Mice of the inbred C57Bl/6J strain displayed high variability in the antibody response of the IgG class to heat-aggregated bovine serum albumin. A cluster analysis (with 1-correlation coefficient as distance) disclosed two groups of mice: those mice than produced an augmented secondary antibody response, and those that did not; another cluster analysis (with difference in antibody concentration as distance) on each of the above groups disclosed small groups of mice, or individual mice, with varying antibody content. Antibody concentration was not associated with ambulation, rearing, grooming, or defecation in the open-field, but ambulation was positively correlated with rearing.
Los ratones de la cepa consanguínea C57Bl/6J mostraron mucha variabilidad en la respuesta de anticuerpos de clase IgG contra la seroalbúmina bovina agregada por el calor. Un análisis de conglomerados, que utilizó como distancia 1-coeficiente de correlación, reveló dos grupos de ratones: aquellos que produjeron una respuesta secundaria aumentada de anticuerpos, y aquellos que no la produjeron. Otro análisis de conglomerados aplicado a cada grupo anterior, y utilizando como distancia la diferencia en la concentración de anticuerpos, reveló grupos pequeños de ratones, o ratones individuales, con concentración diferente de anticuerpos antiseroalbúmina. La concentración de anticuerpos no estaba asociada con estas conductas en el campo abierto: deambulación, incorporación, aseo, o defecación, pero deambulación e incorporación estaban positivamente correlacionadas.
Rodents of an inbred strain display large variations in several behaviors: sucrose preference (Strekalova & Steinbusch, 2010), fear conditioning (Siegmund, Kaltwasser, Holsboer, Czisch, & Wotjak., 2009), decrease in social interaction after social defeat (Krishnan et al., 2007), ambulation in the open-field and the elevated plus-maze (Vidal, 2015). Variations of physiological variables (weight, course of infection, stress response) in inbred animals have also been reported (Gärtner, 2012). This variability, which is not readily explained by genetics or environment, has been termed intangible variation, developmental noise (Blewitt, Chong, & Whitelaw, 2004; Falconer, 1989) or third component (Gärtner, 2012). The author of the present report could not find studies documenting individual differences in the antibody response within an inbred mouse strain; therefore, one goal of this report was to find out if such differences occurred in mice of the C57Bl/6J inbred strain.
A set of correlated behaviors (behavioral syndromes; Sih, Bell, & Johnson, 2004) may represent a temperament trait in animals (Lewejohann, Zipser, & Sachser, 2011). In the C57Bl/6 mouse strain, the antibody response seems uncorrelated with behaviors in the open-field (ambulation, rearing, grooming, and defecation) (Vidal, 2015); therefore, another goal of this report was to find out if the antibody response was correlated with open-field behaviors in each of the groups disclosed by cluster analysis on the antibody response.
MethodSubjectsMale and female mice of the C57Bl/6J strain were purchased from Harlan Iberica (Barcelona, Spain). Seven C57Bl/6J females were mated with three C57Bl/6J males according to this distribution: one male with two females, another male with two other females, and another male with the remaining three females: the offspring were the subjects of this experiment. The males were removed from the females one week before parturition.
Adult mice of the same sex were housed 2–5 per cage, at 21±1°C, under a 12h light-dark cycle (lights on at 8:00h). Food and water were available ad libitum. The illumination on the floor of the mouse room was 154lux whereas the illumination on top of one cage was 96lux.
The experimental procedures were approved by the University of Barcelona Ethics Committee on Animal Experimentation.
Open fieldThe open field was a square enclosure made of gray plastic, 100.0cm×100.0cm×30.0cm. Because preference of the mice for the periphery of the open-field is a correlate of anxiety (Simon, Dupuis, & Costentin, 1994; Treit & Fundytus, 1989), an inner and an outer zone were defined: the inner zone was a (50cm×40cm) rectangle positioned in the center of the open-field, and the outer zone was a 10-cm-wide strip along the walls of the open-field. The apparatus was lit by two neon tubes that yielded 210lux in the center of the field. The open-field test was performed in silence.
ProcedureOpen-field. Each mouse was placed in a corner of the open-field and allowed to move freely for 5min. A video tracking system (Any-maze v. 4.99; Stoelting Co., IL, U.S.A.) recorded ambulation (meters) in each zone; besides, these behaviors were scored manually: number of full-stretch rearings (i.e. the erect mouse stands on its rear paws), number of grooming episodes (i.e. the mouse stands on its rear paws and scratches its face with its forepaws), and number of fecal boli. All the trials took place between 15:00 and 19:00h. The field was washed with disinfectant soap between two mouse sessions.
Male mice took the open-field test at approximately 85, 148, 212, and 287 days of age; female mice took the test at approximately 88, 150, 214, and 289 days of age.
Immunization and antibody measurementEach mouse was injected intraperitoneally (ip.) with 1mg of aggregated bovine serum albumin in 0.10ml of saline; the albumin was aggregated by heating at 67°C for 1h (Passos et al., 1977). Male mice received one injection at approximately 92 days of age, and another one at approximately 156 days; female mice were injected on days 95 and 158 approximately. Male mice were bled when they were about 107, 128, 156, 170, 205, and 282 days old; female mice were bled on days 109, 130, 158, 172, 207, and 284 approximately. Serum concentration of antibodies of the IgG class to aggregated bovine albumin was measured by diffusion-in-gel enzyme-linked immunosorbent assay (DIG-ELISA; Nilsson, Björck, & Ouchterlony, 1985; Vidal, 2002); the IgG concentration in each serum sample was measured in two different dilutions thereof, and the IgG content was the arithmetic mean of both measurements. Concentration of IgG antibodies to bovine serum albumin are expressed as fraction of the concentration in a reference serum.
Statistical analysisTwo cluster analyses were used to disclose individual differences in the antibody response (Guertin, 1966; Skinner, 1978): the first analysis revealed antibody responses of similar shape (i.e. similar kinetics), whereas the second analysis (applied to each of the clusters from the first analysis) revealed differences in the antibody concentration among the sera. For the first cluster analysis, the distance used was 1 minus the correlation coefficient; for the second cluster analysis, the distance was the difference in antibody concentration between any two sera (city-block or manhattan distance); the amalgamation rule was always Ward's method.
The correlation matrix of (six measurements of) antibody with (four measurements of) a given behavior in the open-field was summarized thus: z scores were computed for each measurement of antibody and behavior, the six standardized scores for antibody content were averaged, as were the four standardized scores for the behavior; at the end, the averaged z scores of antibody content were correlated with the averaged z scores of the behavior (the aggregation principle; Epstein, 1979).
The statistical package STATISTICA v.12 (Tulsa, Oklahoma) was used to perform most of the statistical analyses and to produce the graphs. The pvclust package from R (version 3.3.2) was used to perform the cluster analyses.
ResultsVariability of the antibody concentration among the seraThe coefficient of variation at the peak of the primary antibody response (days107 and 109 for males and females) was 104% for male serum, and 131% for female serum; the coefficients of variation in the secondary response (days 170 and 172 for males and females), were 60% and 105%.
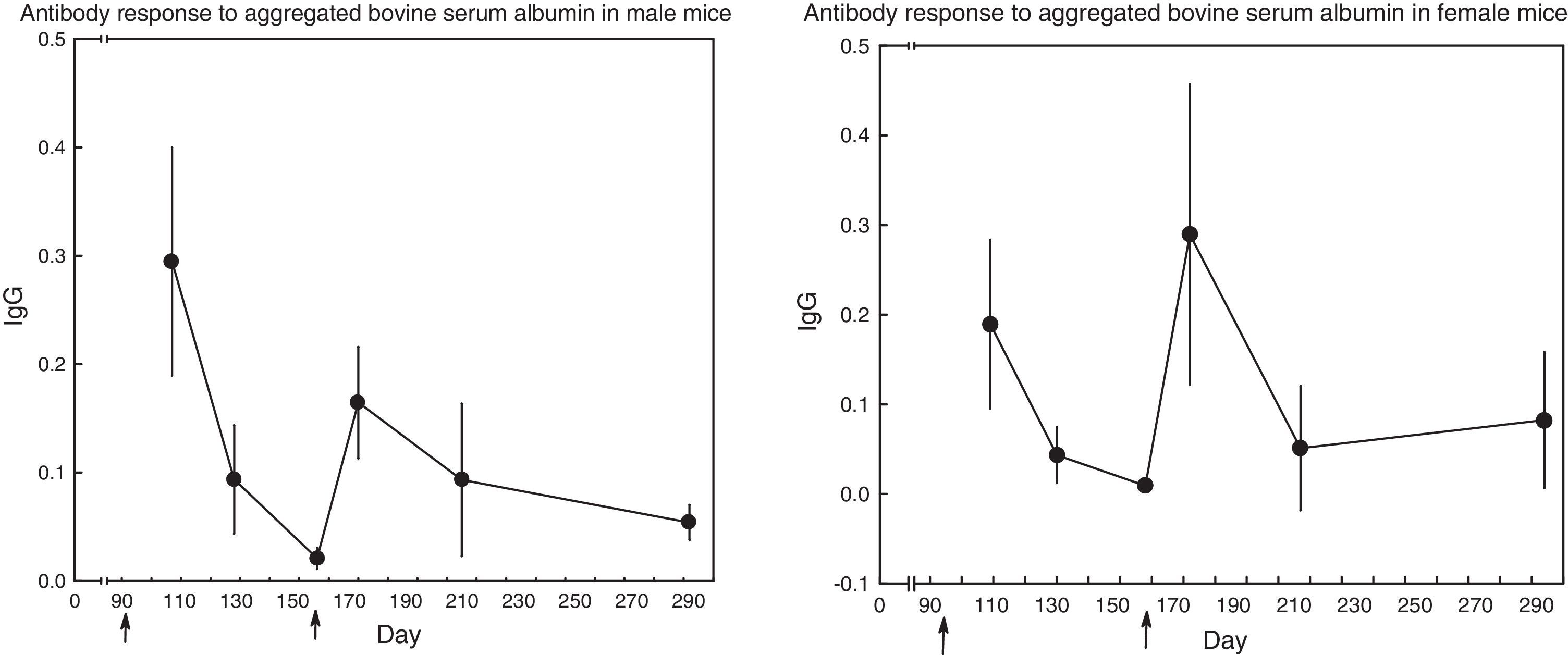
Fig. 1 displays the course of the antibody response in mice of both sexes. In male mice (N=17), the secondary response seemed to be lower than the primary one (t test for repeated measures, day 107 vs. day 170: t(16)=2.40, p=0.03), whereas the opposite seemed to occur in female mice (N=15); nevertheless, the difference did not reach statistical significance: (t test for repeated measures, day 172 vs. day 109: t(14)=1.38, p=0.19).
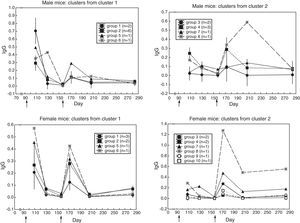
Groups of sera with similar antibody response profileCluster analysis for similar curve shape yielded these groups of sera:
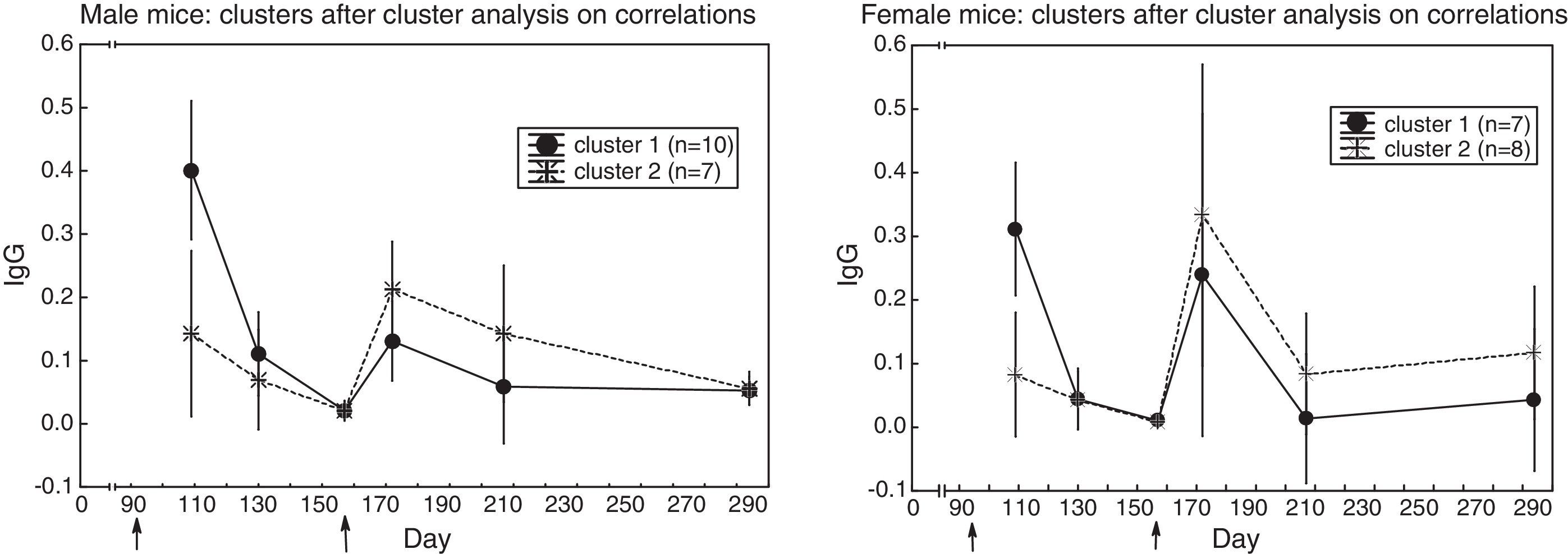
* in male mice (Fig. 2) two clusters emerged: in cluster 1 (N=10), the primary response was higher than the secondary one (t(9)=4.72, p=0.001); in cluster 2 (N=7), the opposite occurred (t(6)=2.77, p=0.032),
* in female mice (Fig. 2) two clusters emerged: in cluster 1 (N=7), the primary response was slightly higher than the secondary one (t(6)=2.69, p=0.036); in cluster 2 (N=8), the secondary response was higher than the primary one (t(7)=2.27, p=0.057); yet, the non-parametric Wilcoxon test for repeated measures yielded Z(8)=2.52, p=0.012.
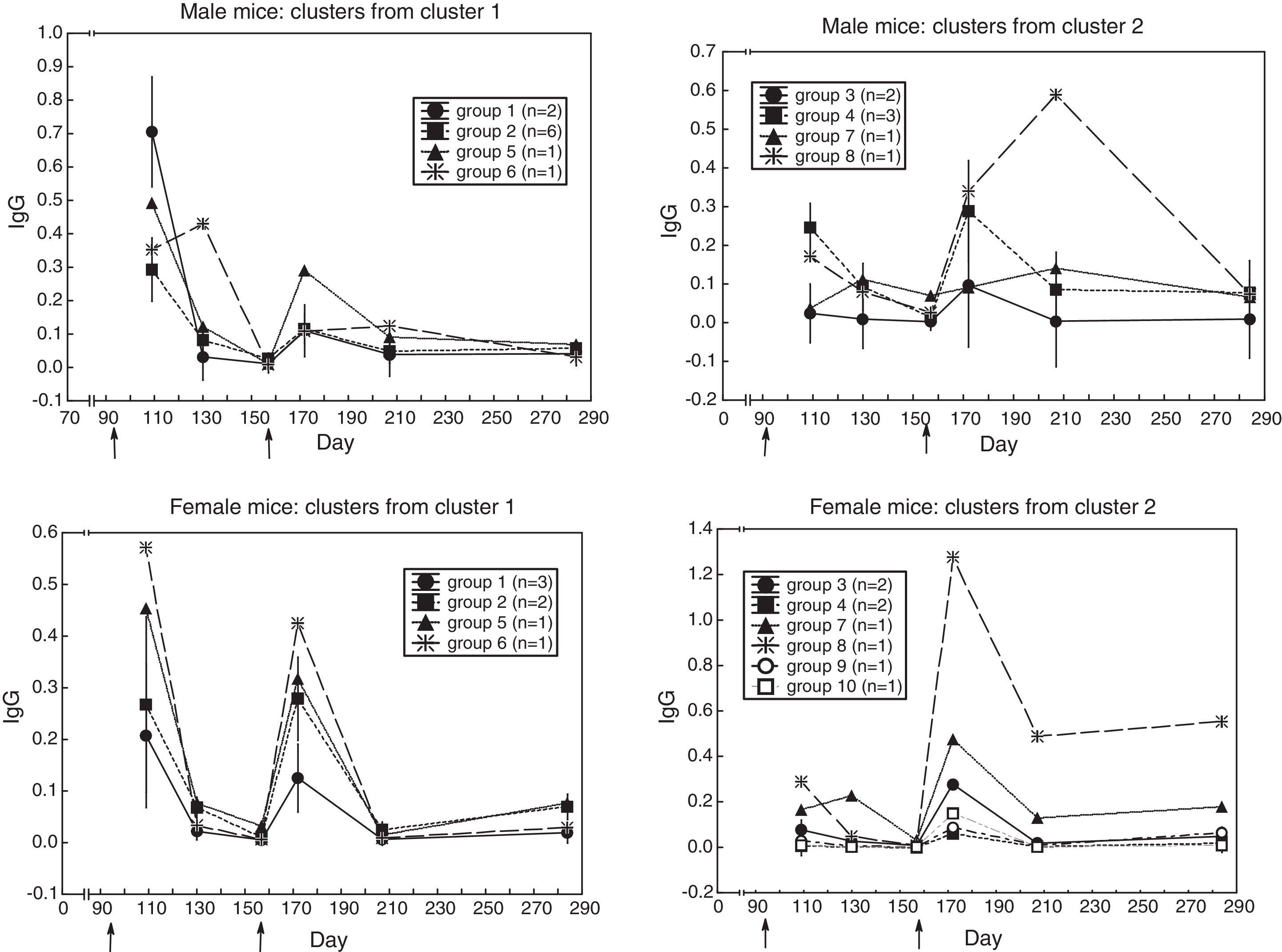
Heterogeneity among the sera with similar antibody response profileTo find out whether the sera with similar response kinetics differed in antibody content, a cluster analysis was performed on each group from the previous cluster analysis. (In the second cluster analysis, the distance was the difference in antibody content between sera along the six measurements; i.e. city-block distance). Fig. 3 shows that similar-kinetics sera are made up of groups of sera and individual sera with different antibody content.
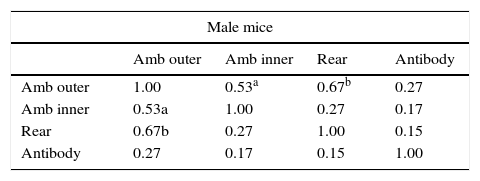
Correlation of antibody concentration and open-field behaviorsFor the whole sample (17 male mice and 14 female mice), Table 1 shows the Pearson correlation coefficients of antibody content and behaviors in the open-field (the variables were standardized and averaged as described in the Statistical analysis section): ambulation in the outer open-field correlated positively and consistently (in both males and females) with rearing and with ambulation in the inner zone of the open-field, whereas antibody content was uncorrelated with any behavior.
Correlation of antibody concentration and open-field behaviors.
| Male mice | ||||
|---|---|---|---|---|
| Amb outer | Amb inner | Rear | Antibody | |
| Amb outer | 1.00 | 0.53a | 0.67b | 0.27 |
| Amb inner | 0.53a | 1.00 | 0.27 | 0.17 |
| Rear | 0.67b | 0.27 | 1.00 | 0.15 |
| Antibody | 0.27 | 0.17 | 0.15 | 1.00 |
| Female mice | ||||
|---|---|---|---|---|
| Amb outer | Amb inner | Rear | Antibody | |
| Amb outer | 1.00 | 0.81c | 0.57d | 0.07 |
| Amb inner | 0.81c | 1.00 | 0.28 | 0.12 |
| Rear | 0.57d | 0.28 | 1.00 | 0.01 |
| Antibody | 0.07 | 0.12 | 0.01 | 1.00 |
Note: These variables are aggregated variables; i.e. the mean of z scores of several measurements of each variable (four for ambulation and rearing; six for antibody content): Amb outer: ambulation in the outer zone of the open-field; Amb inner: ambulation in the inner zone of the open-field; rear: rearing in the open-field; antibody: IgG class antibody content. Number of male mice: 17; number of female mice: 14. Numbers in the table indicate Pearson correlation coefficient.
In the male mice of cluster 1 (N=10), the antibody content was uncorrelated with ambulation, in any zone of the open-field, but correlated with rearing (r=0.76, p=0.01), while in cluster 2 (N=7), the antibody content was uncorrelated with any behavior (maximal absolute-value r (with rearing)=−0.42, p=0.35). In the cluster 1 of female mice (N=7), antibody was correlated with ambulation in the outer open-field (r=0.76, p=0.047), while in cluster 2 (N=7), antibody was uncorrelated with any behavior (maximal absolute-value r (with rear)=0.16, p=0.73).
DiscussionThe (primary and secondary) IgG antibody response to aggregated bovine serum albumin displayed considerable variation among mice (Results and Fig. 1). This fact appeared in one previous report, although it went unnoticed: data from the James and Milne (1972) paper, in C57Bl/6 mice immunized with bovine serum albumin, allow the estimation of coefficients of variation to be in the range of 54% to 100%. A second fact from Fig. 1 was the absence of a typical secondary response: in male mice, the secondary response (on day 170) was lower than the primary one (on day 107), whereas in female mice the primary response (on day 109) and the secondary response (on day 172) were of comparable magnitude (the secondary response seemed higher, but the difference did not reach statistical significance, p=0.189, probably because of the large scatter around both means). The absence of a secondary response could be due, in principle, to the low responsiveness of C57Bl/6 mice to immunization with bovine serum albumin (James & Milne, 1972; Muckerheide, Domen, & Michael, 1987). Nevertheless, results to be shown below revealed that some of the mice mounted an augmented secondary response.
The variability in antibody content of the sera prompted this question: is the antibody response (Fig. 1) made up of a single pattern, or is it made up of several patterns? A cluster analysis that reveals similar profiles disclosed two patterns (in male and female mice): in one of the patterns, the primary response was higher than the secondary response; in the other pattern, the primary response was lower than the secondary one (Fig. 2 and Results). This finding reveals the capacity of some mice to mount a secondary response, and the incapacity of other mice to do so. The coexistence of both patterns explains the absence of a clear booster response in the whole sample (Fig. 1), because, at the peak of the secondary response, both patterns would cancel each other.
One could even ask whether the sera in each of the above patterns have different antibody content. A cluster analysis based on differences in antibody concentration, and applied to each pattern, revealed that each pattern was made up of small groups of sera plus individual sera with varying antibody concentrations (Fig. 3); i.e. one could speak of individual differences in the antibody response. To the knowledge of this paper's author, this is the first time that consistent individual differences in the antibody response of mice of an inbred strain are described; i.e. the antibody response could be a differential (first-order) trait that may be integrated in another (higher-order) trait; the possibility that the antibody response be part, together with behaviors in the open-field, of another trait is explored next.
The last question was: is the antibody response correlated with behaviors in the open-field? If the answer were yes, one could speak of a behavioral syndrome (a kind of temperament trait) that included a form of immunity. Ambulation and rearing in the open-field are known to be positively correlated in random-bred animals (Walsh & Cummins, 1976) and in inbred mice (Carola, D’Olimpio, Brunamonti, Mangia, & Renzi, 2002; Van Abeelen, 1977), but the antibody response was not correlated with those behaviors, either in outbred CD1 mice (Vidal & Rama, 1994) or in C57Bl/6 mice (Vidal, 2015). The results presented here confirm (i) the positive association of ambulation and rearing in the open-field, and (ii) the lack of association (in the whole sample of mice) of the antibody response with ambulation and rearing in the open-field (Table 1 and Results). Nevertheless, the antibody response could be associated with behaviors in each of the clusters from Fig. 2: in fact, the antibody content was correlated with rearing in cluster 1 of male mice, and with ambulation in the periphery of the open-field in cluster 1 of female mice (Results). These results probably were unreliable because (i) they were based on a small sample (7–10 mice per group), (ii) they did not replicate across sexes, and (iii) they did not occur in the whole sample. It is safer to conclude that the behavioral syndrome made up of ambulation and rearing (probably termed activity; Carola et al., 2002) does not include the antibody response.
The high variability in the antibody response reported here beg a question: if the mice are inbred (genetically identical) and have been exposed to the same environmental antigens (because the mice were bred and lived in the same animal room), where do the differences come from? The results of this report do not permit to answer that question.
This work was supported by the Fundación Bosch i Gimpera, Barcelona, Spain [project number 306601]. The funding source had no part in the design and execution of the experiments reported here, nor in the decision to submit the paper for publication.