBC in Mexican mestizos is similar to that in other populations,1,41 but involves a novel linkage disequilibrium susceptibility marker. The possible participation of the HLA-B*39/HLA-DRB1*04 haplo-type as an autochthonous marker in Mexicans is indicated because its frequency is relatively high in the mestizo and indigenous populations and it is associated with autoimmune diseases such as rheumatoid arthritis,50–52 rhu-pus,53 spondyloathropathies,54 Takayasu arthritis,55 and systemic lupus erithrematosus.56 This haplotype is also associated with nonautoimmune diseases such as gallstone disease,57 Chagas’ disease,58 and extrapulmonary tuberculosis.55
The results of this study suggest that an autoimmune mechanism is present in PBC. Molecular mimetism of certain nuclear and mitochondrial proteins may be responsible for the development of autoimmunity and may involve reactive cytotoxic T cells that express CD4 and CD8 receptors and T cells that express the • • •receptor,18 which is directed against the lipoyl domain of the pyru-vate dehydrogenase-complex family.59–61
PBC has a wide spectrum of manifestations, from asymptomatic to cirrhosis. Few studies have addressed this issue and have found several genes that could affect PBC progression to late-stage cirrhosis, principally those involved in immune-response modulation.31 Furthermore, a recent elegant study performed by Pupon et al62 has demonstrated variations in genes involved in detoxification processes via MDR1 transporter, such as ABCB1/ MDR1 and NR1L2/PXR could influence the severity of liver disease and other affecting the response to ursodeoxy-cholic acid, such as SLC4A2/AE2. In our study, one patient had elevated GGT and AlkP levels and a high antinuclear antibody titer. This patient had two alleles in linkage disequilibrium, one of Amerindian origin (B*39-DRB1*04) and one of Southern European origin (B*14-DRB1*01), and both contributed to the development of PBC. This finding suggests that the amount of alleles in disequilibrium in a patient constitutes a risk factor for major clinical manifestations such as a high concentra-tion of autoantibodies and an aggressive disease course. This finding should be confirmed by studies involving large numbers of patients.
In conclusion, Mexican patients with PBC are genetically susceptible to autoimmunity. Mexican mestizo patients with PBC are particularly prone to autoimmunity because of autochthonous alleles (HLA-B39 and HLA-B14) in combination with other alleles of Mediterranean origin (HLA-DR1 and HLA-DR4). The genetic heterogeneity of PBC patients in this series suggests that their genetic background is similar to that of other popula-tions.31,33,35,36,41,42,63 Therefore, although these results were obtained using Mexican mestizo patients, they may be applicable to patients with Caucasoid ancestry.