Background. The incidence of liver cirrhosis is significantly high in Latin population. The high prevalence of nonalcoholic fatty liver disease NAFLD is likely partially responsible for these figures. Liver biopsy is not a practical diagnostic option in this scenario. The validation of noninvasive markers of fibrosis is important in populations with a high prevalence of NAFLD.
Aim. To compare the diagnostic value of noninvasive assessment systems to detect fibrosis in a cohort of Latin patients with biopsy-proven NAFLD.
Material and methods. Patients with biopsy-proven NAFLD were included. Noninvasive evaluations included calculations of NAFLD fibrosis, FIB–4, BARD scores, APRI, and AST/ALT ratio. The sensitivity, specificity, positive predictive value, negative predictive value, and area under the receiver-operating characteristic curve (AUROC) were calculated.
Results. A total of 228 patients (mean age, 48.6 ± 12.7 years) were included. Fifty-one percent were women; 48% were overweight and 23% were obese. The severity of fibrosis was classified as G0, 56.6%; G1, 25%; G2, 6.6%; G3, 7%; and G4, 4.8%. The AUROC values for advanced fibrosis were 0.72 for the NAFLD fibrosis score, 0.74 for FIB–4 score, 0.67 for AST/ALT ratio, 0.66 for APRI score, and 0.65 for BARD score. In 54% of patients with undetermined FIB–4 score and in 60% of patients with undetermined NAFLD fibrosis score, fibrosis was observed in the liver biopsy.
Conclusions. The NAFLD fibrosis, FIB–4, and APRI scores can be used for the noninvasive diagnosis of fibrosis. However, 25% of patients evaluated by these methods have an indeterminate degree of fibrosis.
Nonalcoholic fatty liver disease (NAFLD) refers to a broad spectrum of liver damage that varies from fat deposition in the hepatocytes (steatosis) to chronic inflammatory damage (non-alcoholic steatohepatitis [NASH]). Patients with NASH are at risk of developing fibrosis and liver cirrhosis, and their complications.1 Of note, the presence and severity of fibrosis dictates both overall and liver-related mortality in patients with NAFLD.2 Liver biopsy is the gold standard for assessing fibrosis. Moreover, it provides direct information about inflammation, degree of steatosis, iron deposition, and other findings. However, liver biopsy has several limitations, including its cost, complications, and variability between observers, within the sample and the gastroenterologist’s sampling technique.3–6 Therefore, the development of noninvasive markers for the diagnosis of fibrosis in patients with NAFLD has become important in clinical practice.7 Many non-invasive panels and scoring systems have been developed with variable accuracy. The irregular distribution of fibrosis through the liver indicates that such scoring systems may potentially represent a more accurate reflection of global liver fibrosis. The accuracy of transient elastography (Fibroscan™) has been demonstrated in meta-analysis for the detection of advance liver fibrosis and early liver cirrhosis.8–10
The clinical and biological variables most frequently associated with advanced fibrosis in NAFLD are advanced age, elevated body mass index (BMI), type 2 diabetes mellitus, the metabolic syndrome, increased aspartate aminotransaminase (AST)/alanine aminotransferase (ALT) ratio (AAR), and decreased platelet count.12 These variables are included in some of the evaluation tools for non-invasive assessment of fibrosis. Some of these scores are made based on simple mathematical standardized formulas (e.g., AAR), whereas others such as the FIB–4 and NAFLD fibrosis scores require more complex mathematical algorithms, although these can be calculated easily with on-line calculators (http://nafldscore.com). Compared with other liver diseases, only a limited number of serum markers have been evaluated to predict fibrosis in NAFLD patients. These scores include the AST-to-Platelet Ratio Index (APRI), AAR, BARD score, FIB–4, and NAFLD fibrosis score.7,12 These have been validated against the current gold standard, the liver biopsy, and have been applied in many clinical settings, although the accuracy differs between populations. A valid method for detecting fibrosis in patients with NAFLD is needed for large populations in which liver biopsy is not feasible. Thus, it is essential to develop noninvasive markers of fibrosis in populations with a high prevalence of NAFLD, such as the Latin population.13–18 In Mexico, this disease is estimated to be the most prevalent cause of cirrhosis in women and the second leading cause in men, and it is expected that about one million people will have fatty liver cirrhosis in 2050.19 In Chile, the age-adjusted mortality rate for cirrhosis is among the highest in the world (32/100,000 habitants).20 This is not completely explained by the prevalence of hepatitis C or alcohol consumption which are lower or similar to other populations.21 Thereby, the high prevalence of liver steatosis in a population-based study with ultrasound was 23%21 may explain this difference. This highlights the need to develop a convenient and inexpensive tool for clinical practice. The aim of this study was to compare the diagnostic utility of several scores used in the noninvasive evaluation of fibrosis in patients with NAFLD in the Latin population.
Material and MethodsInformation was obtained about patients with NAFLD undergoing liver biopsy during of cholecystectomy due to abnormal liver test, performed in the Department of Pathology at Medica Sur Clinic Foundation Hospital in Mexico City between January 2005 and December 2010 and in the Department of Gastroenterology at the Pontificia Universidad Católica de Chile between January 2007 and November 2011. The biopsies included were those from patients with the following:
- •
Histopathological diagnosis of NAFLD according to Brunt’s criteria.
- •
Complete data from liver function tests and a blood count within 3 months of the date of the liver biopsy.
- •
Anthropometric measurements (weight, height, and BMI) recorded in the electronic file.
Information was also obtained about comorbidities recorded during the medical history on the day of the liver biopsy.
We excluded patients who exhibited histological evidence or clinical data suggesting the presence of other associated liver diseases (primary biliary cirrhosis, chronic infection with hepatitis B or C, autoimmune hepatitis, sclerosing cholangitis, or overlapping syndrome) or evidence of alcohol intake of more than three drinks of any alcoholic beverage per week.
The clinical parameters obtained were age, sex, weight, height, BMI, and the presence of carbohydrate intolerance or type 2 diabetes mellitus. BMI was calculated as weight (kg)/height (m)2. The diagnosis of type 2 diabetes mellitus was recorded for any patient taking oral hypoglycemic agents or insulin, or if the patient knew that the diagnosis of type 2 diabetes mellitus was reported in the clinical record.
Noninvasive markers of fibrosisThe noninvasive methods used to assess fibrosis were the APRI, AAR, FIB-4, BARD, and NAFLD fibrosis scores. These were calculated using the following equations:
- •
APRI = {AST (IU/1)/[upper normal value of 41 (IU/l)]}/platelet count (x109/l) x 100.22
- •
AAR = AST (IU/l)/ALT (IU/l).
- •
FIB–4 score = age x AST (IU/l)/platelet count (x109/l) x √ ALT (IU/l).23
- •
NAFLD fibrosis score = 1.675 + 0.037 x age (years) + 0.094 x BMI (kg/m2) + 1.13 x abnormal fasting glucose level or diabetes (yes = 1, no = 0) + 0.99 x AAR - 0.013 x number of platelets (x109/l) - 0.66 x albumin concentration (g/dL).24
- •
BARD score = sum obtained from the three variables:25
- °
BMI > 28 = 1 point.
- °
AAR > 0.8 = 2 points.
- °
Diabetes = 1 point.
- °
The cutoff values used for the diagnosis of severe fibrosis were: APRI ≥ 1, AAR ≥ 0.8, NAFLD score ≥ 0.676, BARD score ≥ 2, and FIB-4 score ≥ 3.25.26
Liver biopsyLiver biopsy samples were stained with hematoxylin and eosin, and Masson’s trichrome stain. Biopsies were reviewed by two expert pathologists in each centre, who reached a consensus on the results. The diagnosis of NAFLD was made according to the Brunt criteria, which are described as steatosis, ballooning of hepatocytes, presence of Mallory bodies, inflammation, and fibrosis. NAFLD fibrosis was graded as grade (G) 1, fibrosis in zone 3 and perisinusoidal and/or pericellular fibrosis; grade 2, fibrosis in zone 3 and periportal fibrosis; grade 3, bridging fibrosis; and grade 4, nodule formation and cirrhosis.
The study was approved by the Human Subjects Committee at the Medica Sur Clinic & Foundation, and the Ethics Committee of the Pontificia Universidad Católica de Chile, which were conformed to the ethical guidelines of the 1983 Declaration of Helsinki.
Statistical analysisContinuous variables are described as means and standard deviations; differences between means were analyzed using Student’s t test or the Mann-Whitney U test. Categorical variables are described as numbers and percentages; differences between proportions were analyzed using the χ2 test or Fisher’s exact test. The diagnostic accuracy data were obtained by analyzing 2 × 2 tables for sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The 95% confidence interval (CI) was calculated for each system of noninvasive assessment of fibrosis (APRI, AAR, and FIB–4, BARD, and NAFLD fibrosis scores) using the same method for determining the diagnostic accuracy. Also the conventional likelihood ratios (LR) were calculated. The coefficient of reliability with Cronbach’s alpha was calculated to obtain the internal consistency between the different scores. The area under the curve for advanced fibrosis was determined using receiver-operating characteristic curves (AUROC) for each test. All analyses were performed using SPSS/PC v 16.0 (Chicago, IL). Differences were considered significant with P values < 0.05.
ResultsWe reviewed a total of 243 patients with liver biopsy data and histological diagnosis of NAFLD, and their medical records. We excluded 15 patients who lacked clinical, laboratory, or other secondary diagnostic results. A total of 228 biopsies and patients were analyzed in the study. The patients included 117 women (51%) and 111 men (49%); the median age was 48.6 ± 12.7 years. Sixty-three (27.6%) patients had a normal BMI, one hundred eleven (48.6%) were overweight, 65 (28.5%) had obesity grade I, nine (3.9%) had obesity grade II, two (0.8%) had grade III obesity, and two (0.8%) had morbid obesity. Forty-nine (21.5%) patients had a diagnosis of carbohydrate intolerance or type 2 diabetes mellitus.
Findings related to liver fibrosis degree were as it follows: 56.6 % (129 biopsies) did not display fibrosis (G0), 25% (57) had G1, 6.6% (15) had G2, 7% (16) had G3, and 4.8% (11) had cirrhosis (G4). The biochemical data are shown in table 1.
General characteristics of the total population.
| Variable | n (%) |
|---|---|
| Female | 117 (51) |
| Age (years)* | 48.6 ± 12.7 |
| Normal weight | 63 (27.6) |
| Overweight | 111 (48.6) |
| Obesity | 54 (23.6) |
| Glucose intolerance or diabetes mellitus NAFLD fibrosis grade | 49 (21.5) |
| G0 | 129 (56.6) |
| G1 | 57 (25) |
| G2 | 15 (6.6) |
| G3 | 16 (7) |
| G4 | 11 (4.8) |
| Platelets (103/L)* | 238 ± 95.2 |
| Albumin (g/dL)* | 3.5 ± 0.65 |
| Alanine aminotransferase (ALT) (U/l)* | 73 ± 82.7 |
| Aspartate transaminase (AST) (U/l)* | 57.6 ± 58 |
| Glucose (mg/dL)* | 107.4 ± 25.8 |
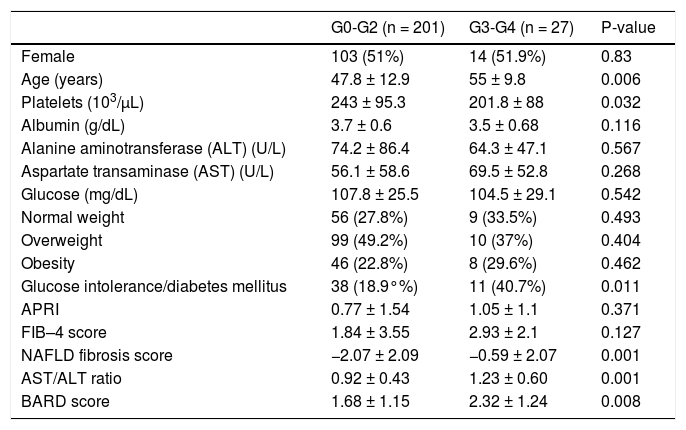
To compare the noninvasive markers of fibrosis with the results of the liver biopsies, we divided the patients into two groups: mild fibrosis (0–2, n = 201, 88.1%) and severe fibrosis (3–4, n = 27, 11.8%). The only variables that differed significantly (p < 0.05) between these two groups were platelet count, age, and the presence of carbohydrate intolerance or type 2 diabetes mellitus (Table 2). The platelet count was higher in the group with mild fibrosis than in the group with severe fibrosis (243 ± 95.3 × 103/μL vs. 201.8 ± 88 × 103/μL, p = 0.032). Patients with the higher degree of fibrosis were older. Glucose intolerance or type 2 diabetes was more prevalent in patients with severe fibrosis (n = 11, 40.7%) than in those with mild fibrosis (n = 38, 18.9%). The ALT and AST levels and AAR, albumin and glucose concentrations, sex, and BMI did not differ significantly between groups.
Characteristics of patients classified according to fibrosis stage.
| G0-G2 (n = 201) | G3-G4 (n = 27) | P-value | |
|---|---|---|---|
| Female | 103 (51%) | 14 (51.9%) | 0.83 |
| Age (years) | 47.8 ± 12.9 | 55 ± 9.8 | 0.006 |
| Platelets (103/μL) | 243 ± 95.3 | 201.8 ± 88 | 0.032 |
| Albumin (g/dL) | 3.7 ± 0.6 | 3.5 ± 0.68 | 0.116 |
| Alanine aminotransferase (ALT) (U/L) | 74.2 ± 86.4 | 64.3 ± 47.1 | 0.567 |
| Aspartate transaminase (AST) (U/L) | 56.1 ± 58.6 | 69.5 ± 52.8 | 0.268 |
| Glucose (mg/dL) | 107.8 ± 25.5 | 104.5 ± 29.1 | 0.542 |
| Normal weight | 56 (27.8%) | 9 (33.5%) | 0.493 |
| Overweight | 99 (49.2%) | 10 (37%) | 0.404 |
| Obesity | 46 (22.8%) | 8 (29.6%) | 0.462 |
| Glucose intolerance/diabetes mellitus | 38 (18.9°%) | 11 (40.7%) | 0.011 |
| APRI | 0.77 ± 1.54 | 1.05 ± 1.1 | 0.371 |
| FIB–4 score | 1.84 ± 3.55 | 2.93 ± 2.1 | 0.127 |
| NAFLD fibrosis score | −2.07 ± 2.09 | −0.59 ± 2.07 | 0.001 |
| AST/ALT ratio | 0.92 ± 0.43 | 1.23 ± 0.60 | 0.001 |
| BARD score | 1.68 ± 1.15 | 2.32 ± 1.24 | 0.008 |
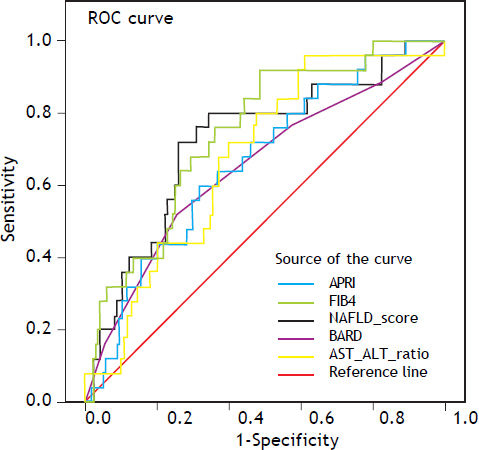
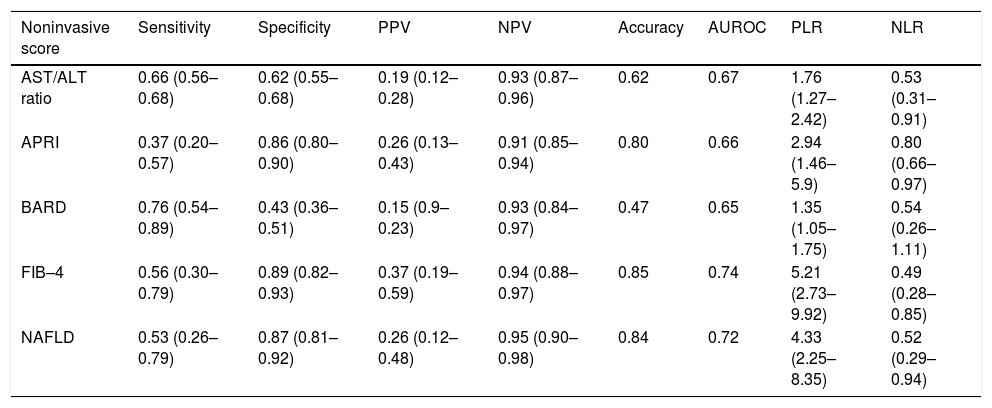
The sensitivity, specificity, PPV, NPV and LH of all noninvasive scores and their diagnostic accuracy and AUROC were analyzed (Table 3). The AAR had a diagnostic accuracy of 0.62 and the AUROC was 0.67 (95%CI, 0.57–0.77). The accuracy in the APRI was 0.80 and the AUROC was 0.66 (95%CI, 0.55–0.77). For the BARD score the accuracy was 0.47 with the AUROC 0.65 (95% CI, 0.52–0.77); the FIB 4 get an accuracy was 0.85 (AUROC 0.74; 95%CI, 0.65–0.84). The NAFLD fibrosis score had diagnostic accuracy of 0.84 and the AUROC was 0.72 (95%CI, 0.60–0.83) (Figure 1).
Comparison between noninvasive markers.
| Noninvasive score | Sensitivity | Specificity | PPV | NPV | Accuracy | AUROC | PLR | NLR |
|---|---|---|---|---|---|---|---|---|
| AST/ALT ratio | 0.66 (0.56–0.68) | 0.62 (0.55–0.68) | 0.19 (0.12–0.28) | 0.93 (0.87–0.96) | 0.62 | 0.67 | 1.76 (1.27–2.42) | 0.53 (0.31–0.91) |
| APRI | 0.37 (0.20–0.57) | 0.86 (0.80–0.90) | 0.26 (0.13–0.43) | 0.91 (0.85–0.94) | 0.80 | 0.66 | 2.94 (1.46–5.9) | 0.80 (0.66–0.97) |
| BARD | 0.76 (0.54–0.89) | 0.43 (0.36–0.51) | 0.15 (0.9–0.23) | 0.93 (0.84–0.97) | 0.47 | 0.65 | 1.35 (1.05–1.75) | 0.54 (0.26–1.11) |
| FIB–4 | 0.56 (0.30–0.79) | 0.89 (0.82–0.93) | 0.37 (0.19–0.59) | 0.94 (0.88–0.97) | 0.85 | 0.74 | 5.21 (2.73–9.92) | 0.49 (0.28–0.85) |
| NAFLD | 0.53 (0.26–0.79) | 0.87 (0.81–0.92) | 0.26 (0.12–0.48) | 0.95 (0.90–0.98) | 0.84 | 0.72 | 4.33 (2.25–8.35) | 0.52 (0.29–0.94) |
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihoodLR) and AUROC are expressed as fractions of 1 with 95% confidence intervals.
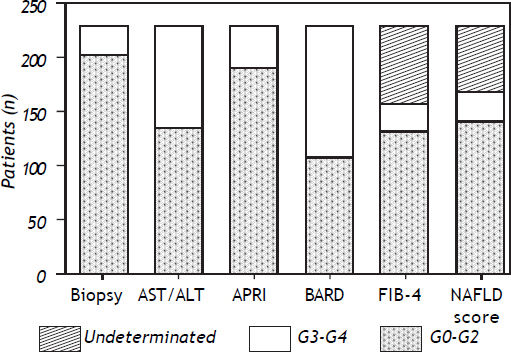
All systems included patients in both the mild fibrosis and severe fibrosis groups (Figure 2). However, the degree of fibrosis could not be determined for all patients in two of the five systems-FIB–4 and NAFLD fibrosis scores. For the biopsies of specimens that showed indeterminate results in the FIB–4 score, 46% of the samples corresponded to G0, 27.3% to G1, 10.9% to G2, 9.5% to G3 and 5.4% to G4. For the biopsies of specimens that showed indeterminate results in the NAFLD fibrosis score, 40% corresponded to G0, 27% to G1, 9.6% to G2, 16% to G3, and 6.4% to G4 (Figure 3). In biopsies with indeterminate results, about 45% of the samples were graded as no fibrosis in the two systems.
The reproducibility coefficient (Cronebach alpha) between NAFLD score and APRI score was 0.48, with the AST / ALT score was 0.58, with BARD score 0.58 and the FIB-4 0.94.
DiscussionNoninvasive methods for detecting fibrosis are used to reduce the need for liver biopsies to identify patients with NAFLD in populations at risk. The purpose of identifying patients by mild fibrosis in NAFLD, is to prevent progression to later stages of fibrosis (cirrhosis) and the development of their complications (hepatocellular carcinoma). Many treatments had been studied to reverse fibrosis from one stage to another, primarily in early stages of the disease, and here is where differentiation between mild and severe fibrosis becomes relevant. Moretto, et al, showed reversal of portal and lobar fibrosis in 16 of 35 morbid obese patients after gastric bypass surgery.27 However, it is important to note, also showed deterioration in the degree of fibrosis in some patients in this and other studies.28
Although there are known variables, such as obesity and diabetes mellitus, that can be used to identify patients at risk of having significant fibrosis, the clinician cannot rely solely on these factors. In this study we found significant differences between patients with mild and severe fibrosis in the prevalence of diabetes mellitus, a finding that has also been reported in other populations.29 By contrast, we found that high BMI was not related to the degree of fibrosis in our cohort, which is already known for the development of fibrosis.30,31 Even though only 23% of our patients had a BMI ≥ 30 kg/m2, only 1.7% of the sample had a BMI > 40 kg/m2. Importantly, 30% of severe fibrosis patients had a BMI < 25 kg/m2. However, because of the retrospective nature of this study, we do not know whether the patients had a higher BMI at some point before the study.
Of the laboratory parameters, the only surrogate marker of advanced fibrosis, that differed between the two groups in our cohort was the platelet count. Blood platelets, by connecting hemostasis and inflammatory processes, participate in the pathogenesis of chronic liver disease. Kondo et al demonstrated pathological findings for the accumulation of platelets in the liver in cases with chronic hepatitis C.32 So, platelets count in chronic liver diseases is modified by many factors, making it surveillance a simple marker to demonstrated progression of the liver disease.
Our data show the importance of screening for fibrosis in patients with fatty liver identified by imaging but with normal laboratory parameters.
The diagnosis of fibrosis in patients with NAFLD is the major predictor of disease progression in these patients. The development of new diagnostic noninvasive fibrosis, are designed to detect these patients early to track suitable to control and prolong the complications that develop in the future. As has been previously determined, the elevation of liver enzymes may or may not be present in patients who have fibrosis, for that reason, other factors have taken importance as predictors of fibrosis in patients with NAFLD (glucose intolerance or diabetes mellitus33), and may have better accuracy in the diagnosis of fibrosis, that together with the enzyme elevations, we can get a better prediction of fibrosis grade. In our cohort we found none difference between two populations (mild fibrosis vs. severe fibrosis) but if there was higher prevalence glucose intolerance or diabetes mellitus in patients with severe fibrosis.
This takes us back to the question of whether clinicians should use noninvasive markers or perform a liver biopsy. Although liver biopsy is the gold standard and complications occurs in only a low percentage of patients, subjecting the patient to this risk is questionable. This is where the role of noninvasive methods becomes important.
The usefulness of the evaluation systems has been evaluated in different NAFLD populations around the world. The AAR was validated for NAFLD and had a reported sensitivity of 52%, specificity of 90%, PPV of 55%, and NPV of 89%.26 When applied to our population, the figures were similar, although the NPV was higher (93%). However, our analysis showed a diagnostic accuracy of 0.62, which raises questions about its effectiveness in diagnosing fatty liver disease in Latino population.
The APRI was originally developed for assessing fibrosis in patients infected with hepatitis C virus, but this method has been validated recently for NAFLD.34,35 One study that assessed the usefulness of this system26 in 145 patients found a sensitivity of 27%, specificity of 89%, PPV of 37%, and NPV of 95%. Another assessment of the NAFLD in a French cohort showed a sensitivity of 66%, specificity of 90%, PPV of 72%, and NPV of 87%; these are the highest sensitivity and PPV values reported.36 Our results are similar to the values for sensitivity and NPV reported by McPherson.26 Extrapolating these results to our population and comparing the diagnostic accuracy suggest that APRI can reliably exclude the presence of severe fibrosis. The APRI has an advantage in that it uses two variables available in routine practice and a simple formula for the calculation, although it is unable to obtain values for indeterminate fibrosis.
The NAFLD fibrosis score was created to evaluate fibrosis in fatty liver. In 138 patients included in a previous study, this system showed sensitivity, specificity, PPV, and NPV of 22.7%, 100%, 81.3%, and 100%, respectively.24 Other studies26,29 have reported sensitivity, specificity, PPV, and NPV in the ranges of 22–78%, 58–100%, 30–81%, and 92–100%, respectively. We found a sensitivity of 53%, specificity of 87%, low PPV of 26%, and NPV of 95%. The values obtained in our study are comparable with those obtained in different geographic locations and with data from a more recent study of the English population, which obtained a diagnostic accuracy of 0.84. This provides even greater statistical power supporting the use of this marker for detecting advanced fibrosis in our population. However, one drawback of this marker is the need for a calculator to produce the value because the formula is complex. However, this method is applicable to Latino populations because of its high diagnostic accuracy for severe fibrosis.
The BARD score includes variables such as the presence of diabetes, which as shown in this study, is a predictor of fibrosis. This score identifies patients at increased risk of advanced fibrosis. Some studies have shown a low sensitivity and PPV26,37 and Ruffillo, et al.29 reported an NPV < 90%. By contrast, the original validation study was reproduced by McPherson, et al.,26 who reported a PPV and NPV for advanced fibrosis of 44% and 95%, respectively. In our sample, the BARD score had a sensitivity, specificity, and PPV < 80%; although the NPV was 93%, the accuracy was low (0.47). These data show that the BARD score has poor diagnostic value for advanced fibrosis.
The FIB-4 system has shown interesting results in studies published from around the world. The sensitivity was reported as 85%, specificity 65%, PPV 75–80%, and NPV 95%.26,38 We found different results in our study: a lower sensitivity and PPV, but an NPV of 94% and accuracy of 0.85.
In our study, of the samples that produced indeterminate values in the FIB–4 and NAFLD scores, 3.5% were graded as G4. In the patients whose status remains undetermined, there is no consensus about the need for liver biopsy, although this group would be the most likely candidate for biopsy for adequate staging of fibrosis as part of appropriate surveillance and monitoring. According to our data, the likelihood will be classified as undetermined and then subjected to biopsy but will not have fibrosis is about 50%; the other 50% of such patients will have some stage of fibrosis. Also, we obtained a coefficient of reliability between these two scores of 0.94, which indicates a reproducibility of the results with the two different diagnostic methods.
One limitation of the study is the number of study population, however there is not a constraint to evaluate the diagnostic accuracy as well as the validation study of the NAFLD score by Angulo, et al., where the number of patients was 253 and the prevalence of advanced fibrosis was 14%.24 Another important limitation is the lack of some data, as the hip circumference and waist/hip ratio, which are important for Latino population.39
ConclusionThe APRI, FIB-4 and NAFLD fibrosis scores have a higher accuracy and may be used for the noninvasive diagnosis of advanced fibrosis in NAFLD in the Mexican and Chilean population, mainly to rule out severe fibrosis. These tools are very useful in countries with high prevalence of overweight and obesity, as Mexico or Chile having a higher risk of developing fatty liver and fibrosis.
However, 25% of patients evaluated by these methods have an indeterminate degree of fibrosis and may need to be evaluated by a second noninvasive system or even by an invasive procedure such as a liver biopsy. Future research to assess the clinical implications of non-invasive markers is needed.
AcknowledgementThis work was partially supported by Medica Sur & Clinic and Foundation and by grants from the Chilean National Fund for Research in Science and Technology (FONDECYT #1110455 to MA and # 11100113 to CB) and the National Council for Scientific and Technological Research (CONICYT Chile, Project ACT79 to MA).