Hyponatremia is common in patients with decompensated cirrhosis and is associated with increased mortality. Tolvaptan, a vasopressor V2 receptor antagonist, can increase free water excretion, but its efficacy and safety in cirrhotic patients remain unclear.
Material and MethodsWe studied the usage and safety of tolvaptan in cirrhotic patients in a real-life, non-randomized, multi-center prospective cohort study. Forty-nine cirrhotic patients with hyponatremia were treated with tolvaptan 15 mg daily, and 48 patients not treated with tolvaptan in the same period served as controls. Improvement in serum sodium level was defined as an increase in serum sodium from < 125 to ≥ 125 mmol/L or from 125-134 to ≥ 135 mmol/L on day 7.
ResultsTwenty-three (47%) patients in the tolvaptan group and 17 (35%) in the control group had normal serum sodium on day 7 (p = 0.25). Serum sodium improved in 30 (61%) patients in the tolvaptan group and 17 (35%) patients in the control group (p = 0.011). Adverse events occurred in 46-47% of patients in both groups, and tolvaptan was not associated with worsened liver function. No patient with normal serum sodium on day 7 died within 30 days of treatment, whereas 16% of those with persistent hyponatremia died (p = 0.0019).
ConclusionIn conclusion, short-term tolvaptan treatment is safe and can improve serum sodium level in cirrhotic patients with hyponatremia. Normalization of serum sodium level is associated with better survival.
Hyponatremia is a common and important complication of decompensated liver cirrhosis, and cirrhotic patients with hyponatremia have increased mortality.1,2 Severe hyponatremia also complicates fluid management in cirrhosis. The standard treatment of ascites includes salt restriction and diuretics, such as spironolactone and furo-semide.3 These treatments, however, aggravate hy-ponatremia and, in cases of severe hyponatremia, treatment has to be interrupted. In contrast, sodium replacement for severe hyponatremia leads to fluid retention and rapid as-cites accumulation.
In addition to the effect of diuretics, pathophysiologi-cal changes in cirrhosis contribute to the development of hyponatremia. In response to increased hepatic vascular resistance, there is adaptive vasodilatation in the splanchnic as well as the systemic circulation.4 The resulting drop in effective circulating volume activates the renin-angi-otensin-aldosterone system and antidiuretic hormone (ar-ginine vasopressin),5 the latter leading to free water retention and hyponatremia.
Therefore, vasopressin receptor V2 antagonists (vaptans) were developed to address the pathophysiology of hyponatremia. This class of drugs has been shown to reverse hyponatremia in patients with syndrome of inappropriate antidiuretic hormone secretion, congestive heart failure, and cirrhosis.6-10 A systematic review of 12 trials showed that vaptans effectively increased serum sodium levels and reduced body weight and the need for paracen-tesis in cirrhotic patients, with a neutral effect on surviv-al.11 Unfortunately, satavaptan was voluntarily withdrawn because of concerns about its safety and efficacy. In a subsequent 3-year trial in patients with autosomal dominant polycystic kidney disease, patients receiving tolvaptan were more likely to have elevated liver enzymes and to discontinue treatment than controls.12 Nonetheless, because hepatotoxicity has only been reported with long-term but not short-term treatment, the use of tolvaptan for edema and hyponatremia in cirrhosis is a possibility in some countries, such as China and Japan. Therefore, a study on the short-term use of these agents is urgently needed.
This prospective cohort study was conducted to record the real-life usage, efficacy, and safety of tolvaptan in cir-rhotic patients in China.
Material and MethodsStudy design and patientsThis was a multicenter, open-label, non-randomized, prospective cohort study. Consecutive cirrhotic patients who received tolvaptan for the treatment of hyponatremia were recruited. Cirrhotic patients with hyponatremia who declined tolvaptan treatment during the same period served as controls. The decision to use tolvaptan was a joint decision between the doctors and patients. We included adult hospital patients aged 18-70 years with cirrhosis confirmed by histology or unequivocal clinical or radiological evidence of portal hypertension and hy-ponatremia. Hyponatremia was defined as serum sodium level < 135 mmol/L in the general population but arbitrarily as < 130 mmol/L in cirrhotic patients, according to the European Association for the Study of the Liver (EASL) guidelines. Because chronic asymptomatic hy-ponatremia is common in cirrhotic patients, particularly in the presence of ascites,13,14 we included patients with serum sodium levels < 130 mmol/L or patients with sodium 130-134 mmol/L and other symptoms. Symptom assessment was at the discretion of individual investigators but usually included general and neurological symptoms, such as malaise and dizziness. Patients were excluded if they had: clinical features of hypovolemia; systolic blood pressure < 90 mmHg; variceal bleeding in the last 6 months; hepatic encephalopathy or infected ascites in the last 2 weeks; serum creatinine > 309 μmol/L (3.5 mg/dL); urinary tract obstruction; severe cardiopulmonary, neurological, gastrointestinal, endocrine, or metabolic diseases; and estimated life expectancy < 1 month. All patients provided informed written consent. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by the institutions’ human research committees.
Study interventionPatients who received at least one dose of tolvaptan were included in the active study group. The recommended treatment duration was 7 days; treatment for over 30 days was not allowed. The starting dose of tolvaptan was 15 mg daily. With close monitoring of the fluid status and serum sodium, the dose of tolvaptan could be adjusted daily at 15 mg intervals up to a dose of 60 mg daily.
Patients not receiving tolvaptan served as the control group. They received the standard of care, including monitoring of fluid status and serum sodium, fluid restriction, and/or sodium replacement in cases of severe hyponatremia. Sodium replacement was mostly given in patients with serum sodium level < 120 mmol/L; only six patients received sodium replacement in this study.
In both groups, the use of other diuretics, such as spironolactone and furosemide, was allowed with close monitoring of the fluid status, electrolyte levels, and renal function.
Clinical assessmentAt baseline, medical history, vital signs, and physical examination findings were recorded. Blood was taken for liver biochemistry, serum creatinine, urea, sodium, potassium, glucose, complete blood picture, and prothrombin time. The Cockcroft-Gault equation was used to estimate the glomerular filtration rate.15 Hepatitis B surface antigen and anti-hepatitis C virus antibody were checked to determine the etiology of cirrhosis. The EQ-5D-3L questionnaire was used to assess the quality of life.16 All assessments were repeated on day 7, and follow-up on day 30 was optional.
Study outcomesThe primary outcome was the proportion of patients with normal serum sodium level (135-145 mmol/L) on day 7. Secondary outcomes included serum sodium level on day 7 and day 30, mortality, and changes in sodium, renal function, body weight, and quality of life from baseline until day 7 and day 30. Adverse events were recorded daily during hospitalization and then on day 7 and day 30.
Statistical analysisIn a sub-analysis of the Study of Ascending Levels of Tolvaptan trials, 41% of cirrhotic patients receiving tolvaptan had normal serum sodium on day 4 compared with 11% of patients in the placebo group.6,10 Assuming that the proportion of patients with normal serum sodium on day 7 was 40% in the tolvaptan group and 20% in the control group, a sample size of around 118 patients per group would achieve 90% power in detecting the difference at a 5% significance level. Nevertheless, because of slow patient recruitment, the protocol was amended after an investigator meeting on 28 December 2013. Assuming that the proportion of patients with normal serum sodium on day 7 was 40% in the tolvaptan group and 10% in the control group, a sample size of around 38 patients in each group would achieve 80% power in detecting a difference at a 5% significance level. Allowing for a dropout rate of 20%, a total sample size of 96 patients was required.
Continuous variables were summarized as mean ± standard deviation or median (range) and compared using the unpaired t test or Wilcoxon rank sum test as appropriate. Changes of continuous variables over time were analyzed using paired t test and Wilcoxon signed rank test. Categorical variables were compared using the chi-square test or Fisher’s exact test. In the intention-to-treat (ITT) analysis, patients with missing serum sodium results during follow-up were handled using the last-observation-carried-forward method and classified as having persistent hyponatremia. The per-protocol (PP) population only included the observed cases; missing data were not imputed. A two-sided p-value < 0.05 was considered statistically significant.
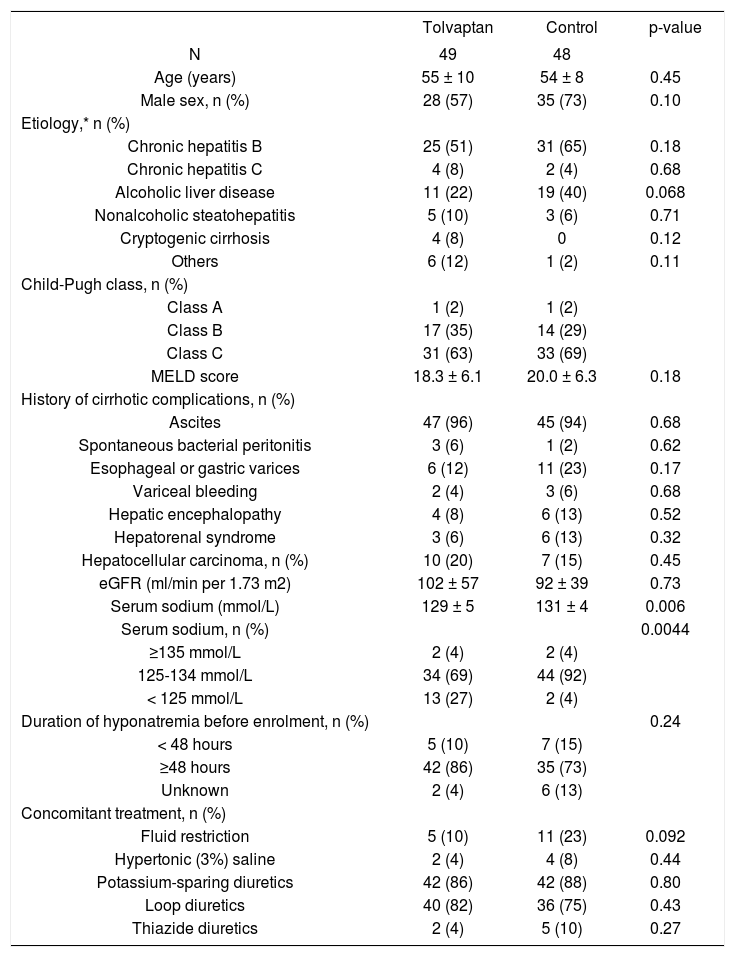
ResultsFrom May 2013 to September 2014, 100 cirrhotic patients were screened, and 97 patients were enrolled and formed the ITT population (Supplementary figure). Four patients, two in each group, no longer had hyponatremia at baseline. In addition, six patients, three in each group, did not attend follow-up on day 7. These 10 patients were excluded from the PP analysis (Supplementary figure). The cohort mainly represented middle-age male patients (Table 1). Chronic hepatitis B and alcoholic liver disease were the leading causes of cirrhosis. The majority of patients had Child-Pugh class C cirrhosis and was on potassium-sparing diuretics and loop diuretics. More patients in the tolvaptan group had serum sodium < 125 mmol/L; the two groups were otherwise well matched.
Baseline characteristics of patients in the tolvaptan and control groups.
| Tolvaptan | Control | p-value | |
|---|---|---|---|
| N | 49 | 48 | |
| Age (years) | 55 ± 10 | 54 ± 8 | 0.45 |
| Male sex, n (%) | 28 (57) | 35 (73) | 0.10 |
| Etiology,* n (%) | |||
| Chronic hepatitis B | 25 (51) | 31 (65) | 0.18 |
| Chronic hepatitis C | 4 (8) | 2 (4) | 0.68 |
| Alcoholic liver disease | 11 (22) | 19 (40) | 0.068 |
| Nonalcoholic steatohepatitis | 5 (10) | 3 (6) | 0.71 |
| Cryptogenic cirrhosis | 4 (8) | 0 | 0.12 |
| Others | 6 (12) | 1 (2) | 0.11 |
| Child-Pugh class, n (%) | |||
| Class A | 1 (2) | 1 (2) | |
| Class B | 17 (35) | 14 (29) | |
| Class C | 31 (63) | 33 (69) | |
| MELD score | 18.3 ± 6.1 | 20.0 ± 6.3 | 0.18 |
| History of cirrhotic complications, n (%) | |||
| Ascites | 47 (96) | 45 (94) | 0.68 |
| Spontaneous bacterial peritonitis | 3 (6) | 1 (2) | 0.62 |
| Esophageal or gastric varices | 6 (12) | 11 (23) | 0.17 |
| Variceal bleeding | 2 (4) | 3 (6) | 0.68 |
| Hepatic encephalopathy | 4 (8) | 6 (13) | 0.52 |
| Hepatorenal syndrome | 3 (6) | 6 (13) | 0.32 |
| Hepatocellular carcinoma, n (%) | 10 (20) | 7 (15) | 0.45 |
| eGFR (ml/min per 1.73 m2) | 102 ± 57 | 92 ± 39 | 0.73 |
| Serum sodium (mmol/L) | 129 ± 5 | 131 ± 4 | 0.006 |
| Serum sodium, n (%) | 0.0044 | ||
| ≥135 mmol/L | 2 (4) | 2 (4) | |
| 125-134 mmol/L | 34 (69) | 44 (92) | |
| < 125 mmol/L | 13 (27) | 2 (4) | |
| Duration of hyponatremia before enrolment, n (%) | 0.24 | ||
| < 48 hours | 5 (10) | 7 (15) | |
| ≥48 hours | 42 (86) | 35 (73) | |
| Unknown | 2 (4) | 6 (13) | |
| Concomitant treatment, n (%) | |||
| Fluid restriction | 5 (10) | 11 (23) | 0.092 |
| Hypertonic (3%) saline | 2 (4) | 4 (8) | 0.44 |
| Potassium-sparing diuretics | 42 (86) | 42 (88) | 0.80 |
| Loop diuretics | 40 (82) | 36 (75) | 0.43 |
| Thiazide diuretics | 2 (4) | 5 (10) | 0.27 |
In the tolvaptan group, the mean duration of tolvaptan treatment was 6.3 ± 3.7 days (range 1-20 days). Only 13 (27%) patients received tolvaptan for 7 days or more, and only five (10%) patients continued tolvaptan treatment after discharge from the hospital. The mean cumulative dose of tolvaptan received was 82.5 ± 28.9 mg (range 15-150 mg). No patient received more than 15 mg of tolvaptan per day during the treatment period.
The primary outcome of normal serum sodium level of 135-145 mmol/L on day 7 was achieved in 23 (47%) patients in the tolvaptan group and 17 (35%) patients in the control group (p = 0.25) (Table 2). Both the ITT and PP analysis yielded similar results. The two groups also had a similar serum sodium level of 134 ± 5 mmol/L on day 7. However, given that the tolvaptan group had a significantly lower serum sodium level at baseline, the tolvaptan group had a greater increase in serum sodium level from baseline to day 7 than the control group (increase in serum sodium 5 ± 6 mmol/L vs. 2 ± 5 mmol/L; p = 0.0062). Significantly more patients in the tolvaptan group showed improvement in serum sodium on day 7 (Table 2). One (2%) patient in the tolvaptan group had increased serum sodium of 147 mmol/L on day 7, but he was asymptomatic for hypernatremia, and the level returned to normal later.
Biochemical changes in the tolvaptan and control groups.
| Variable | Tolvaptan | Control | p-value |
|---|---|---|---|
| N | 49 | 48 | |
| Day 7 results | |||
| Serum sodium on day 7 (mmol/L) | 134 ± 5 | 134 ± 5 | 0.51 |
| Change in serum sodium from baseline to day 7 (mmol/L) | 5 ± 6 | 2 ± 5 | 0.0062 |
| Normal serum sodium on day 7, n (%) | |||
| ITT | 23 (47) | 17 (35) | 0.25 |
| PP | 22 (50) | 16 (37) | 0.23 |
| Improved serum sodium on day 7,* n (%) | |||
| ITT | 30 (61) | 17 (35) | 0.011 |
| PP | 30 (68) | 17 (40) | 0.0099 |
| eGFR on day 7 (ml/min per 1.73 m2) | 99.5 ± 53.8 | 86.8 ± 35.5 | 0.51 |
| Change of eGFR from baseline to day 7 (ml/min per 1.73 m2) | -2.6 ± 21.2 | -4.1 ± 17.9 | 0.87 |
| Day 30 results† | |||
| Serum sodium on day 30 (mmol/L) | 13 ± 8 | 135 ± 4 | 0.27 |
| Change in serum sodium from baseline to day 30 (mmol/L) | 3 ± 10 | 3 ± 5 | 0.82 |
When stratified by baseline serum sodium level, all patients with baseline serum sodium level < 125 mmol/L in the tolvaptan group had serum sodium level ≥ 125 mmol/L on day 7 (Table 3). In contrast, only one of the two patients with baseline serum sodium level < 125 mmol/L had improved serum sodium level on day 7. For patients with baseline serum sodium level of 125-134 mmol/L, 56% had day 7 serum sodium ≥ 135 mmol/L in the tolvaptan group compared with 42% in the control group (p = 0.21).
Baseline and day 7 serum sodium level in the tolvaptan and control groups (per-protocol analysis).
| Day 7 serum sodium (mmol/L) | <125 | 125-134 | ≥135 | Total |
|---|---|---|---|---|
| Tolvaptan group | ||||
| Baseline serum sodium < 125 mmol/L | 0 | 8 (67%) | 4 (33%) | 12 |
| Baseline serum sodium 125-134 mmol/L | 2 (6%) | 12 (38%) | 18 (56%) | 32 |
| Total | 2 (5%) | 20 (45%) | 22 (50%) | 44 |
| Control group | ||||
| Baseline serum sodium < 125 mmol/L | 1 (50%) | 1 (50%) | 0 | 2 |
| Baseline serum sodium 125-134 mmol/L | 2 (5%) | 22 (54%) | 17 (42%) | 41 |
| Total | 3 (7%) | 23 (53%) | 17 (4%) | 43 |
There was a trend that the estimated glomerular filtration rate (eGFR) was higher at baseline and on day 7 in the tolvaptan group than in the control group (Tables 1 and 2). The mean decline in eGFR was only 2.6 mL/min per 1.73 m2, which was not significantly higher than that in the control group (Table 2). From baseline to day 7, there was a small but significant decrease in body weight from 64.3 ± 12.7 kg to 63.5 ± 11.3 kg in the tolvaptan group (p = 0.0093) and from 62.8 ± 11.5 kg to 62.1 ± 11.4 kg in the control group (p = 0.0039) (between groups p = 0.56).
Because hyponatremia was more severe in patients in the tolvaptan group, these patients reported more problems with mobility and self-care at baseline (Table 4). Nonetheless, the two groups had similar quality of life on day 7, and patients in the tolvaptan group had a greater improvement in self-care (Table 4). The health status, as measured by the EQ-5D index, increased from 0.50 ± 0.26 to 0.57 ± 0.25 from baseline to day 7 in the tolvaptan group (p = 0.045) but remained static at 0.59 ± 0.30 at baseline and 0.61 ± 0.32 on day 7 in the control group (p = 0.14) (between groups p=0.77). Similarly, the global EQ 100mm visual analog scale increased from 53.9 ± 17.3 to 61.5 ± 18.9 in the tolvaptan group (p = 0.0014) but remained static at 62.5 ± 19.0 and 62.9 ± 20.2 in the control group (p = 0.54) (between groups p = 0.011).
Change in quality of life (EQ-5D-3L) from baseline to day 7.
| Verificar columnas en el original. GRACIAS | Tolvaptan (n = 49) | Control (n = 48) | Between-group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dimension | Baseline | N | No problems | Some problems | Extreme problems | N | No problems | Some problems | Extreme problems | p-value |
| Day 7 | n(%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||
| Mobility | No problems Some problems Extreme problems Within-group p-value | 43 | 8 (-18.6%) 2 (-4.65%) 0 (0%) 0.7212* | 2 (-4.65%) 20 (-46.51%) 1 (-2.33%) | 1 (-2.33%) 2 (-4.65%) 7 (-16.28%) 0.9776* | 41 | 15 (-36.59%) 2 (-4.88%) 0 (0%) | 3 (-7.32%) 12 (-29.27%) 1 (-2.44%) | 0 (0%) 1 (-2.44%) 7 (-17.07%) | 0.8546† |
| Self-Care | No problems Some problems Extreme problems Within-group p-value | 43 | 9 (-20.93%) 5 (-11.63%) 0 (0%) 0.6386* | 8 (-18.6%) 11 (-25.58%) 1 (-2.33%) | 0 (0%) 3 (-6.98%) 6 (-13.95%) 0.8013* | 41 | 20 (-48.78%) 2 (-4.88%) 0 (0%) | 2 (-4.88%) 10 (-24.39%) 0 (0%) | 0 (0%) 1 (-2.44%) 6 (-14.63%) | 0.0044† |
| Usual activities | No problems Some problems Extreme problems Within-group p-value | 43 | 6 (-13.95%) 2 (-4.65%) 0 (0%) 0.0620* | 1 (-2.33%) 21 (-48.84%) 0 (0%) | 1 (-2.33%) 6 (-13.95%) 6 (-13.95%) 0.2719* | 41 | 9 (-21.95%) 1 (-2.44%) 0 (0%) | 6 (-14.63%) 15 (-36.59%) 2 (-4.88%) | 0 (0%) 1 (-2.44%) 7 (-17.07%) | 0.9029† |
| Pain/ Discomfort | No problems Some problems Extreme problems Within-group p-value | 43 | 13 (-30.23%) 3 (-6.98%) 0 (0%) 0.2335* | 8 (-18.6%) 13 (-30.23%) 1 (-2.33%) | 1 (-2.33%) 3 (-6.98%) 1 (-2.33%) 0.4020* | 41 | 13 (-31.71%) 3 (-7.32%) 0 (0%) | 7 (-17.07%) 14 (-34.15%) 1 (-2.44%) | 1 (-2.44%) 2 (-4.88%) 0 (0%) | 0.7696† |
| Anxiety/ Depression | No problems Some problems Extreme problems Within-group p-value | 43 | 19 (-44.19%) 2 (-4.65%) 0 (0%) 1.0000* | 2 (-4.65%) 17 (-39.53%) 1 (-2.33%) | 0 (0%) 1 (-2.33%) 1 (-2.33%) 0.9536* | 41 | 17 (-41.46%) 4 (-9.76%) 0 (0%) | 4 (-9.76%) 12 (-29.27%) 2 (-4.88%) | 0 (0%) 1 (-2.44%) 1 (-2.44%) | 0.1421† |
Adverse events were reported in 23 (47%) patients in the tolvaptan group and 22 (46%) patients in the control group (Table 5). Specifically, although increased alanine aminotransferase was previously noted in patients taking long-term tolvaptan,12 in this study, alanine aminotrans-ferase elevation occurred in none of the patients in the tolvaptan group and one (2%) patient in the control group. Serious adverse events, mainly from prolonged hospitali-zation and death, occurred in four (8%) patients in the tolvaptan group and seven (15%) patients in the control group. All of the serious adverse events were judged to be related to the underlying cirrhosis and not to the study treatment.
Adverse events, serious adverse events and deaths in the tolvaptan and control groups.
| Tolvaptan | Control | |
|---|---|---|
| N | 49 | 48 |
| Patients with adverse events, n (%) | 23 (47) | 22 (46) |
| Patients with serious | 4 (8) | 7 (15) |
| adverse events, n (%) | ||
| Gastrointestinal hemorrhage | 0 | 2 (4) |
| Peritoneal hemorrhage | 0 | 1 (2) |
| Liver failure | 2 (4) | 1 (2) |
| Hepatic encephalopathy | 1 (2) | 1 (2) |
| Spontaneous bacterial peritonitis | 1 (2) | 0 |
| Pancytopenia | 0 | 1 (2) |
| Complications of hepatocellular carci | noma 0 | 2 (4) |
| Subcutaneous hemorrhage | 1 (2) | 0 |
| Death, n (%) | 3 (6) | 5 (10) |
Three (6%) patients in the tolvaptan group and five (10%) patients in the control group died within 30 days (p = 0.36 by log-rank test) (Figure 1A). In the tolvaptan group, two patients died of liver failure, and one patient died of extensive subcutaneous hemorrhage. In the control group, the causes of death included hepatocellular carcinoma (n = 2), gastrointestinal hemorrhage (n = 1), peritoneal hemorrhage (n = 1), and liver failure with hepatic encephalopathy (n = 1). Again, none of the deaths were considered to be related to the study treatment.
Kaplan-Meier curve of 30-day survival in (A) the tolvaptan and control groups (p = 0.36), (B) patients with and without normalization of serum sodium level (135-145 mmol/L) on day 7 (p = 0.0019), and (C) patients with and without improvement of serum sodium level on day 7 (p = 0.013). Footnote: Improved serum sodium was defined as an increase of serum sodium from < 125 mmol/L to ≥ 125 mmol/L in patients with baseline serum sodium < 125 mmol/L, or from < 135 mmol/L to ≥ 135 mmol/L in patients with baseline serum sodium 125-134 mmol/L.
Although there was no significant difference in mortality between the treatment groups, changes in serum sodium level were strongly associated with 30-day mortality. None of the 40 patients with normal serum sodium on day 7 died, compared with eight of 51 (16%) patients with persistent hyponatremia on day 7 who died (p = 0.0019, Figure 1B). Similarly, only one of 48 (2%) patients with improved serum sodium level on day 7 died, compared with seven of 43 (16%) patients without improvements who died (p = 0.013, Figure 1C). The patient who died despite improvement in sodium had serum sodium level 119 mmol/L at baseline and 130 mmol/L on day 7.
DiscussionWe set out to investigate the real-life usage of tolvaptan in patients with cirrhosis and hyponatremia in light of recent hepatotoxicity data.12 Based on prospective multi-center data collection, we found that Chinese doctors have been using tolvaptan judiciously. There were few prescriptions, and all doctors prescribed tolvaptan at the starting dose of 15 mg daily for a short finite period. Tolvaptan was well tolerated and was not associated with increased adverse events, hepatotoxicity, or mortality. Sixty-one percent of patients on tolvaptan had improved serum sodium level. In addition, improvements in serum sodium level could be used as a predictor of short-term mortality.
Our findings are consistent with previous clinical trials that tested the use of tolvaptan in various types of hyperv-olemic and euvolemic hyponatremia.6-10 In the Study of Ascending Levels of Tolvaptan in Hyponatremia (SALT) studies involving mainly patients with congestive heart failure, cirrhosis, and syndrome of inappropriate antidiu-retic hormone secretion, tolvaptan treatment increased serum sodium from 128.5 to 133.9 mmol/L in 4 days.6 Subsequent studies further showed that tolvaptan could improve not only hyponatremia but also edema and ascites in patients with cirrhosis.17-21 Interestingly, two Japanese dose-finding studies suggested that the optimal dose of tolvaptan for cirrhotic patients can be as low as 7.5 mg daily, although edema rather than hyponatremia was the primary focus of both studies.17,19 In our study, 61% of patients had improved serum sodium level on day 7 of tolvaptan treatment; half also had normal serum sodium level. Although the proportion of patients with normal serum sodium level on day 7 did not differ significantly in the tolvaptan and control groups, it is important to note that most doctors used tolvaptan sparingly and patients in the tolvaptan group had more severe hyponatremia at baseline.
As tolvaptan can induce profound diuresis, its renal safety must be scrutinized. In our study, tolvaptan use was not associated with a greater decline in eGFR or acute kidney injury. Similarly, tolvaptan had limited impact on the blood urea nitrogen or serum creatinine in the major phase II and III clinical trials.6-8 During long-term treatment in patients with autosomal dominant polycystic kidney disease, tolvaptan actually prevented deterioration in kidney function.12 In contrast to loop diuretics and thi-azide diuretics, V2 antagonists increase free water excretion. Thus, the effect is evenly distributed across the intravascular, interstitial, and intracellular compartments, which may reduce the impact on renal perfusion.
Another major concern when using tolvaptan in cir-rhotic patients is potential hepatotoxicity. This worry stems from data showing liver enzyme elevation during long-term tolvaptan treatment.12 In our study, tolvaptan was not associated with worsened liver function, and none of the treated patients had elevations in alanine ami-notransferase. Similarly, there has been no hepatic safety signal in the pivotal short-term clinical trials,6-8 suggesting that short-term tolvaptan use is probably safe.
In our study, tolvaptan had a neutral effect on 30-day mortality. Nonetheless, the change in serum sodium level was an important prognostic marker. No patient with normalization of serum sodium level on day 7 died within 30 days, and only one patient with improved serum sodium level died. Hyponatremia is common in patients with de compensated cirrhosis and is associated with increased risk of hepatic encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome, and overall mortality.22-24 Some investigators also advocate the inclusion of serum sodium level in the model for end-stage liver disease (MELD).2-25 Our study further highlights the importance of assessing serial serum sodium levels. An improvement in serum sodium level signifies better prognosis regardless of the choice of treatment.
Our study involved multiple centers and is representative of the real-life clinical management in China. Compared with clinical trials, an observational study design allowed for less restricted patient inclusion and could thus achieve a higher external validity. Nevertheless, our study also had a few limitations. First, like all real-life cohorts, the treatment regimens were heterogeneous. The relatively small proportion of patients with normalization of serum sodium level was due to conservative prescription of tolvaptan, where some doctors might only aim for an improved but not necessarily normal serum sodium level. Second, there was imbalance between the treatment groups. Negative bias was introduced because patients in the tolvaptan group had lower baseline serum sodium level than the control group, but it supported the efficacy of tolvaptan in correcting hyponatremia. Third, the general indication of tolvaptan is for the management of severe hy-pervolemic hyponatremia (< 125 mmol/L). In the present study, only 13 patients in the tolvaptan group and two patients in the control group had such low sodium levels. Our findings should be interpreted cautiously and require independent validation. Finally, the sample size was relatively small and was adjusted because of slow patient recruitment. Nonetheless, the cohort reflects the practice in different parts of China and provides valuable real-life data. The prognostic impact of response to hyponatremia treatment also warrants further study.
In conclusion, short-term tolvaptan treatment was safe, and it improved serum sodium level in 60% of cirrhotic patients with hyponatremia. This is notable, because normalization of serum sodium level is associated with better survival.
Abbreviations- •
EASL: European Association for the Study of the Liver.
- •
eGFR: estimated glomerular filtration rate.
- •
ITT: intention-to-treat.
- •
MELD: Model for End-Stage Liver Disease.
- •
PP: per-protocol.
- •
SALT: Study of Ascending Levels of Tolvaptan in Hy-ponatremia.
Ji-Dong Jia has received speaker honorariums and consultancy fees from Novartis, Gilead Sciences, Bristol-Myers Squibb, Merck, Moehringer Ingelheim, and Roche. Xie Wen has received speaker honoraria from Abbott Laboratories, Bristol-Myers Squibb, Merck, Novartis, Roche, and Sanofi-Aventis. Xiaojin Wang has received speaker honoraria and consultancy fees from Bristol-Myers Squibb, Novartis, and Roche. Wei Lu has received a research grant from Novartis. Vincent Wong has served as an advisory board member for AbbVie, Gilead Sciences, Jans-sen, Otsuka, and Roche and as a consultant for Merck and Novomedica and received paid lecture fees from Abbott, Echosens, Gilead Sciences, Novartis, and Roche.
Financial SupportThis study was a company-sponsored, investigator-initiated study. The participating sites received financial support from Otsuka for laboratory and study-related expenses. Otsuka also provided support on database management. The investigators, however, remained independent in data interpretation and manuscript preparation.