Vanishing bile duct syndrome (VBDS) is a rare disorder and requires a liver biopsy for a diagnosis. The condition has not been reported in children with toxic epidermal necrolysis (TEN). The etiology of VBDS in our patient with TEN is most likely from drug hypersensitivity. A high index of suspicion will prompt clinicians to start more specific investigations and treatments. The use of immunosuppressive agents, intravenous immunoglobulin and ursodeoxycholic acid has not been consistently successful in these patients. A new approach with biologic agents such as anti-tumor necrosis factor-α may be a promising therapy and reduce severe adverse outcomes.
Abbreviations:
Vanishing bile duct syndrome (VBDS)
Toxic Epidermal Necrolysis (TEN)
Stevens Johnson syndrome (SJS)
Trimethoprim-Sulfamethoxazole (TMP-SMX)
Peribiliary Vascular Plexus (PVP)
IntroductionVanishing bile duct syndrome (VBDS) has been reported in a number of conditions such as drug administration, toxins, primary biliary cirrhosis, chronic allograft rejection, graft-versus-host disease, Langerhans’ cell histiocytosis and Alagille Syndrome.1-3 The more acute form of VBDS is related to drug use. Although rare, vanishing bile duct syndrome (VBDS) is associated with toxic epidermal necrolysis (TEN) in adults and Stevens Johnson syndrome (SJS) in both children and adults.1,2 When intrahepatic bile ducts vanish, they do not usually regenerate. As a result, liver failure develops following biliary cirrhosis.3 There is evidence, however, that some selected patients experiencing VBDS associated with TEN or SJS improve after immunosuppressive therapy.2,4
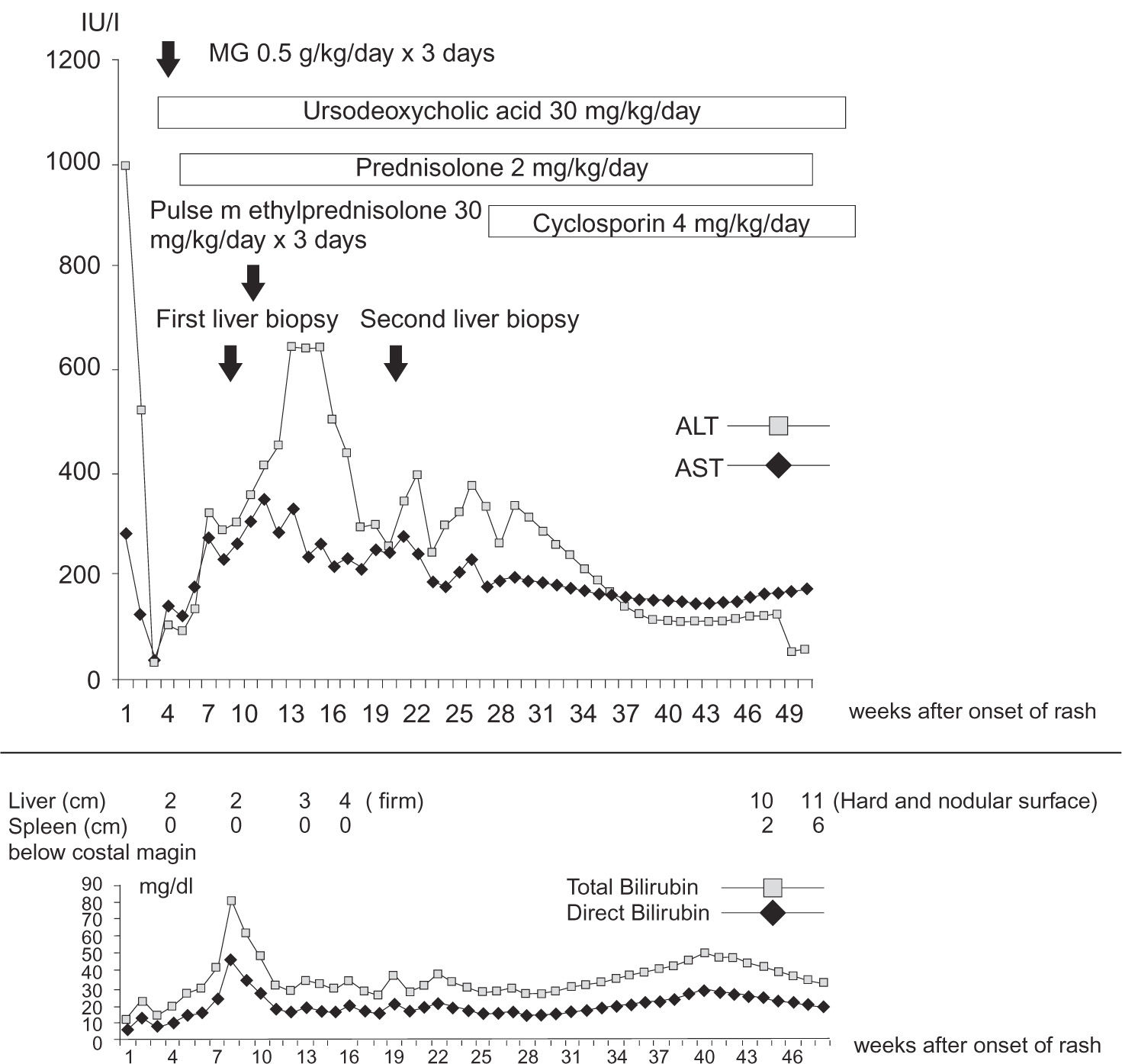
Case reportA previously healthy 7-year-old girl was hospitalized with a diagnosis of acute viral infection after a 4-day period of fever reaching 39oC. The hospital course was complicated with pulmonary edema, myocarditis and sepsis which required ventilatory support. A fourteen-day course of ceftazidime and trimethoprim-sulfamethazole and a seven-day course of doxycycline were administered empirically due to positive serology for scrub typhus and leptospirosis. No history of travel and exposure to contaminated water or soil was noted. Two weeks after the onset of the illness, she developed a fever including generalized erythematous papules that affected the oral cavity, conjunctivae, anus and genitalia. The skin rash shortly evolved into blisters. Drug hypersensitivity reaction was diagnosed. Due to oral mucosal involvement and inability to eat, parenteral nutrition was started. Dexamethasone was administered. Cholestasis was noted one week after the onset of rash (Figure 1). She was then transferred to our institution for continuation of care. The physical findings on admission included icteric sclerae, jaundice in nondesquamated areas, generalized maculopapular rash with blistering on her trunk and extremities, back, and face, pseudomembranes with symblepharon of both eyes and hepatomegaly. A diagnosis of toxic epidermal necrolysis was made. The leukocyte count was 3,960 mm-3 with 53% neutrophils, 35% lymphocytes, 11% monocytes and 1% eosinophils. Platelet count was 525,000 mm3, and hemoglobin was 8.9 g/dL, hematocrit 27.3%. MCV 77 μm3, MCH 25 pg and MCHC 32 g/dL. The red blood cell morphology was unremarkable. Urine examination was unremarkable except the presence of bile. Other test results included: serum aspartate aminotransferase, 70 U/L (normal 4-40 U/L); alanine aminotransferase, 109 U/L (normal 4-40 U/L); alkaline phosphatase, 441 U/L (normal 30-300 U/L), total bilirubin 10.4 mg/dL (normal 0.2-1.2 mg/dL); direct bilirubin 9 mg/dL (normal 0-0.2 mg/dL), γ- glutamyl transpeptidase (GGT) 880 U/L (normal 7-50 U/L); albumin 2.3 g/dL (normal 3.5-5.5 g/dL); globulin 3.5 g/dL (normal 1.5-3.5 U/L); normal prothrombin and partial thromboplastin time and normal serum electrolytes. The erythrocyte sedimentation rate was 68 mm/h. Electrolytes, BUN and creatinine were within normal limits. Serology for hepatitis A, B, C, Epstein-Barr viruses, cytomegalovirus and human immunodeficiency virus was negative. The immunoglobulin concentrations were normal. The abdominal sonogram demonstrated a patent extrahepatic bile duct, absence of stones, normal intrahepatic bile ducts and liver echogenicity. An eye examination did not show evidence of posterior embryotoxon. Antinuclear antibody (ANA) titer was 1:160, speckled type and antineutrophil cytoplasmic antibody (ANCA) was positive. Both anti-smooth muscle antibody and anti-liver/kidney microsomal antibody were negative. Antimitochondrial antibody was not performed. Endoscopic retrograde cholangiopancreatogram did not demonstrate any abnormality of the pancreatobiliary system. Intravenous immunoglobulin was administered for three consecutive days at a dose of 0.5 g/kg and prednisolone (2 mg/ kg/day) and ursodeoxycholic acid (20 mg/kg/day) were started.
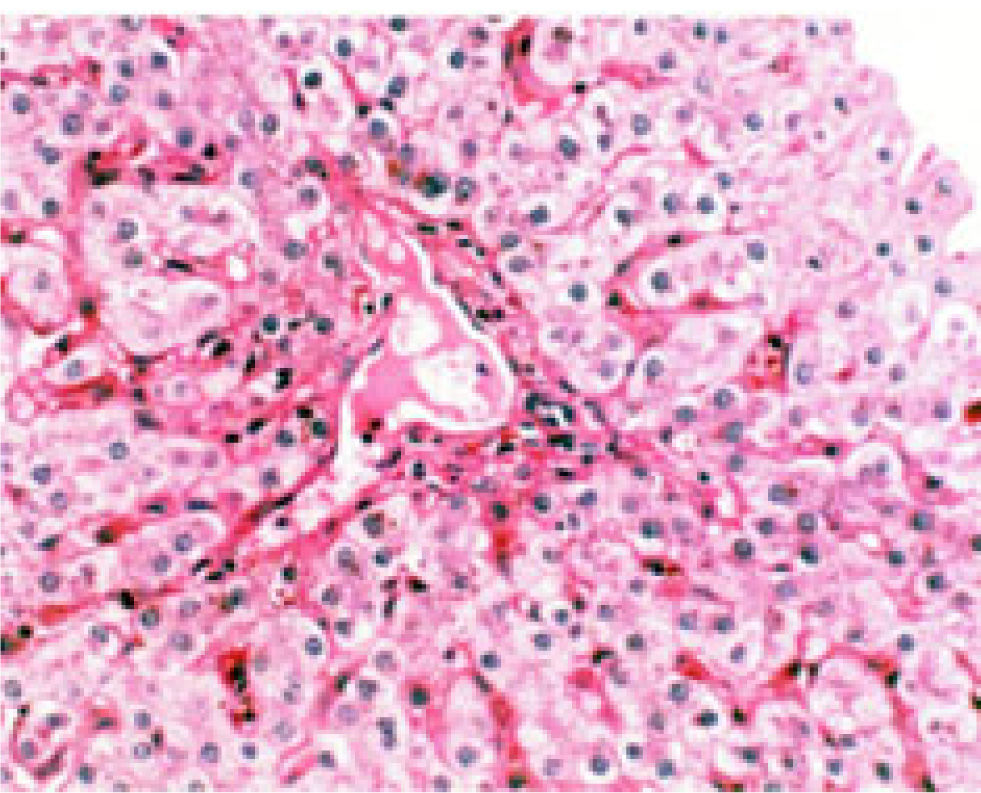
A percutaneous liver biopsy performed at 12 weeks after the onset of TEN showed portal inflammation with predominant polymorphonuclear cells and no intralobar bile ducts in at least 10 portal areas on H&E stain (Figure 2), whereas cytokeratin 7 staining rarely identified bile ducts (not shown). Neither viral cytopathic effect nor an organism was identified. There was no significant hepatocellular damage. There was no evidence of autoimmune hepatitis or sclerosing cholangitis. Repeat ANA and ANCA were negative. The diagnosis of VBDS associated with TEN was made. No clinical or biochemical improvement of cholestasis was observed despite treatment with prednisolone for 15 weeks (Figure 1). Pulse methylprednisolone at 30 mg/kg/day was administered for three consecutive days (Figure 1). A repeat liver biopsy performed 8 weeks after the first one failed to reveal interlobular bile ducts. Cyclosporin A was administered at increasing doses up to 4 mg/kg/day with optimal serum levels for over 24 weeks. Twenty weeks after the child developed dyspnea of unknown etiology. No significant pulmonary arteriovenous shunting was detected. Radiographic studies of the chest were unremarkable. In spite of all immunosuppressive treatment the patient progressed to chronic liver disease and awaits liver transplantation.
DiscussionVanishing bile duct syndrome (VBDS) is not commonly encountered in clinical practice. VBDS is the final outcome of factors acting in an isolated or combined manner such as immunological, idiosyncratic, metabolic, infectious, vascular, developmental and/or chemical mechanisms.3 The common target is located at the interlobular bile ducts which have a diameter of 20-80 μm and blood supply is exclusively provided by the hepatic arterial branches, the so-called peribiliary vascular plexus (PVP). The PVP is often compromised through various previously discussed mechanisms. The disappearance of more than 50% of interlobular bile ducts in the portal areas is defined as intrahepatic bile duct loss.5 There is literature, though, that has reported VBDS to have reversed over a period of time in a very small number of patients.2,6,7
TEN and SJS are an uncommon immune complex-mediated hypersensitivity reaction, associated with drug use and certain infections, which present with marked involvement of skin and mucosal surfaces. Gastrointestinal manifestations of TEN and SJS include diffuse inflammation, erosion and ulceration of the mucosa leading to dysphagia, impaired alimentation, vomiting, bleeding and diarrhea. 8-10 Ductopenia-related jaundice is rare and VBDS has been reported in 2 children with SJS only, but not in those with TEN.1,2
Trimethoprim-sulfamethazole (TMP-SMX), ampicillin, amoxicillin-clavulanic acid, phenytoin sodium and phenobarbital have been reported in adult patients with both VBDS and TEN.10 Signs of drug-associated TEN usually begin 1 to 3 weeks after initiation of drug therapy. Therefore TMP-SMX is more likely to play a major role in the development of the multiorgan involvement in our patient.
The fact that few people develop this phenomenon raises the hypothesis of genetic predisposition, particularly HLA haplotypes or lack of protective mechanisms. The association of VBDS with TEN or SJS also leads to an interesting theory that the drug, acting as a hapten, produces autoantibodies against a common target antigen, epithelial cytokeratin, present in bile duct epithelium, skin, conjunctival epithelium and orogenital mucosa.1 The presentation of immunogenic peptides by class II HLA molecules on macrophages might represent the first step of immune damage by the generation of cytotoxic T cells and/or antibody-producing B cells.11 Paquet et al hypothesized two steps of TEN pathomechanism.12 The reactive drug metabolites stimulate direct cytotoxic effects on epithelial cells during the initial step when apoptosis occurs intracellularly via the CD95 and tumor necrosis factor-α (TNF-α) systems. In the amplification step, TNF-α acts as an autocrine or paracrine messenger increasing its own production. This further induces the expression of class II MHC, adhesion molecules and releases more chemotactic factors with a result of epithelial destruction. The production of cytokines particularly TNF-α by inflammatory cells around portal tracts may increase HLA expression, compromising the peribiliary vascular plexus (PVP), which in turn interferes with bile duct proliferation and the basement membrane matrix.11 Interestingly, the child in this report had hypergammaglobulinemia and evidence of polyclonal B lymphocyte activation (positive serology for scrub typhus and leptospirosis) which have been previously reported to be associated with a significantly increased risk of SJS, particularly when exposed to certain drugs.11
Leptospirosis is a worldwide zoonotic disease which can manifest with fever, oculocutaneous lesions, portal inflammation and cholestatic jaundice as seen in this case.13 Renal impairment usually occurs as a severe complication but did not occur in this patient. Microscopic visualization of Leptospira by light microscopy commonly performed to identify the bacteria in clinical tissues14 did not demonstrate the bacteria on two of her liver biopsies. However, the study by dark-field microscopy and immunofluorescence of liver tissue was not done in this patient. In an animal model, Nally et al studied liver tissues from guinea pigs infected with Leptospira under microscopy with H&E staining.15 Large numbers of Leptospira and activated Kupffer cells were observed with increased inflammation in portal tracts and no bile duct injury or damage was noted. Based on overall clinical presentation and results from two liver biopsies, leptospirosis is unlikely to be the etiology of VBDS and TEN.
Autoantibodies have been found in patients developing drug-associated hepatitis and VBDS. Anti-smooth muscle antibodies were positive at the titer of 1:640 in a 57-yearold Samoan-American man without previous abnormal liver function receiving TMP-SMX and developing VBDS.16 Given his liver histopathology and the lack of response to corticosteroids, autoimmune hepatitis was unlikely to be the cause of VBDS, as seen in our patient.
Drug-associated cholestasis usually improves after discontinuation of the drug. The improvement can be delayed due to drug distribution in adipose tissue. The prognosis of drug-induced small bile duct injury remains unpredictable, partly because liver injury is not well detected at an early phase. A single study indicated that the development of acute cholangitis with ductular and periductular degeneration is likely an early event when sequential liver biopsies were studied in a group of adults.17 Liver histopathology often reveals a late stage of damage, indicating an irreversible outcome. Although there is no specific therapy for VBDS, some authors claimed that the use of immunosuppressive medications or ursodeoxycholic acid resulted in a regain of intralobar bile ducts,2,4,6 whereas others did not.1,7 Ursodeoxycholic acid has shown to improve the outcome in a few individuals with VBDS from prochlorperazine and chlorpromazine, 6,7 but not in those who took other medications. Smith et al reported an improvement of cholestasis in a child with VBDS with a high dose of ursodeoxycholic acid.18 The better response to ursodeoxycholic acid in those patients suggests different mechanisms of drug-induced bile duct injury. In addition, a milder degree of the liver damage or an early phase of VBDS can be reversible with immunosuppression.2 A new therapeutic approach with anti-TNF-α antibodies is promising but its efficacy requires confirmation in a trial of a large group of patients with TEN.12 Although the natural course of VBDS is not favorable, an attempt to regain bile ducts is mandatory.
In conclusion, a judicious use of any drugs should be practiced in patients of all age groups. It is essential to monitor clinical manifestations of liver injury and/or liver function tests, especially in drugs known to cause hepatobiliary damage. The offending drug must be withdrawn when facing its serious side effects. Although the result of immunosuppressive drugs is largely disappointing, an early use of steroids and IVIG is encouraged in patients with TEN or SJS to prevent permanent liver and other organ damage after evaluation of risks and benefits. Anti-TNF-α antibodies may reverse TEN evolutions and improve the outcome. Liver transplantation is the ultimate rescue when developing liver failure.
AcknowledgementsThe authors thank Carlos Lifschitz, M.D. for reviewing the manuscript.