Following Zuckerman's psychobiological personality model, a series of genetic association hypotheses between some polymorphisms of Serotonin, Testosterone, Dopamine, MAO-A and COMT, and three temperamental traits (Impulsive Unsocialized Sensation Seeking, Neuroticism, and Extroversion-Sociability) were tested. In order to surpass previous limitations of this kind of studies: 1) the measurement of the phenotype was improved by using eight personality scales, and 2) some statistical analyses were conducted to test conjointly the effects of the considered polymorphisms. 252 participants from two samples (147 male inmates, and 105 male healthy volunteers) were analyzed. A tendency in the theoretical expected direction is reported when several polymorphisms are considered conjointly. The discussion section recommends applying more integrative frameworks to explore the complex paths from genetics to behavior.
Partiendo del modelo de personalidad psicobiológico de Zuckerman se planean una serie de hipótesis de la asociación genética de diferentes polimorfismos de la serotonina, testosterona, dopamina, MAO-A y COMT, y tres rasgos temperamentales (Búsqueda impulsiva de sensaciones no socializada, Neuroticismo y Extroversión-sociabilidad). Para superar limitaciones anteriores de este tipo de estudios: 1) la medida fenotípica se mejoró usando ocho escalas para medir los constructos de personalidad, y 2) algunos análisis estadísticos fueron usados para estudiar conjuntamente los efectos de los polimorfismos. La muestra analizada se compuso de 252 participantes de dos muestras de hombres, una de 147 presos y otra de 105 voluntarios sanos. Los resultados informan que cuando se consideran conjuntamente todos los polimorfismos estu-diados se observa una tendencia en la dirección de las hipótesis formuladas. La sección de la discusión recomienda aplicar variables más integradoras para explorar los caminos complejos de las genéticas a la conducta.
Marvin Zuckerman's personality model is rooted in the psychobiological tradition of the study of personality (Matthews & Deary, 1998; Stelmack, 2004; Zuckerman & Aluja, 2014). He sets out to explain the differences in basic personality traits on the grounds of the role played by neurotransmitters, gonadal hormones, and neuroregulater brain enzymes. Specifically, he places the main emphasis on the role of monoamines (dopamine, noradrenaline [also called catecholamines]) and serotonin [also known as indoleamine]) (Zuckerman, 2005). In general, dopamine (Do) circuits in the brain would be involved in appetitive approach and exploration, serotonin (5-HT) in behavioral control and inhibition of approach in response to signals of danger, and norephinephine (Ne) would be linked to arousal, alarm and fear (for a review of the multiple studies supporting this model at the biological level, see Zuckerman, 2005). Zuckerman's model also recognizes the role of gonadal hormones, especially testosterone, in the observed differences in personality and behaviour. This role is supported by a great deal of research (i.e. Aluja & García, 2005; Archer, 2006; Roberti, 2004).
As is well known, enzymes regulate the action of neurotransmitters. For instance, synaptically released dopamine is inactivated by re-uptake into presynaptic terminals followed by re-storage or degradation by monoamine oxidase (MAO). Equally, Catechol-O-methyltransferase (COMT) degrades catecholamines such as dopamine and norepinephrine. On the other hand, released serotonin (5-HT) is also inactivated by re-uptake into terminals followed by re-storage or degradation by MAO. Monoamino oxidase normally destroys monoamine transmitters within presynaptic cells. MAO exists in two forms: A and B. In the brain, noradrenaline and serotonin are delaminated mainly by MAO-A, and dopamine by MAO-B (Halligan, Kischka, & Marshall, 2004). Azmitia and Whitaker-Azmitia (1995) also describe that MAO-A affects preferentially the serotonergic system.
The specific paths from biology to personality differences are set out in Zuckerman's psychopharmacological model (Zuckerman, 2005, p. 270). This psychopharmacological model describes the hypothethised causal paths from neurotransmitters (Dopamine, Serotonin, Norepinephrine and Gamma-Aminobutyric acid [GABA]), enzymes (as MAO-B), and metabolites (as Dopamine beta hydroxylase [DBH]) and gonadal hormones to three temperamental basic traits included in Zuckerman's personality model (Zuckerman, Kuhlman, Joireman, Teta, & Kraft, 1993): Impulsive Unsocialized Sensation Seeking (P-ImpUSS), Neuroticism-Anxiety (N-Anx) and Sociability (Sy). Zuckerman's model suggests that P-ImpUSS is related to high approach (high dopamine level), low inhibition (low serotonin level), and low arousal (low norepinephrine level). N-Anx is mainly related to high arousal (high norepinephrine) and low GABA, and Sy should be associated with gonadal hormones (high testosterone) and high approach (high dopamine).
In the last two decades, there has been great interest in studying the genetic basis of personality traits. This approach rests on two aspects: 1) High heredability of personality traits (Loehlin, 1992) and 2) the great amount of evidence of the relationships between biological markers and psychological variables (i.e.: Stelmack, 2004; Zuckerman, 2005). Thus, the particular candidate genes to be studied can be selected according to what is known about the biochemical or neurological correlates of behavior in animals or humans. It is logical to expect genes to affect personality through biology. Hence, considering the strong evidence establishing the biological basis of personality traits, a similar strong association between specific genes and certain traits is also to be expected (Benjamin, Ebstein, & Belmaker, 2002).
The general hypothesis is that some alleles of certain polymorphisms would be associated with high (or low in some cases) levels of neurotransmitters, neuroregulator enzymes and gonadal hormones and, therefore, they should have an impact on the differences in personality traits. For the present paper, we focus on some polymorphism associated with relevant variables considered in the Zuckerman model: Serotonin, Testosterone, Dopamine, MAO-A, and COMT.
With regard to serotonin, the 5-HTTLPR polymorphism has received special attention. It maps on the promoter region of SCL6A4 gene affecting the transcriptional activity of 5-HTT. Studies using human cell lines expressing the two different genotypes indicate that the L allele is associated with higher transcriptional efficiency of the promoter than is the S allele (Aluja, García, Blanch, De Lorenzo, & Fibla, 2009; Bennett et al., 2002; Lesch et al., 1996). On the contrary, carriers of the S allele exhibit a reduced rate of 5-HT uptake. On the other hand, the role of the 5-HTTVNTR polymorphism in serotonin transmission remains rather less clear. Some studies suggest that individuals homozygous for the long allele of the 5-HTTVNTR appeared to have lower affinity of 5-HT uptake than individuals heterozygous for the short allele (Kaiser et al., 2002). If this were so, the 5-HTTVNTR 12/12 genotype would have a negative effect on serotonin neural transmission. However, other authors point out that 5-HTTVNTR 12 allele may be functionally related to a better serotoninergic neural transmission. This being so, the presence of S allele of the 5-HTTLPR and the 12/12 genotype of 5-HTTVNTR would be associated with low serotonin and, following Zuckerman's model, with low inhibition and high P-ImpUSS.
Concerning testosterone, it has been reported that two polymorphisms (CAG and GGN) of the human androgen receptor (AR) influence gene expression (Kittles et al., 2001). Indeed, short alleles of both polymorphisms, especially for CAG, have been associated with an increased expression of the gene (i.e. an increase of testosterone; Ding, Xu, Menon, Reddy, & Barrack, 2005). In this sense, several studies have examined the associations of AR gene polymorphisms with disruptive behavior and disinhibited personality traits. Comings, Chen, Wu, and Muhleman (1999) found that a lower risk for attention-deficit/hyperactivity disorder, conduct disorder and oppositional defiant disorder is associated with a haplotype formed by long alleles of both CAG and GGN repeat. Jönsson et al. (2001) found significant associations of shorter CAG repeats with traits related to dominance and aggression of the Karolinska Scales of Personality (KSP). The AR exon 1 repeat polymorphisms have also been examined for association with Psychoticism (a strong marker of P-ImpUSS (Zuckerman, Kuhlman, Thornquist, & Kiers, 1991). Turakulov, Jorm, Jacomb, Tan, and Easteal (2004) found that males with short CAG alleles had significantly higher Eysenck's Psychoticism scores. Therefore, subjects carrying the short version of the two polymorphisms are expected to have higher levels of testosterone and, following the Zuckerman model, high scores on P-ImpUSS and Sy.
Variation in dopamine release and in the density of its varied receptors regulates cortical excitability, intensity of sensory pleasure, and reactions to novel events. Greater dopamine activity in the cortex also implies that a new event will produce a proportionally smaller rise in dopamine. These findings imply that children or adults with greater cortical dopamine activity might have lower preference for novel experiences than those with less dopamine activity (Kagan & Snidman, 2007).
Many polymorphisms associated with dopamine levels have been investigated. We focus on the dopamine 2 receptor (DRD2), since it has been related with behavioral desinhibition, a core concept of Sy and, above all, P-ImpUSS (Zuckerman, 1994). Lower D2 receptor availability seems to be associated with lower performance in inhibition tasks (Hamidovic, Dlugos, Skol, Palmer, & de Wit, 2009). Elsewhere, Munafò, Matheson and Flint (2007) concluded that the presence of the A1 allele of one of the most studied polymorphisms of DRD2 (the TaqIA, whose alleles are A1 and A2) is a risk factor for alcoholism. Therefore, the presence of A1 allele on the TaqIA polymorphism of the DRD2 should be associated with high dopamine levels and, following the Zuckerman model, high Extroversion and P-ImpUSS scores.
Two neuroregulator enzymes will be analyzed in the present paper: MAO-A and COMT. The former preferentially affects serotonin (Azmitia & Whitaker-Azmitia, 1995). The MAO-A gene polymorphism classifies individuals as low (allele 3) or high (allele 4) MAO activity types. Shih, Chen, and Ridd (1999) reported that eliminating the MAO-A gene (an experimental design to produce low activity) results in increases in serotonin and norepinephrine in some cerebral areas. Sabol, Hu, and Hamer (1998) reported that high activity type transcribes 2-10 times more efficiently than the low repeat allele. Although this impact of the genotype on the biological activity of the enzyme seems firmly established, the association between the activity of the gene and psychological variables is somewhat puzzling. On one hand, low MAO expression appears to be a risk factor for developing aggressive behaviors and impulsivity in adulthood (Caspi et al., 2002; Huang et al., 2004). On the other hand, other authors have found an association of the high activity alleles with higher impulsivity and aggression in a community sample of males (Manuck, Flory, Ferrell, Mann, & Muldoon, 2000). In any case, if any significant relationship between MAO-A and psychological measures is reported, this is usually between the low activity alleles of MAO-A and measures of impulsivity/aggression or related concepts such as Cluster B personality disorders (Jacob et al., 2005).
The Catechol-O-methyltransferase (COMT) helps to maintain the appropriate levels of dopamine and norepinephrine in the prefrontal cortex. Human gene polymorphism in the COMT has two alleles (Val and Met). Heterozygous individuals (Val/Met) exhibit intermediate levels of COMT activity compared to homozygous individuals (Egan et al., 2001). It seems that the Met allele is associated with decreased activity of the enzyme COMT. Thus, individuals homozygous for the Met allele (Met/Met) are 40% less active than individuals homozygous for the Val allele (Val/Val) in the prefrontal cortex (Chen et al., 2004). Likewise, Tunbridge et al. (2007) results show that Met/Met subjects have dopamine basal levels higher than heterozygous subjects and those with double allele Val.
Some studies have addressed the association between COMT and various phenotypes related to impulsive antisocial behavior. Thus, Lachman, Nolan, Mohr, Saito, and Volavka (1998) reported an association between the Met allele and measures of aggression in patients diagnosed with schizophrenia and a history of repeated violence. Other studies using psychiatric patients have obtained similar results (Volavka, Bilder, & Nolan, 2004). Similarly, a recent study that linked psychopathic characteristics with different polymorphisms in a sample of adolescents with attention deficit hyperactivity disorder has found a relationship between COMT and problems interpreting the emotions of others (Fowler et al., 2009). Using personality measures, an association between COMT and sensation seeking or reward dependence has also been reported (Tsai, Hong, Yu, & Chen, 2004).
Zuckerman's model predicts that high levels of dopamine and low levels of norepinephrine reduce the threshold necessary for a sensation seeking and aggressive behavior. Starting from this biological causal premise, the hypotheses are simple: if aggressive/disinhibited behavior is provoked by a high dopaminergic activity, then a lower activity of MAO-A and COMT (i.e. less able to “eliminate” dopamine) should be related to an increase in P-ImpUSS; and, going a step further, the alleles that are associated with less activation of these enzymes (low transcription activity) should be associated with disinhibited personality, sensation-seeking and antisocial behavior (Volavka et al., 2004).
Munafò et al. (2003) conducted a meta-analysis with all studies (from 1995 to 2002) that linked genetic polymorphisms and personality variables. Phenotypic measures were divided into three categories: Approach, Inhibition and Aggression. After entering various covariates such as age, sex or ethnicity of the participants in each study, only the effect of serotonin (5-HTT) on the inhibition system remained significant. In light of these disappointings results, Munafò et al. (2003) suggested the following changes in molecular genetic studies of personality: 1) Using a composition of conceptually related phenotypic measures from various biological models of personality (i.e., it is not desirable to use a single questionnaire, for example, the scale of Novelty Seeking from Cloninger's TCI or TCI-R to describe the population at the phenotypic level); 2) using group comparison designs to increase the power of the studies; 3) investigating the interactions between different genetic markers.
With regard to the first suggestion, Reuter, Schmitz, Corr, and Hennig (2006) found that Val/Val carriers scored higher on Extroversion and NS1 facet (exploratory excitability) of TCI than subjects with one copy of MET allele. They could not find the same results in the total Novelty Seeking scale and the other three facets. Therefore, they concluded that genetic studies should focus on subfactors (or different scales and measures) of the same construct. Burt, McGue, Iacono, Comings, and MacMurray (2002) found no relationship between the DRD2 and scales assessed by the Multidimensional Personality Questionnaire (MPQ). However, they did not include an explicit measure of sensation seeking or antisocial behavior, but a scale named Control that measures the opposite pole of impulsivity, so the lack of association could be produced by the specific and unique phenotypic measure applied. The results of both studies reinforce the idea raised by Munafò et al. (2003) about the necessary improvement of the phenotypic characterization of subjects.
On the other hand, when Reuter et al. (2006) explored the role of the interaction between the COMT and DRD2 TaqIA, they found an interaction between both genes. Thus, high levels on Gray's Sensitivity to Reward trait were associated with two genetic profiles (Met Homozygous/Homozygous A2) and (homozygous Val/Homozygous A1). It should be remarked that both profiles were associated with high dopaminergic activity. This is also congruent with Zuckerman's model since Sensitivity to Reward correlates highly with Extroversion (Aluja & Blanch, 2011; Torrubia, Ávila, Moltó, & Caseras, 2001).
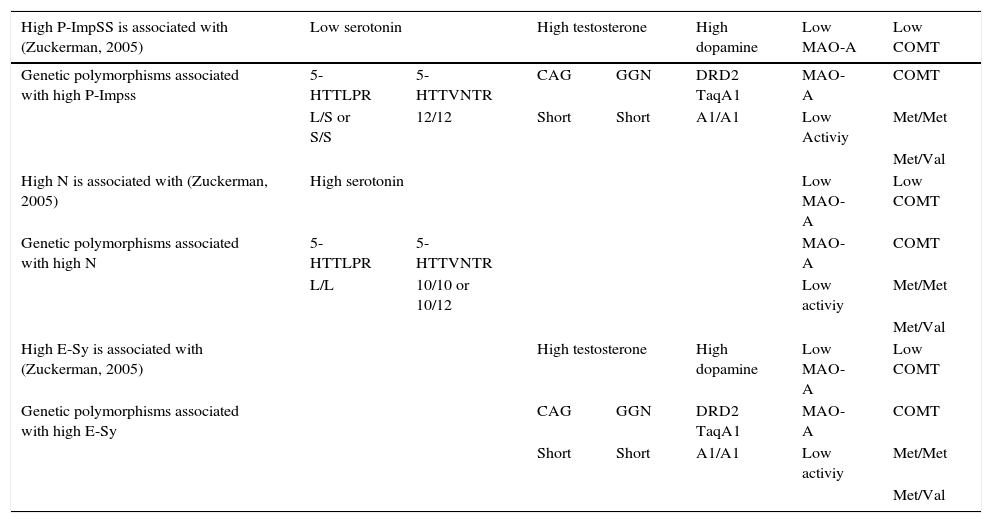
Table 1 shows the association hypotheses between the high or low levels of some biological markers (Serotonin, Testosterone, Dopamine, MAO-A and COMT) and the high scores on the three temperamental traits considered in Zuckerman's psychopharmacological model (Zuckerman, 2005): Impulsive Unsocialized Sensation Seeking (P-ImpUSS), Neuroticism (NE) and Extroversion-Sociability (E-Sy). In addition, considering the commented literature about the impact of some alleles on the biological activity of these neurotransmitters, enzymes and testosterone, Table 1 also includes the specific alleles of the seven polymorphisms considered in the present study (5-HTTLPR and 5-HTTVNTR for Serotonin, CAG and GGN for Testosterone, DRD2-TaqIA for Dopamine, MAO-A and COMT) that should be associated with the expected high or low biological levels.
Neurotransmitter, testosterone and enzyme levels associated with high scores on Impulsive Unsocialized Sensation Seeking (P-ImpUSS), Neuroticism (N), and Extroversion-Sociability (E-Sy) according to Zuckerman's psychobiological model (2005), and specific genetic polymorphisms and allelic variations associated with these levels*
| High P-ImpSS is associated with (Zuckerman, 2005) | Low serotonin | High testosterone | High dopamine | Low MAO-A | Low COMT | ||
|---|---|---|---|---|---|---|---|
| Genetic polymorphisms associated with high P-Impss | 5-HTTLPR | 5-HTTVNTR | CAG | GGN | DRD2 TaqA1 | MAO-A | COMT |
| L/S or S/S | 12/12 | Short | Short | A1/A1 | Low Activiy | Met/Met | |
| Met/Val | |||||||
| High N is associated with (Zuckerman, 2005) | High serotonin | Low MAO-A | Low COMT | ||||
| Genetic polymorphisms associated with high N | 5-HTTLPR | 5-HTTVNTR | MAO-A | COMT | |||
| L/L | 10/10 or 10/12 | Low activiy | Met/Met | ||||
| Met/Val | |||||||
| High E-Sy is associated with (Zuckerman, 2005) | High testosterone | High dopamine | Low MAO-A | Low COMT | |||
| Genetic polymorphisms associated with high E-Sy | CAG | GGN | DRD2 TaqA1 | MAO-A | COMT | ||
| Short | Short | A1/A1 | Low activiy | Met/Met | |||
| Met/Val | |||||||
Summing up, following Munafò et al. (2003) suggestions, the research design of the present study includes two characteristics: 1) Subjects will be described at the psychological level using several personality scales related with the three temperamental traits. It is also advisable to use multivariate analysis to capture the common variance of the related scales to define better the psychological constructs (Loehlin, 2003), 2) it is aimed to explore the interaction of some genetic markers to explain better the differences in each trait. Note that P-ImpUSS would be linked to the seven polymorphisms included in the present study, N to four and E-Sy to five (Table 1). It should be also remarked that Zuckerman's model also predicts a lack of association between some polymorphisms and certain traits. For instance, no association is expected between Dopamine or Testosterone and Neuroticism.
Material and methodsSubjectsThe total sample of the present study is composed of 258 participants from two different samples. The first one consisted of 153 male inmates of the Spanish Penitentiary Centre of Ponent (Lleida). The mean age was 33.31 (S.D: 8.6). Exclusion criteria for participating in the study were: 1) Non Caucasian, 2) presence of psychotic or affective disorder diagnosed by the psychiatric staff of the penitentiary, 3) cognitive disability or language difficulties, and 4) being a relative of one of the participants in the study. 95.9% of the sample was Caucasian Spanish, and the remaining 4.1% were of European Caucasian extraction (three Portuguese, one Croatian, one French, and one Rumanian). Before administering the protocol, it was confirmed that all non-native Spanish subjects had a good enough level of Spanish to answer properly. No reward was given for participating.
The second one was made up of 105 male volunteers recruited from students and university staff. The mean age was 26.71 (S.D: 9.68). All subjects were Caucasian Spanish. They signed an informed consent, and received a monetary reward (30 Euros) for participating. From now on, this sample will be named “Control” for descriptive purposes only. The present study was approved by the ethical committee of the University.
Psychological variablesEight personality scales were applied to both samples: Impulsive Sensation Seeking, Neuroticism-Anxiety, and Sociability scales from the Zuckerman-Kuhlman Personality Questionnaire (ZKPQ), Barratt's Impulsivity scale, Eysenck's Psychoticism, and Neuroticism, Extroversion and Conscientiousness scales from the NEO-FFI.
- •
ZKPQ: The ZKPQ measures Zuckerman's Alternative Five: Impulsive Sensation Seeking, Neuroticism-Anxiety, Activity, Aggression-Hostility, and Sociability (Zuckerman et al., 1993). This test is composed of 89 items (plus 10 items from an Infrequency scale) with a dichotomous (True-False) answer format. A short version of 69 items validated for the Spanish population was administered in the present study. This short version has similar psychometric properties to the original one (Aluja, García, & García, 2003), with alpha reliability coefficients between .71 and .79 for males. For the present study, only the Impulsive Sensation Seeking (ImpSS), Neuroticism-Anxiety (N-Anx) and Sociability (Sy) scales were analyzed.
- •
Barratt Impulsivity Scale (BIS-10). The BIS-10 is an inventory made up of 34 items with an answer format of a 4-point Likert-type scale (Barratt, 1985). It comprises of three subscales, and a total score for Impulsivity. Only the latter (Imp) will be analyzed in the present study. Psychometric properties of the Spanish translation were similar to the original British scale (Luengo, Carrillo-de-la-Peña, & Otero, 1991).
- •
Psychoticism: The short version of the Psychoticism scale (P) derived from the Eysenck Personality Questionnaire was applied in the present study (EPQ-RS). It is a 12-item scale with a dichotomous (True-False) answer format. Alpha reliability of the Spanish version was .62 for males (Eysenck & Eysenck, 1997).
- •
NEO-FFI: The NEO-FFI is a shortened version of 60 items (12 by scale) of the NEO-PI-R (Costa & MacCrae, 1999), distributed in five scales: Neuroticism (N), Extraversion (E), Openness (O), Agreeableness (A), and Conscientiousness (C). The NEO-FFI has an answer format of a 5-point Likert-type scale (0-4). Alpha reliability coefficients in the Spanish version range between .71 and .82 (Costa & Mac-Crae, 1999). Only Neuroticism (NEO-FFI_N), Extraversion (NEO-FFI_E) and Conscientiousness (NEO-FFI_C) scales were analyzed in the present sample.
DNA from inmates and control samples was obtained from buccal snaps using the BuccalAmp DNA extraction kit (Epi-centre, Madison, USA). Details about the procedures for genotyping the following polymorphism may be found in the corresponding reference: 5-HTTLPR and 5-HTTVNTR (García, Aluja, Fibla, Cuevas, & García, 2010), and CAG and GGN (Aluja, García, Blanch, & Fibla, 2011).
The genotypes for the DRD2 TaqIA/ANKK1 (rs1800479) polymorphism was determined by an allelic discrimination assay based on fluorogenic 5_nuclease activity:
TaqMan Single Nucleotide Polymorphism (SNP) Genotyping Assay (Applied Biosystems INC). The conditions for DRD2/ ANKK1 Taq1A were the following: initial denaturation 94 ¿C 5min, 35 cycles consisting of 94 ¿C 1min, 50 ¿C 1min, 72 ¿C 1min, and final extension step on 72 ¿C 10min. Subsequently, for allele detection PCR products were accomplished by overnight incubation with 3 U of TaqI restriction endonuclease, resolved in 7% PAAG and visualized by staining with ethidium bromide. After incubation with TaqI, the A1-allele remains intact (310bp) while the A2-allele is cleaved (130bp and 180bp).
The MAO A coding gene (Xp11.4–Xp11.3) presents a well-characterized functional polymorphism consisting of a variable number of tandem repeats (VNTR). It was amplified using primers designed by the Web Primer site (http://www.seq.yeastgenome.org/cgi-bin/web-primer) that flanked the polymorphic region located approximately 1200bp upstream from the translation start site (GeneBank accession number AJ004833). Sequences of the primers are as follows: MAO A forward: 5′-CCAGAAACATGAGCACAAACG-3′, and MAO A reverse: 5′-ATTCGGACAGGCTGTAGGAG-3′. The forward primer was labelled with FAM fluorophore. Polymerase chain reaction (PCR) was carried out in a final volume of 25 μl including 200 ng of genomic DNA, 12pmol of each primer, 200 μM of dNTPs, 1.5mM of MgCl2 and 1 U of Taq DNA polymerase (Promega, Madison, WI, USA). Cycling conditions started with an initial denaturation at 94 μC for 3min, followed by 30 s at 94 ¿C, 45 s at 58 ¿C and 60 s at 72 ¿C for 30 cycles,with a final extension at 72 ¿C for 4min. The PCR products were mixed with 1.5 μl of formamide, 0.5 μl of loading buffer, 0.5 μl of fluorescently labelled size standard GENESCAN 350-TAMRA and then electrophoresed for 150min in the ABI automated DNA sequencer 377 (Applied Biosystem, Foster City, CA). Data were collected and analyzed using the ABI GENESCAN 672 software (Applied Biosystem, Foster City, CA).
COMT val158met genotypes were determined by restriction fragment length polymorphism. A 109-base-pair polymerase chain reaction (PCR) product was generated in 40 cycles with an annealing temperature of 50 ¿C by using primers Comt1nt 1881 50-CTCATCACCATCGAGAGATCAA-30 and Comt2nt 1989 50-CCAGGTCTGACAACGGGTCA-30. The val 158 and met158 alleles were discriminated by digesting the PCR product with NlaIII at 37 ¿C for 4h, followed by a native 10% polyacrylamide gel electrophoresis. The val158 homozygotes (86 and 23 base pairs), met158 homozygotes (68 and 18 base pairs) and val158met heterozygotes (86, 68, 23 and 18 base pairs) were visualized by silver staining.
AnalysisPsychological factors will be described after a factor analysis using principal components and Varimax rotation method. Since Zuckerman's psychopharmacological model was developed for three traits of Zuckerman's personality model (Impulsive Unsocialized Sensation Seeking [P-ImpUSS], Neuroticism-Anxiety [N] and –Extroversion-Sociability [E-Sy]), three factors were retained. Factor scores were computed using the regression method. A series of t-tests was computed to compare mean differences on scales and factors between the subjects group with the genetic polymorphisms associated with high scores and the remaining subjects. In order to explore simultaneously the interaction among all polymorphisms, a new variable containing the number of Genetic Factors (GF) was computed according to the number of alleles associated with high scores presented in every subject (see Table 1). Note that this variable is computed three times by subject, one for each trait. It is expected that the larger the number of genetic factors associated with high scores, the higher the mean scores will be. All analyses were performed for the total sample and, for replication purposes, for the inmate and control samples separately.
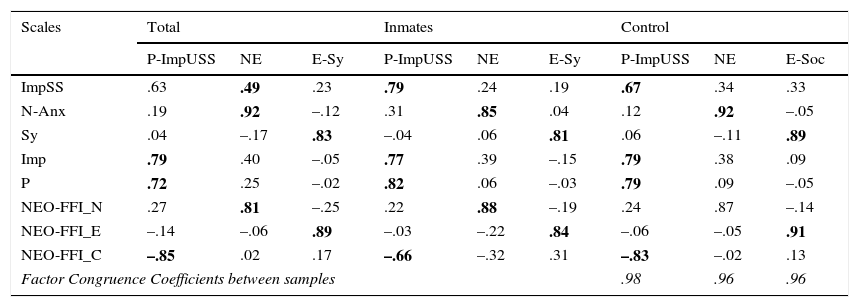
ResultsAs a first necessary step, samples should be properly psychologically described on the basis of the three psychological factors raised by Zuckerman in his psychobiological model: Impulsive Unsocialized Sensation Seeking (P-ImpUSS), Neuroticism (N-Anx), and Extroversion-Sociability (Sy). Table 2 shows the three factor solution for the total, inmate and control samples. Zuckerman's Impulsive Sensation Seeking (ImpSS), Barratt's Impulsivity (Imp), Eysenck's Psychoticism (P), and Conscientiousness (C) load on the first factor (P-ImpUSS). Zuckerman's Neuroticism-Anxiety and Neuroticism scale from the NEO-FFI load on the second one (N-Anx). Note that ImpSS and Imp also present high secondary loadings on this factor. However, both scales will not be considered in this factor for theoretical reasons. Finally, only Sociability from ZKPQ and Extroversion from the NEO-FFI load on the third factor (Sy). Factor solution is highly congruent with Zuckerman's theory (Aluja, García, & García, 2002; Zuckerman et al., 1993). Further more, since congruence coefficients were larger than .95, it is demonstrated that the three factor solution is identical in both samples.
Rotated Factor solutions in the Total, Inmates and Control samplesa,b,c
| Scales | Total | Inmates | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P-ImpUSS | NE | E-Sy | P-ImpUSS | NE | E-Sy | P-ImpUSS | NE | E-Soc | |
| ImpSS | .63 | .49 | .23 | .79 | .24 | .19 | .67 | .34 | .33 |
| N-Anx | .19 | .92 | –.12 | .31 | .85 | .04 | .12 | .92 | –.05 |
| Sy | .04 | –.17 | .83 | –.04 | .06 | .81 | .06 | –.11 | .89 |
| Imp | .79 | .40 | –.05 | .77 | .39 | –.15 | .79 | .38 | .09 |
| P | .72 | .25 | –.02 | .82 | .06 | –.03 | .79 | .09 | –.05 |
| NEO-FFI_N | .27 | .81 | –.25 | .22 | .88 | –.19 | .24 | .87 | –.14 |
| NEO-FFI_E | –.14 | –.06 | .89 | –.03 | –.22 | .84 | –.06 | –.05 | .91 |
| NEO-FFI_C | –.85 | .02 | .17 | –.66 | –.32 | .31 | –.83 | –.02 | .13 |
| Factor Congruence Coefficients between samples | .98 | .96 | .96 | ||||||
ZKPQ: ImpSS (Impulsive Sensation Seeking), N-Anx (Neuroticism-Anxiety), Sy (Extroversion-Sociability); Imp: Impulsivity; P (Psychoticism); NEO-FFI: N (Neuroticism), E (Extroversion), C (Conscientiousness).
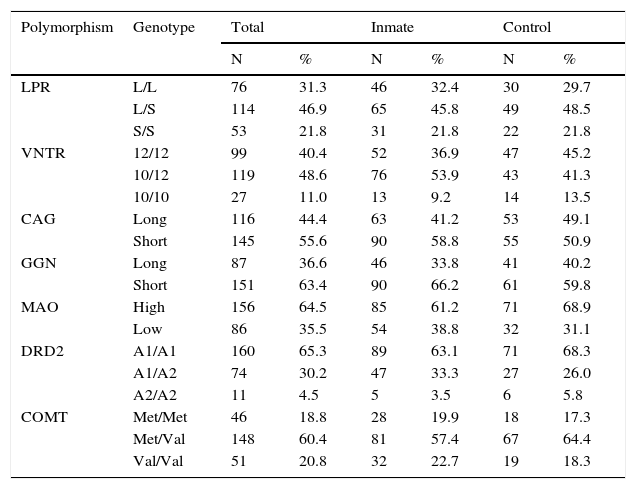
Table 3 shows the allelic frequencies for the seven polymorphisms considered in the total, inmate and control samples. Note that the homozygote condition for the A2 allele of the DRD2 presents an extremely low frequency. Thus, the subjects would split into two groups: 1) Homozygous for the allele A1, 2) carrying an allele A2.
Allelic frequencies in the total, inmate and control samples.
| Polymorphism | Genotype | Total | Inmate | Control | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| LPR | L/L | 76 | 31.3 | 46 | 32.4 | 30 | 29.7 |
| L/S | 114 | 46.9 | 65 | 45.8 | 49 | 48.5 | |
| S/S | 53 | 21.8 | 31 | 21.8 | 22 | 21.8 | |
| VNTR | 12/12 | 99 | 40.4 | 52 | 36.9 | 47 | 45.2 |
| 10/12 | 119 | 48.6 | 76 | 53.9 | 43 | 41.3 | |
| 10/10 | 27 | 11.0 | 13 | 9.2 | 14 | 13.5 | |
| CAG | Long | 116 | 44.4 | 63 | 41.2 | 53 | 49.1 |
| Short | 145 | 55.6 | 90 | 58.8 | 55 | 50.9 | |
| GGN | Long | 87 | 36.6 | 46 | 33.8 | 41 | 40.2 |
| Short | 151 | 63.4 | 90 | 66.2 | 61 | 59.8 | |
| MAO | High | 156 | 64.5 | 85 | 61.2 | 71 | 68.9 |
| Low | 86 | 35.5 | 54 | 38.8 | 32 | 31.1 | |
| DRD2 | A1/A1 | 160 | 65.3 | 89 | 63.1 | 71 | 68.3 |
| A1/A2 | 74 | 30.2 | 47 | 33.3 | 27 | 26.0 | |
| A2/A2 | 11 | 4.5 | 5 | 3.5 | 6 | 5.8 | |
| COMT | Met/Met | 46 | 18.8 | 28 | 19.9 | 18 | 17.3 |
| Met/Val | 148 | 60.4 | 81 | 57.4 | 67 | 64.4 | |
| Val/Val | 51 | 20.8 | 32 | 22.7 | 19 | 18.3 | |
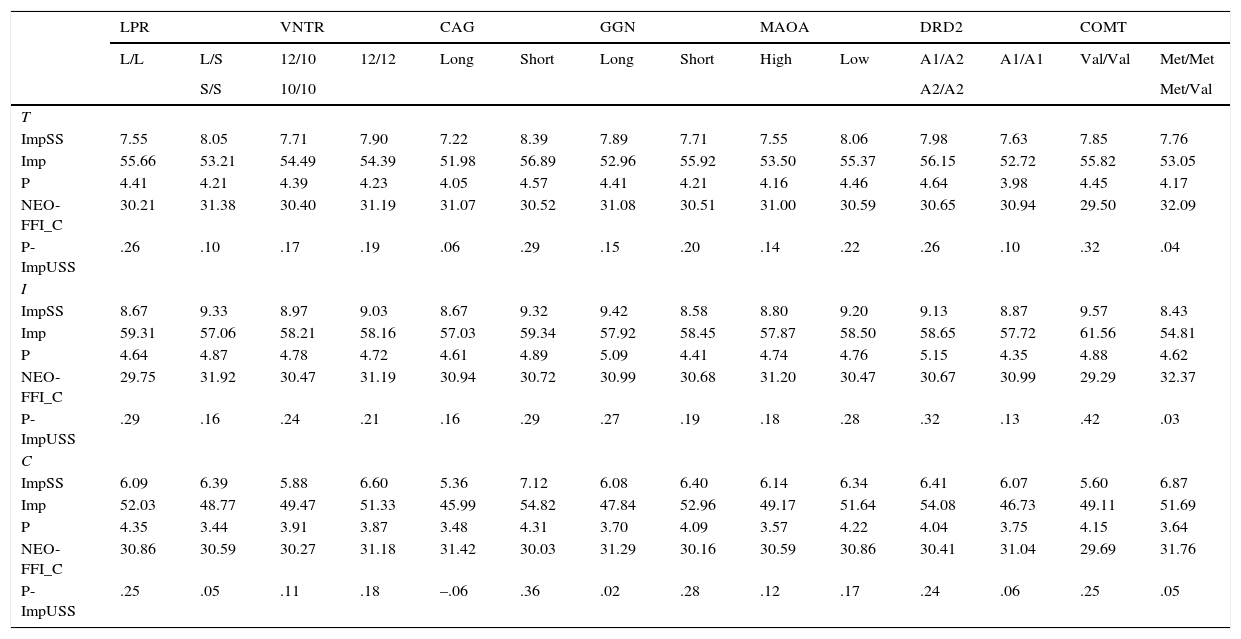
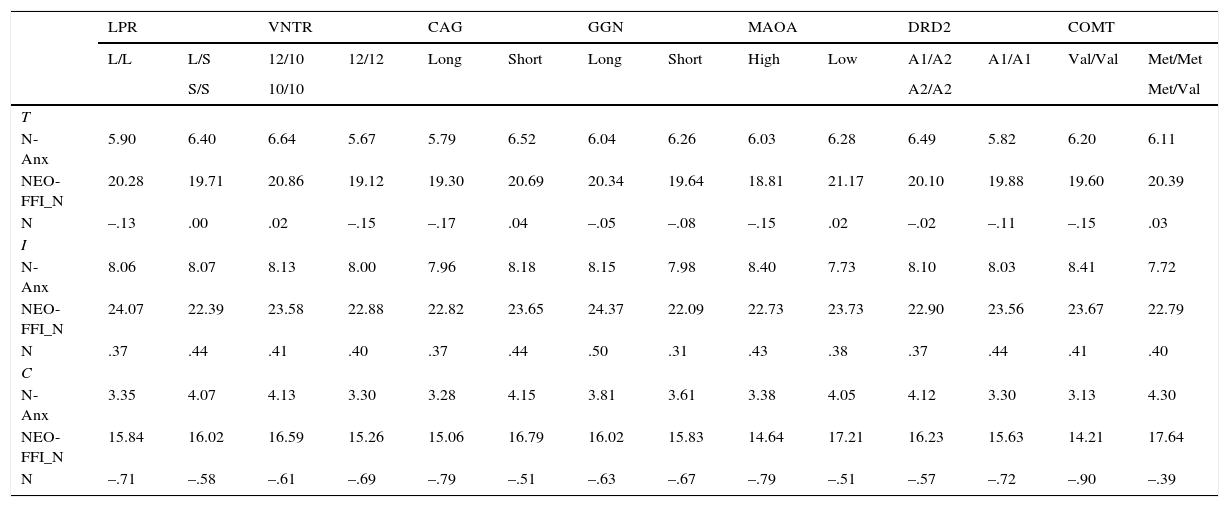
Tables 4, 5 and 6 show the mean scores for the two genetic groups of every polymorphism. According to the hypotheses raised in Table 1, the second column of every polymorphism always represents the genetic group associated with higher scores on the corresponding trait. Hence, for every polymorphism and psychological variable, the value on the right is always expected to be the largest. Focusing on Table 4, genetic groups associated with higher scores on P-ImpUSS were as follows: LPR (L/S or S/S), VNTR (12/12), CAG (Short), GGN (Short), MAO-A (Low), DRD2 (A1/A1), and COMT (Met/Met or Met/Val). The exact number of subjects in any group can be computed after Table 3. No mean differences were observed at the 0.01 level. Also, no clear tendency was observed for the four scales and the psychological factor. It should be remarked that mean differences on the Conscientiousness scale are expected to be the opposite to the remaining scales giving the theoretical definition of this trait and, of course, the negative loading reported in Table 2.
Means for the personality scales and the factor scores of the Impulsive Unsocialized Sensation Seeking construct (P-ImpUSS) in the total (T), inmate (I) and control (C) samples*
| LPR | VNTR | CAG | GGN | MAOA | DRD2 | COMT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L/L | L/S | 12/10 | 12/12 | Long | Short | Long | Short | High | Low | A1/A2 | A1/A1 | Val/Val | Met/Met | |
| S/S | 10/10 | A2/A2 | Met/Val | |||||||||||
| T | ||||||||||||||
| ImpSS | 7.55 | 8.05 | 7.71 | 7.90 | 7.22 | 8.39 | 7.89 | 7.71 | 7.55 | 8.06 | 7.98 | 7.63 | 7.85 | 7.76 |
| Imp | 55.66 | 53.21 | 54.49 | 54.39 | 51.98 | 56.89 | 52.96 | 55.92 | 53.50 | 55.37 | 56.15 | 52.72 | 55.82 | 53.05 |
| P | 4.41 | 4.21 | 4.39 | 4.23 | 4.05 | 4.57 | 4.41 | 4.21 | 4.16 | 4.46 | 4.64 | 3.98 | 4.45 | 4.17 |
| NEO-FFI_C | 30.21 | 31.38 | 30.40 | 31.19 | 31.07 | 30.52 | 31.08 | 30.51 | 31.00 | 30.59 | 30.65 | 30.94 | 29.50 | 32.09 |
| P-ImpUSS | .26 | .10 | .17 | .19 | .06 | .29 | .15 | .20 | .14 | .22 | .26 | .10 | .32 | .04 |
| I | ||||||||||||||
| ImpSS | 8.67 | 9.33 | 8.97 | 9.03 | 8.67 | 9.32 | 9.42 | 8.58 | 8.80 | 9.20 | 9.13 | 8.87 | 9.57 | 8.43 |
| Imp | 59.31 | 57.06 | 58.21 | 58.16 | 57.03 | 59.34 | 57.92 | 58.45 | 57.87 | 58.50 | 58.65 | 57.72 | 61.56 | 54.81 |
| P | 4.64 | 4.87 | 4.78 | 4.72 | 4.61 | 4.89 | 5.09 | 4.41 | 4.74 | 4.76 | 5.15 | 4.35 | 4.88 | 4.62 |
| NEO-FFI_C | 29.75 | 31.92 | 30.47 | 31.19 | 30.94 | 30.72 | 30.99 | 30.68 | 31.20 | 30.47 | 30.67 | 30.99 | 29.29 | 32.37 |
| P-ImpUSS | .29 | .16 | .24 | .21 | .16 | .29 | .27 | .19 | .18 | .28 | .32 | .13 | .42 | .03 |
| C | ||||||||||||||
| ImpSS | 6.09 | 6.39 | 5.88 | 6.60 | 5.36 | 7.12 | 6.08 | 6.40 | 6.14 | 6.34 | 6.41 | 6.07 | 5.60 | 6.87 |
| Imp | 52.03 | 48.77 | 49.47 | 51.33 | 45.99 | 54.82 | 47.84 | 52.96 | 49.17 | 51.64 | 54.08 | 46.73 | 49.11 | 51.69 |
| P | 4.35 | 3.44 | 3.91 | 3.87 | 3.48 | 4.31 | 3.70 | 4.09 | 3.57 | 4.22 | 4.04 | 3.75 | 4.15 | 3.64 |
| NEO-FFI_C | 30.86 | 30.59 | 30.27 | 31.18 | 31.42 | 30.03 | 31.29 | 30.16 | 30.59 | 30.86 | 30.41 | 31.04 | 29.69 | 31.76 |
| P-ImpUSS | .25 | .05 | .11 | .18 | –.06 | .36 | .02 | .28 | .12 | .17 | .24 | .06 | .25 | .05 |
Means for the personality scales and the factor scores (N) of the Neuroticism construct in the total (T), inmate (I) and control (C) samples*
| LPR | VNTR | CAG | GGN | MAOA | DRD2 | COMT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L/L | L/S | 12/10 | 12/12 | Long | Short | Long | Short | High | Low | A1/A2 | A1/A1 | Val/Val | Met/Met | |
| S/S | 10/10 | A2/A2 | Met/Val | |||||||||||
| T | ||||||||||||||
| N-Anx | 5.90 | 6.40 | 6.64 | 5.67 | 5.79 | 6.52 | 6.04 | 6.26 | 6.03 | 6.28 | 6.49 | 5.82 | 6.20 | 6.11 |
| NEO-FFI_N | 20.28 | 19.71 | 20.86 | 19.12 | 19.30 | 20.69 | 20.34 | 19.64 | 18.81 | 21.17 | 20.10 | 19.88 | 19.60 | 20.39 |
| N | –.13 | .00 | .02 | –.15 | –.17 | .04 | –.05 | –.08 | –.15 | .02 | –.02 | –.11 | –.15 | .03 |
| I | ||||||||||||||
| N-Anx | 8.06 | 8.07 | 8.13 | 8.00 | 7.96 | 8.18 | 8.15 | 7.98 | 8.40 | 7.73 | 8.10 | 8.03 | 8.41 | 7.72 |
| NEO-FFI_N | 24.07 | 22.39 | 23.58 | 22.88 | 22.82 | 23.65 | 24.37 | 22.09 | 22.73 | 23.73 | 22.90 | 23.56 | 23.67 | 22.79 |
| N | .37 | .44 | .41 | .40 | .37 | .44 | .50 | .31 | .43 | .38 | .37 | .44 | .41 | .40 |
| C | ||||||||||||||
| N-Anx | 3.35 | 4.07 | 4.13 | 3.30 | 3.28 | 4.15 | 3.81 | 3.61 | 3.38 | 4.05 | 4.12 | 3.30 | 3.13 | 4.30 |
| NEO-FFI_N | 15.84 | 16.02 | 16.59 | 15.26 | 15.06 | 16.79 | 16.02 | 15.83 | 14.64 | 17.21 | 16.23 | 15.63 | 14.21 | 17.64 |
| N | –.71 | –.58 | –.61 | –.69 | –.79 | –.51 | –.63 | –.67 | –.79 | –.51 | –.57 | –.72 | –.90 | –.39 |
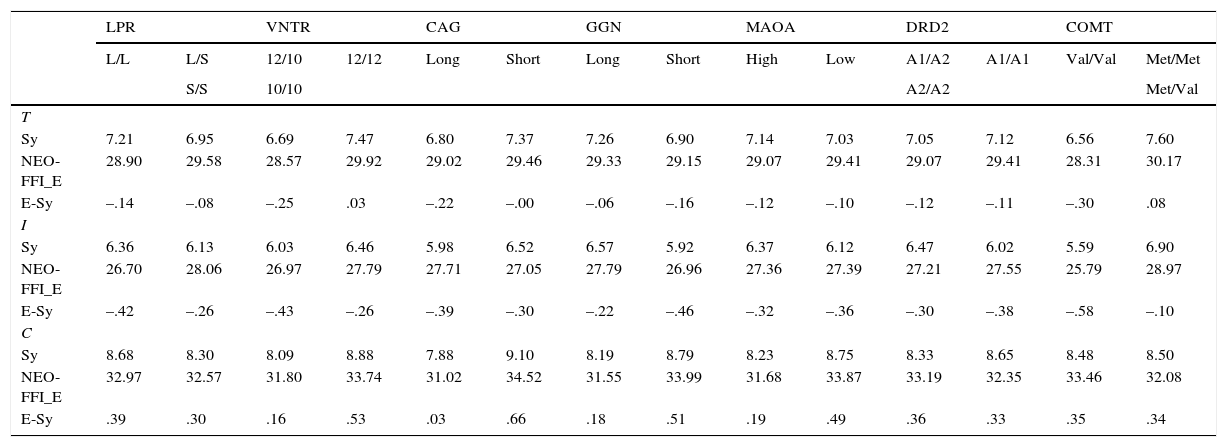
Means for the personality scales and the factor scores (E-Sy) of the Extroversion-Sociability construct in the total (T), inmate (I) and control (C) samples*
| LPR | VNTR | CAG | GGN | MAOA | DRD2 | COMT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L/L | L/S | 12/10 | 12/12 | Long | Short | Long | Short | High | Low | A1/A2 | A1/A1 | Val/Val | Met/Met | |
| S/S | 10/10 | A2/A2 | Met/Val | |||||||||||
| T | ||||||||||||||
| Sy | 7.21 | 6.95 | 6.69 | 7.47 | 6.80 | 7.37 | 7.26 | 6.90 | 7.14 | 7.03 | 7.05 | 7.12 | 6.56 | 7.60 |
| NEO-FFI_E | 28.90 | 29.58 | 28.57 | 29.92 | 29.02 | 29.46 | 29.33 | 29.15 | 29.07 | 29.41 | 29.07 | 29.41 | 28.31 | 30.17 |
| E-Sy | –.14 | –.08 | –.25 | .03 | –.22 | –.00 | –.06 | –.16 | –.12 | –.10 | –.12 | –.11 | –.30 | .08 |
| I | ||||||||||||||
| Sy | 6.36 | 6.13 | 6.03 | 6.46 | 5.98 | 6.52 | 6.57 | 5.92 | 6.37 | 6.12 | 6.47 | 6.02 | 5.59 | 6.90 |
| NEO-FFI_E | 26.70 | 28.06 | 26.97 | 27.79 | 27.71 | 27.05 | 27.79 | 26.96 | 27.36 | 27.39 | 27.21 | 27.55 | 25.79 | 28.97 |
| E-Sy | –.42 | –.26 | –.43 | –.26 | –.39 | –.30 | –.22 | –.46 | –.32 | –.36 | –.30 | –.38 | –.58 | –.10 |
| C | ||||||||||||||
| Sy | 8.68 | 8.30 | 8.09 | 8.88 | 7.88 | 9.10 | 8.19 | 8.79 | 8.23 | 8.75 | 8.33 | 8.65 | 8.48 | 8.50 |
| NEO-FFI_E | 32.97 | 32.57 | 31.80 | 33.74 | 31.02 | 34.52 | 31.55 | 33.99 | 31.68 | 33.87 | 33.19 | 32.35 | 33.46 | 32.08 |
| E-Sy | .39 | .30 | .16 | .53 | .03 | .66 | .18 | .51 | .19 | .49 | .36 | .33 | .35 | .34 |
Mean scores for the Neuroticism factor (N) and the two scales are reported in Table 4. In this case, the target genetic groups were: CAG (Short), GGN (Short), MAO-A (Low), and COMT (Met/Met or Met/Val). No mean differences and no tendency in the expected direction were observed. On the other hand, in spite of this lack of direct association between Serotonin, Dopamine and Neuroticism in Zuckermans's psychobiological model, results for these polymorphisms were also reported. In this case, no mean differences were expected.
Table 6 shows the results for the Extroversion-Sociability factor (E-Sy) and the two corresponding scales. The genetic groups associated with high scores on this factor were: CAG (Short), GGN (Short), MAO-A (Low), DRD2 (A1/ A1), and COMT (Met/Met or Met/Val). Likewise, no mean differences at the 0.01 level or a clear tendency were observed. On the other hand, in spite of the lack of direct association between Serotonin and E-Sy in Zuckermans's psychobiological model, results for those polymorphisms were also reported. In this case, no mean differences were expected.
García et al. (2010) and Munafò et al. (2003) suggest that the analysis of one polymorphism only is not a promising approach, meaning that several polymorphisms must be considered simultaneously. From a theoretical standpoint, Zuckermans's model clearly supports this view since differences in each trait are linked to several biological variables. Therefore, a new series of analyses was conducted considering the number of genetic factors. It is expected that the larger the number of genetic factor associated with high scores, the higher the mean scores will be. It should be remarked that seven polymorphism are considered in the present paper for P-ImpUSS, four for N-Anx and five for E-Sy.
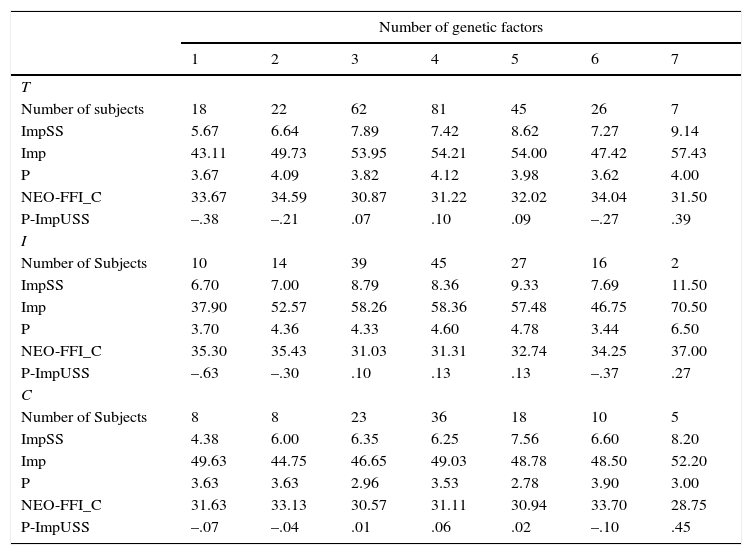
Table 7 shows the mean differences for the seven groups formed on the basis on the number of genetic factors for Impulsive Unsocialized Sensation Seeking (P-ImpUSS). Note that the greater the number of factors, the larger the mean scores. This pattern is reproduced for ImpSS, Imp, P-ImpUSS and, as expected, in the opposite direction for C. Moreover, this pattern is replicated across inmate and control samples. The exception is the group with 6 genetic factors, which usually shows lower mean scores (larger for C) than the previous groups.
Means for the personality scales and the factor scores (P-ImpUSS) of the Impulsive Unsocialized Sensation Seeking construct as a function of the number of Genetic factors for higher scores (GF) in the total (T), inmate (I) and control (C) samples.
| Number of genetic factors | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| T | |||||||
| Number of subjects | 18 | 22 | 62 | 81 | 45 | 26 | 7 |
| ImpSS | 5.67 | 6.64 | 7.89 | 7.42 | 8.62 | 7.27 | 9.14 |
| Imp | 43.11 | 49.73 | 53.95 | 54.21 | 54.00 | 47.42 | 57.43 |
| P | 3.67 | 4.09 | 3.82 | 4.12 | 3.98 | 3.62 | 4.00 |
| NEO-FFI_C | 33.67 | 34.59 | 30.87 | 31.22 | 32.02 | 34.04 | 31.50 |
| P-ImpUSS | –.38 | –.21 | .07 | .10 | .09 | –.27 | .39 |
| I | |||||||
| Number of Subjects | 10 | 14 | 39 | 45 | 27 | 16 | 2 |
| ImpSS | 6.70 | 7.00 | 8.79 | 8.36 | 9.33 | 7.69 | 11.50 |
| Imp | 37.90 | 52.57 | 58.26 | 58.36 | 57.48 | 46.75 | 70.50 |
| P | 3.70 | 4.36 | 4.33 | 4.60 | 4.78 | 3.44 | 6.50 |
| NEO-FFI_C | 35.30 | 35.43 | 31.03 | 31.31 | 32.74 | 34.25 | 37.00 |
| P-ImpUSS | –.63 | –.30 | .10 | .13 | .13 | –.37 | .27 |
| C | |||||||
| Number of Subjects | 8 | 8 | 23 | 36 | 18 | 10 | 5 |
| ImpSS | 4.38 | 6.00 | 6.35 | 6.25 | 7.56 | 6.60 | 8.20 |
| Imp | 49.63 | 44.75 | 46.65 | 49.03 | 48.78 | 48.50 | 52.20 |
| P | 3.63 | 3.63 | 2.96 | 3.53 | 2.78 | 3.90 | 3.00 |
| NEO-FFI_C | 31.63 | 33.13 | 30.57 | 31.11 | 30.94 | 33.70 | 28.75 |
| P-ImpUSS | –.07 | –.04 | .01 | .06 | .02 | –.10 | .45 |
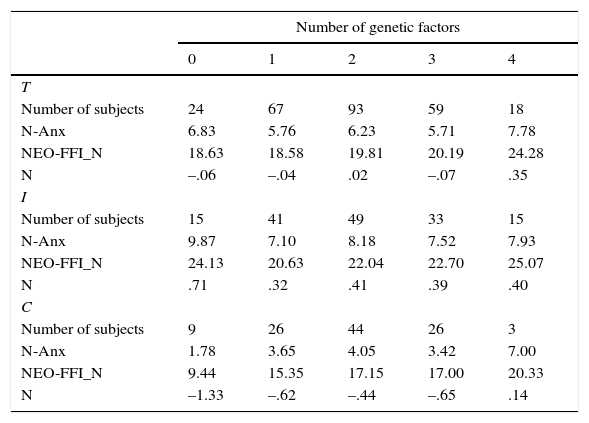
Table 8 shows the results for the interaction of the four polymorphisms associated with Neuroticism. The increase in the scores is clearly observed for the Neuroticism factor and the NEO-FFI_N scale, and for the total and control samples. However, no such increase is observed for Zuckerman's N-Anx scale and for the inmate sample.
Means for the personality scales and the factor scores (N) of the Neuroticism construct as a function of the number of Genetic risk factors (GRF) in the total (T), inmate (I) and control (C) samples.
| Number of genetic factors | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| T | |||||
| Number of subjects | 24 | 67 | 93 | 59 | 18 |
| N-Anx | 6.83 | 5.76 | 6.23 | 5.71 | 7.78 |
| NEO-FFI_N | 18.63 | 18.58 | 19.81 | 20.19 | 24.28 |
| N | –.06 | –.04 | .02 | –.07 | .35 |
| I | |||||
| Number of subjects | 15 | 41 | 49 | 33 | 15 |
| N-Anx | 9.87 | 7.10 | 8.18 | 7.52 | 7.93 |
| NEO-FFI_N | 24.13 | 20.63 | 22.04 | 22.70 | 25.07 |
| N | .71 | .32 | .41 | .39 | .40 |
| C | |||||
| Number of subjects | 9 | 26 | 44 | 26 | 3 |
| N-Anx | 1.78 | 3.65 | 4.05 | 3.42 | 7.00 |
| NEO-FFI_N | 9.44 | 15.35 | 17.15 | 17.00 | 20.33 |
| N | –1.33 | –.62 | –.44 | –.65 | .14 |
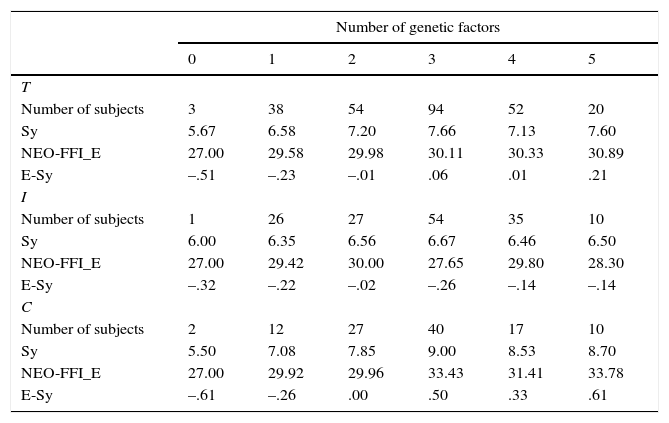
Table 9 shows the results for the Extroversion-Sociability factor. An increase is found for the total sample, and it is replicated in both subsamples, being especially intense for the control sample. It is also observed for the factor scores and the two psychometric scales.
Means for the personality scales and the factor scores (E-Soc) of the Extroversion-Sociability construct as a function of the number of Genetic risk factors (GRF) in the total (T), inmate (I) and control (C) samples.
| Number of genetic factors | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| T | ||||||
| Number of subjects | 3 | 38 | 54 | 94 | 52 | 20 |
| Sy | 5.67 | 6.58 | 7.20 | 7.66 | 7.13 | 7.60 |
| NEO-FFI_E | 27.00 | 29.58 | 29.98 | 30.11 | 30.33 | 30.89 |
| E-Sy | –.51 | –.23 | –.01 | .06 | .01 | .21 |
| I | ||||||
| Number of subjects | 1 | 26 | 27 | 54 | 35 | 10 |
| Sy | 6.00 | 6.35 | 6.56 | 6.67 | 6.46 | 6.50 |
| NEO-FFI_E | 27.00 | 29.42 | 30.00 | 27.65 | 29.80 | 28.30 |
| E-Sy | –.32 | –.22 | –.02 | –.26 | –.14 | –.14 |
| C | ||||||
| Number of subjects | 2 | 12 | 27 | 40 | 17 | 10 |
| Sy | 5.50 | 7.08 | 7.85 | 9.00 | 8.53 | 8.70 |
| NEO-FFI_E | 27.00 | 29.92 | 29.96 | 33.43 | 31.41 | 33.78 |
| E-Sy | –.61 | –.26 | .00 | .50 | .33 | .61 |
To sum up, there is a gradual increase in mean scores as a factor. An increase is found for the total sample, and it is function of the number of genetic factors associated with high replicated in both subsamples, being especially intense for scores on the three factors. As it has been commented above, the control sample. It is also observed for the factor scores this pattern is somewhat replicated across subsamples and and the two psychometric scales. psychological measures, the results being less clear for the inmate sample, and for some scales such as the N-Anx. Factor scores are normalized, so they are forced to be an average of 0. Note that groups with a medium number of genetic factors (2-4 range) usually present averages around zero, the lowest and highest means usually being associated with groups with the lowest of highest number of factors, respectively.
Discussion and conclusionsFollowing Zuckermans's psychobiological model, personality traits are caused by differences in monoamines, related enzymes and testosterone. Previous biological studies support this model (see a revision on Stelmack, 2004; Zuckerman, 2005; Zuckerman & Aluja, 2014). However, as far as we know no study had tested it from a genetic approach only. Overall, the results are congruent with Zuckerman's psychobiological complex model.
Zuckerman's model argues against simple systems that identify one personality trait with one neurotransmitter. Furthermore, it may be assumed that this simplistic view is behind the problems to replicate the results about the genetic basis of personality traits and other psychological variables (Hamer, 2002), and it can account for the failure to find main significant effects on the personality traits (Munafò et al., 2003). Congruent with these problems, the results of the present study show that none of the seven polymorphisms had a significant effect on the differences in the three temperamental traits when they are considered separately.
In an attempt to solve this lack of replication, Munafò et al. (2003) recommend the study of the interaction of several polymorphisms rather than the association between one scale and one polymorphism. In this sense, analyzing the interaction between the COMT, 5-HTTLPR and DRD4 has already produced positive results. For instance, Strobel, Lesch, Jatzke, Paetzold, and Brocke (2003) found that subjects with the Val/Val, L/L, 7- genotype scored significantly lower on the Novelty Seeking scale. The relevance of this result is that it was obtained in the same population where previously no main effect of the DRD4 (presence vs absence of allele 7) was observed. Results of the present study reproduce the same pattern. As mentioned earlier, although no main effect of each polymorphism alone is observed, a tendency in the theoretical expected direction is found when several polymorphisms are considered conjointly.
In this sense, the more genetic factors are considered, the higher scores on the three traits. This is especially true for P-ImpUSS and E-Sy, but less clear for NE. The observed pattern is replicated for both samples for P-ImpUSS, thus providing evidence that P-ImpUSS is associated with a genetic profile of low serotonin (L/S or S/S from 5-HTTLPR, 12/12 from 5-HTTVNTR), high testosterone (Short alleles of CAG and GGN), high dopamine (A1/A1 from DRD2 TaqA1), low MAO-A activity, and low COMT Activity (One copy of Met Allele). The increase in high scores for NE and E-Soc is only observed in the control sample. There are two reasons for the less clear results for NE: 1) Note that the main biological variables affecting NE from Zuckerman's model (GABA, norepinephrine and endorphines) are not considered in the present study, and 2) it is not clear which allele of GGN would be functionally associated with high levels of testosterone. In this sense, Aluja et al. (2011) report an association of the long haplotype of GGN with impulsivity personality.
Munafò et al. (2003) also recommends using several phenotypic measures. Previous studies have already confirmed the worth of such recommendations by using an index composed of various disinhibited personality measures (Aluja et al., 2011; García et al., 2010). Note that the differences in the two genetic groups for every polymorphism are usually larger in the factor scores. In fact, most of the larger differences (although non significant) were observed for this composite score. It should be noted that factor scores are supposed to be a better measure of the temperamental trait than the scales separately, since they capture the common psychological variance of the related scales eliminating the specific variance of each scale (Loehlin, 2003).
Related to the previous point, in a recent meta-analysis, Calati et al. (2011) reported an association of the Val/Val variant with measures of anger (an emotion closely linked to the Neuroticism trait). However, Zuckerman's model predicts the opposite, that is to say, an association between the Met allele and negative emotions, such as anger or depression, linked to Neuroticism. Notice that Zuckerman's model predict psychopathological tendencies (Aluja, Gar-cía, Cuevas, & García, 2007). Reported results in the present paper are more congruent with Zuckerman's view. In this sense, Rujescu, Giegling, Gietl, Hartmann, and Moller (2003) found that homozygous Met/Met individuals outwardly expressed their anger. They also reported that Val allele homozygotes tended to express their anger inwardly. This dual way of expressing the anger is a reminder of the need to improve the phenotypic definition of psychological constructs. A better definition of what is being measured is essential to understand properly the results of genetic association studies in the personality field.
Interpretation of results in regard to the role of enzymes is somewhat confusing. In the case of MAO-A, low activity genotype would be associated with a greater efficiency of the enzyme and, therefore, with a better deamination of the neurotransmitter, that is to say, less activity of the neurotransmitter. Thus, according to Zuckerman's model, low MAO-A activity should produce high serotonin levels, and therefore more inhibition and less P-ImpUSS. However, some studies have reported the contrary, and low MAO-A activity has been associated with high P-ImpUSS or related phenotypes such as antisocial and psychopathic tendencies (Caspi et al., 2002; Tikkanen et al., 2011). The results of the present study are more congruent with this last view, since Low Activity subjects tend to score higher on P-ImpUSS factor and scales.
On the other hand, low MAO-A should produce higher levels of Neuroticism through the increase in Serotonin levels. The present study goes some way to supporting this hypothesis, since subjects with low MAO-A Activity tend to present higher scores on Neuroticism scales and factor, especially in the control sample. In any case, this contradictory role of the MAO-A to account for differences in some personality traits has already been observed by other authors (Manuck et al., 2000), and it remains an unsolved question.
A similar argument may be raised for the role of COMT. Following Zuckerman's model and the theoretical impact of COMT on neurotransmitter levels, one copy of the MET allele (i.e., less enzymatic activity) should be associated with higher levels of Dopamine and Norepinephrine. However, it should be noted that, in Zuckerman's model, high dopamine is associated with high P-ImpUSS, but high Norepinephrine is associated with the opposite (high arousal and, thus, lower P-ImpUSS). In the present study, subjects with the Val/Val genetic profile tend to score high on the P-ImpUSS factor and related scales. This result is congruent with Zuckerman's model since VAL/VAL profile is associated with more COMT activity, less Norepinephrine, less arousal and, consequently, high P-ImpUSS. This pattern supports the role of Norepinephrine in the observed differences in P-ImpUSS.
As other authors have emphasized (Kagan & Snidman, 2007), the number of possible neurochemical profiles that can influence behavior is much larger than the number of ways a class of behavior may be displayed. Finally, it is important to appreciate the extraordinary specificity of brainmind relationships. For instance, the different responses that are often regarded as equivalent indexes of a fear state are mediated by distinct neural circuits and, therefore, probably create different states of fear (Fanselow, 1994). Simplistic views of the impact of genetic differences on personality traits should therefore be discarded. In this sense, more complex frameworks of the relationships among biological variables (at both biological and genetic levels) and psychological traits should be considered. Therefore, Marvin Zuckerman's psychobiological model represents a useful framework to explore the relationships between genetics, biology and personality. This model may provide the necessary theoretical framework to explore the complex paths from genetics to behavior.
This research was supported by the Spanish Ministry of Education and Science through the “Ramon y Cajal” program (first author). Also, necessary financial support was given by the University of Lleida (Orgànica 1606 [Projecte: TR 265]), the Autonomous Government of Catalonia (Dirección General de Instituciones Penitenciarias and DURSI'S Program d’ajuts per a accions especials de recerca [Aces’03]) to the second author), and the Spanish “Fondo de Investigaciones Sanitarias” (PI-050303) (second author). We would also express our sincere acknowledgment to the voluntary participants of this study as well as to the staff, especially to the medical staff, of the Centre Penitenciary of Ponent (Lleida, Spain).