Introduction
Inosine (IN) is a nucleoside, derived from adenosine by deamination.1 Adenosine converts to inosine by the action of adenosine deaminase. Then, a nucleoside phosphorylase divides IN in hypoxanthine and pentose. In turn, hypoxanthine is oxidized to xanthine by the xanthine oxidase, a flavoprotein containing Fe and Mo. Xanthine is a substrate of xanthine oxidase, which converts it to uric acid, the terminal product of purines’ degradation, to be eliminated essentially by urine.
Researches of the group of Bethesda, USA, have extensively studied the electrophysiological,2,3 pharmacological,4 and therapeutical5 characteristics of adenosine, as well as its action on the specific elements of the atrioventricular conduction system.6,7 Myocite receptors for adenosine have also been identified.8 Moreover, purines modulate the phenomenon of cellular death produced by free oxygen radicals.9 On its side, adenosine acts as an effective antiarrhythmic on supraventricular tachycardias and on certain ventricular tachyarrhythmias, particularly on those induced by catecholamines, probably due to inhibition of adenylate cyclase.10,11,12
We have already pointed out the antiarrhythmic action of adenosine on certain clinical13 and experimental14 ventricular tachycardias. In our experimental series, the antiarrhythmic effect of adenosine was generally biphasic: one, early and fleeting; the other, delayed.
Herein, we aim at establishing whether IN, derived from adenosine, has effects on experimental ventricular tachyarrhythmias, notwithstanding that some authors15 consider it “mostly inactive”.
Methods and materials
This study was realized on 92 mongrel dogs weighing between 13 and 17 Kg. All animals were anesthetized with 30 mg/kg intravenous sodium pentobarbital, connected with an artificial respiration system, and received continuous infusion of Hartmann solution. After opening the thorax to expose the heart on the open pericardium, 1ml-1.5 ml of 70% phenol -depending on the weight of the animal- were injected into the free left ventricular wall close to the apex. This produced a circumscribed, well defined myocardial lesion. Some 30min-60 min later, a ventricular tachycardia, generally left, was induced by introducing small crystals of aconitine (Sigma) into the myocardium, next to the damaged area. Some 15 min-30 min later, an inosine bolus (12 mg) was injected through the superior vena cava. At the end of injection, a transient fall of the systolic arterial pressure always occurred, as in dogs treated with adenosine.14 The IN’s action was investigated in 63 dogs, while another 29 dogs did not receive it. In 34 of those 63 animals, the ventricular tachycardia (VT) was due to the crystals of aconitine inserted into myocardium, in other 16, the VT was produced only by myocardial damage caused by phenol and in 13, the VT was presented spontaneously without myocardial damage neither aconitine.
In all the experiments, leads II, aVR or aVL, the intraventricular right (IVD) and left (IVI) unipolar leads, as well as the unipolar lead on the wall of superior vena cava, were recorded. These were registered by a VR6 polygraph of Electronics for Medicine Co., with a speed of 100 mm/sec. These traces were obtained under control conditions and with VT. After the injection of IN, they were obtained immediately and every 5 min for the first 15 min, then every 30 min for the next hour or more. Nevertheless, traces were continuously monitored on the polygraph screen. The systolic blood pressure was measured at the same time using a U shaped mercury manometer attached to the right femoral artery, because variations in blood pressure were of more interest than its absolute values.
The recovery of sinus rhythm (SR) within 15 min after the IN injection was considered an early effect. If the same occurred between 30 and 60 min, it was considered a late effect. Recovery of SR was considered fleeting if lasting only a few seconds, and transitory when it lasted few minutes or more. This recovery was always followed by the return of VT. This finding is in favor of a cause-effect relation and against some self-limited tachyarrhythmias.
At the end of each experiment, the weight of the heart was established to determine the approximate dose of used IN in milligrams per gram of myocardium (0.07mg/g- 0.10 mg/g). Liquid volume, administered to each animal, and released to urine, was measured.
Statistical analysis
To contrast the distribution proportions of the animals treated with inosine that went into SR, and those of the control animals Table 1, the data were submitted to the χ2 statistical test, which revealed 27.22; and 24.88 with Yates correction. The statistical level of significance was p = 0.0000006, and with Fisher’s Exact Test, p = 0.000004. Calculations were performed using Epi Info version 6.04b of CDC & WHO (2007) Geneve, Switzerland. The confidence intervals limits for proportions with a 0.95 significant level (ICL = Inferior Confidence Limit; SCL = Superior Confidence Limit) were consulted from “Confidence Limits for p”, in Scientific Tables; Documents Geigy. Konrad Diem, Basel 1965, p 8.
Results
Control animals showed neither fleeting nor transitory recovery of SR after the VT was unchained. This arrhythmia quickly accelerated until originating a ventricular fibrillation in 5 to 20 minutes. Figure 1 shows the electrocardiographic evolution, in a control dog, of ventricular tachycardia with atrio-ventricular dissociation, toward ventricular fibrillation.
Figure 1. Electric evolution of ventricular tachycardia, with atrioventricular dissociation, due to aconitine in phenol-damaged myocardium, toward the ventricular fibrillation in a control dog.
Antiarrhythmic effects of inosine
Table 1 shows the different evolution of ventricular tachyarrhythmias in the IN-treated dogs with respect to those developed in control dogs, regarding SR recovery. Inosine reestablished SR in 36 of the 63 treated animals (57.14%, ICL 44.05%; SCL 69.54%), whereas SR was not reestablished in 27 (42.86%). This difference is not statistically significant. However, concerning SR recovery between animals receiving and those not receiving IN (control group), a very significant difference was revealed by the analysis of propor tions and confidence limits Table 2 as well as by the distribution of χ2, and p values.
Analyzing the experiments distributed in three subgroups Table 3, it can be noted that IN antiarrhythmic effects were obtained in 26 of 34 animals in which the VT was originated by aconitine in the phenol-damaged myocardium (76.47%), in 13 of 16 animals, in which VT was solely due to phenol (81.25%) and in 6 of 13 dogs with VT spontaneously originated (46.15%). Not statistically significant difference between SR recovery in these subgroups was found.
Examples
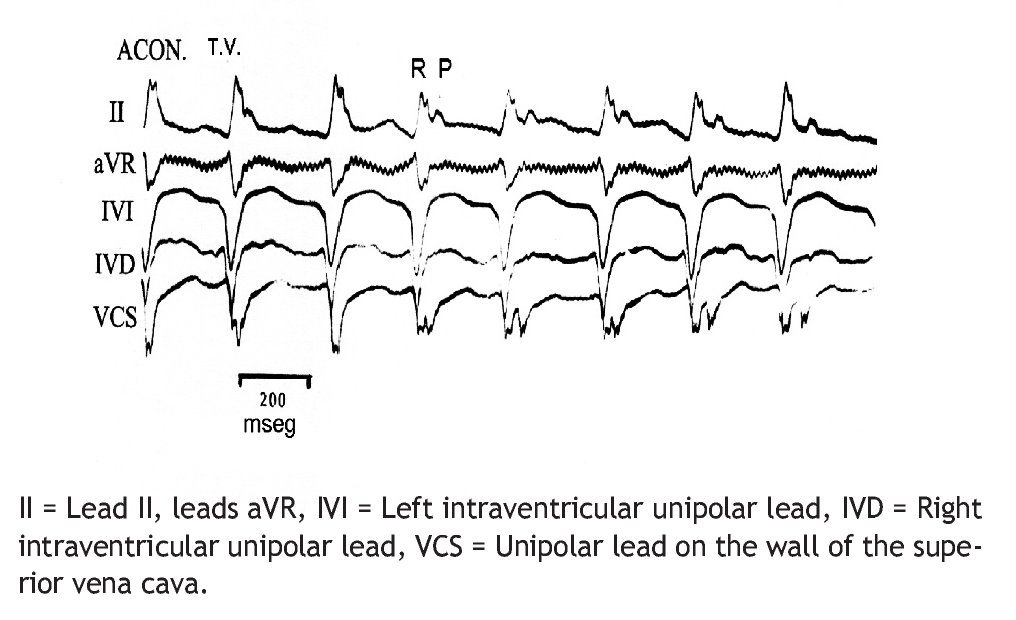
Figure 2 reproduces the electrocardiographic tracings of a 273/min VT, with atrioventricular dissociation, induced by aconitine in the ventricular phenol-damaged myocardium.
Figure 2. Ventricular tachycardia (VT), 273/min, with AV dissociation, due to aconitine in damaged myocardium (ACON).
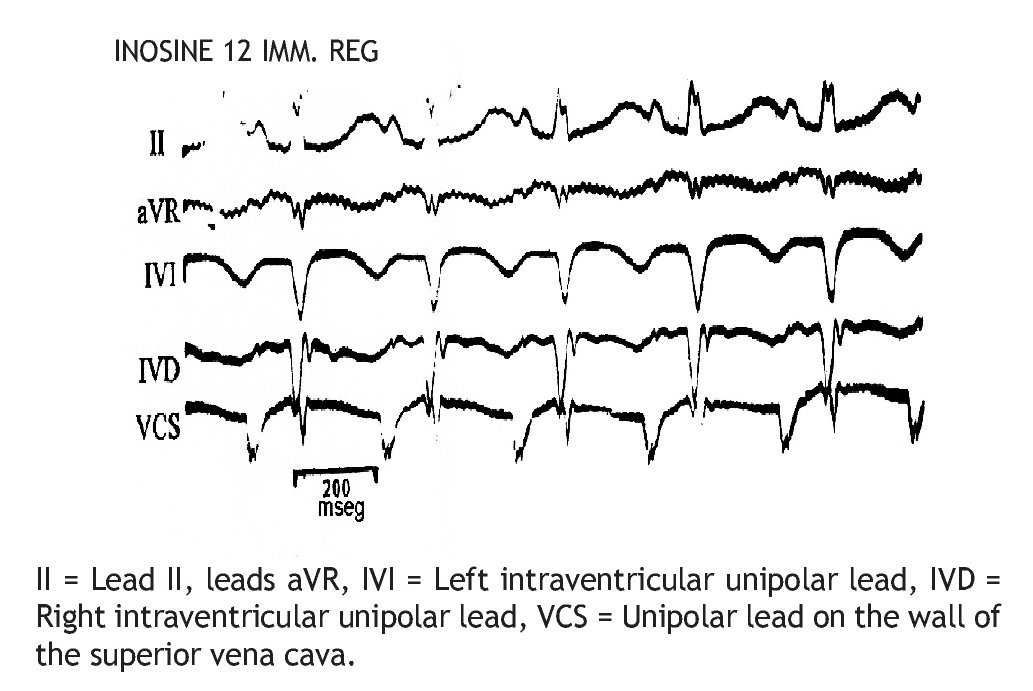
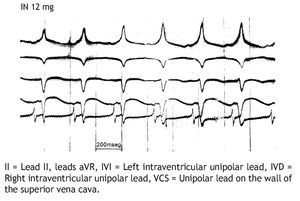
The injection of 12 mg of IN through the superior vena cava Figure 3 immediately pro duced SR recovery (200/min).
Figure 3. Same experiment of Figure 2, immediate recovery (IMM REG) of the sinus rhythm (SR), 200/min, after injection of 12 mg IN, through superior vena cava.
Figure 4 exhibits a left ventricular isorhythmic tachycardia, 166/min, and the morphology of ventricular complexes corresponding to an advanced degree RBBB, due only to myocardial damage by phenol. P waves patched to R waves can be seen.
Figure 4. Left ventricular isorhythmic tachycardia, 166/min, 15 min after the myocardial damage was caused. Note the atrial electrical phenomenon (P wave) linked with the ventricular complex (R wave).
Forty-five minutes after the injection of 12 mg of inosine through the superior vena cava Figure 5 a late and fleeting SR, 162/mln, appeared. Now the RBBB morphologies are absent and P waves precede the ventricular complexes with constant intervals.
Figure 5. Same experiment of Figure. 4, 45 minutes after the bolus injection of 12 mg of IN through superior vena cava, a late and fleeting sinus rhythm, 162/min, was recorded.
Arrhythmogenic effects of inosine
Under the light of its pro-arrhythmic effects, IN, admin istered to dogs with VT, produced atrial tachycardia in 10 dogs, causing also atrial flutter and fibrillation, as well as ventricular pre-excitation phenomena in two animals.
Concerning the atrioventricular conduction system, the mentioned nucleoside induced ventricular-atrial block of the second degree with progressive periods of Luciani-Wenckebach type in two animals, similar to that observed previously with adenosine.14
Examples
Figure 6 shows, in its superior section, an atrial tachycardia, 273/min, due to inosine. This tachycardia evolved towards an atrial flutter, 545/min, with variable atrioventricular block (intermediate section) and, finally, to atrial fibrillation (inferior section).
Figure 6. Superior section: supraventricular tachycardia 273/min due to inosine. Intermediate section: this tachycardia evolved toward atrial flutter (FL. AUR.) 545/min and a variable AV block. Inferior section: atrial fibrillation (FIBR.AUR.) presented with a mean ventricular frequency of 272/min
Figure 7 gives an example of both arrhythmogenic and antiarrhythmic actions of inosine in the same animal (a dog of 15.8 kg). During a VT, 230/min, due only to myocardial damage by phenol, the first dose of 12 mg of inosine, injected through the superior vena cava, reduced the frequency of this tachycardia to 193/min (not presented in this figure). A second dose of inosine, 5 min after its administration (superior section) originated an atrial flutter, 500/min, with a variable atrioven tricular block (early arrhythmogenic effect of inosine). Thirty minutes later (inferior section) a transient SR, l50/min, with prolonged QTc:MV + 0.06 sec appeared, which corresponds to a late antiarrhythmic effect.
Figure 7. Superior section: 5 min after the bolus injection of the second dose of 12 mg of inosine (IN) through the superior vena cava, an atrial flutter (Fl AUR) appeared with a frequency of 5OO/min and a variable AV block. It is an early arrhythmo genic effect. Inferior section: 30 min later, a transient SR 15O/min, appeared: late antiarhythmic effect.
Figure 8 reproduces the manifestation of ventricular pre-excitation: presence of delta wave in the initial portion of the R wave and shortening of the P-R interval, after the administration of 12 mg of IN during an experimental ventricular tachycardia, 300/min.
Figure 8. Electrocardiographic signs of the ventricular preexcitation: delta waves (Δ) and shortening of the atrioventricular interval (P-R), due to the administration of 12 mg of IN during a ventricular tachycardia, 300/mln, due to myocardial damage induced by phenol.
Figure 9 clearly shows, on its last strip, a retrograde ventricular-atrial block of second degree, with progressive periods of Luciani-Wenckebach type: progressive prolongation of ventricular-atrial intervals (V-a) until the interrupted passage of the retrograde activation impulse, and absence of the small atrial wave (a) after the fifth ventricular com plex (V). This phenomenon was seen on the immediate recordings (IMM REG) after the administration of 12 mg of IN during a ventricular tachycardia, 273/min.
Figure 9. In the last strip of these tracings, we can see a retrograde ventricular-atrial block, with progressive periods of Luciani-Wenckebach. This phenomenon presented after the administration of 12 mg of inosine through the superior vena cava during a ventricular tachycardia, 273/min.
Discussion
In 1987, Lubbe et al.16 pointed out that all the nucleosides derived from adenine have an anticatecholaminic action. When these nucleosides reach a sufficient concentration, they are able to suppress the origin of ventricular arrhythmias due to catecholamines.10,11 These purine compounds, by means of incompletely defined mechanisms, can act as antagonists of the increased myocardial vulnerability mediated by AMPc, which contributes to the genesis of the ventricular fibrillation during the early phase of myocardial ischemia.
The results of our study objectively show that IN has anti arrhythmic and arrhythmogenic effects similar to those of adenosine, from which it is derived. Concerning the relation chemical structure-biological activity, this fact suggests that adenosine deamination does not remove the potential biological activity of this derivate. The IN’s antiarrhythmic effects are exerted on atrial and ventricular arrhythmias, probably due to the activation of A1 receptors and through other intracellular mechanism.17 Its action could be opposed by blockers of these receptors.
On the contrary, IN’s arrhythmogenic effects can be due to sympathetic reflexes, similar to those mentioned by Belardinelli et al.3 concerning adenosine.
Furthermore IN could increase the outward potassium current, like adenosine and acetylcholine, shortening the refractory period of myocytes. In fact, a physiologist of our Institute,18 has ob served that, in mouse myocytes, low doses of IN –similar to those used in this study– impel the outward potassium current, while high doses of this nucleoside increase the inward calcium current in myocardial cells. These observations could satisfactorily explain the origin of the atrial flutter and its passage to atrial fibrillation. On the other side, IN, as well as adenosine,19 can allow for the manifestation of a ventricular preexcitation coincident with bradycardia or nodal block, also promoting the conduction through some accessory pathway, usually non-functional: a secondary mechanism to a sympathetic activation.
Inosine apparently acts on the same adenosine receptors of myocy tes. These, A1, A2A and A38, are stored on the surface of protein G, regulating the effects by stimulating these receptors. Protein G activates the K and Ca-β channels20 and, probably, phospholypase C. The receptor A1, coupled to a protein G system, reduces the levels of cyclic adenosine monophosphate (cAMP), opposing the increase of adenylate cyclase activity. Inosine could also act on other intra cellular and sarcolemmal receptors that are influenced by adenosine, as well as on muscarinic channels of potassium (KACh, KADO), which me diate its chronotropic and dromotropic effects in atria but not in ventricles. When adenosine and IN are accumulated in the extracellular spaces, the outward current of intracellular potassium could increase, thereby inducing a fall in cellular conduction speed.7
We present here the antiarrhythmic and arrhythmogenic effects of IN, an adenosine derivate, which seems to act on the same receptor of adenosine and has a similar action. These effects of IN are shown in this study; although certain authors21 suggest that va riations in adenosine’s structure implicate the loss of important interactions, and others9 describe its intracellular action independent from other adenosine receptors.
Concerning the arrhythmogenic effects of IN, we have observed that atrial tachycardia, flutter, and fibrillation present in treated animals, as well as in those that received adenosine,22,23 are probably due to a shortening of the atrial refractory period. It is worthwhile to mention that, during the treatment of ventricular arrhythmias with intravenously applied IN, electrocardiographic signs of ventricular preexcitation are manifested, as occurs with adenosine.24 This fact already reported by other authors, regarding adenosine,25,26 can occur coincidentally with bradycardia or heart block, or could be due to a facil itated conduction through some accessory pathway usually not functional: a secondary phenomenon due to sympathetic activation.
Limitations of the study
The number of experiments included in each subgroup is rather small. A larger number of experiments could confirm the observations already made and support our results. On the other side, it is necessary to state that we did not find specific studies on the electrophysiology and pharmacology of IN, whereas there are numerous studies concerning adenosine. For this reason, we must rely on publications on the characteristics and ef fects of adenosine. lt would be suitable to study also the action of IN infusions.
Conclusions
Inosine, a derivate from adenosine, has antiarrhythmic and arrhythmogenic effects similar to those of adenosine. Its antiarrhythmic action is exerted on atrial and ventricular tachycardias, probably due to the activation of myocyte’s receptors, which is opposed by their blockers.
The arrhythmogenic effects of IN are probably due to sympathetic reflexes stimulated by this nucleoside, similar to those stimulated by adenosine.
On the other hand, IN can produce a ventricular preexcitation coincident with bradycardia or heart block, or a facilitated conduction through an accessory, usually not functional pathway. The arrhythmogenic effects of IN can be due to sympathetic reflexes, similar to those stimulated by adenosine.
Concerning the alterations of atrioventricular and ventricular-atrial conduction, IN, as well as adenosine, could reduce the volt age and duration of the action potentials, particularly in the cells of the central region of the atrio-ventricular node.
ABBREVIATIONS
LIV: Left intraventricular lead
RIV: Right intraventricular lead
SVC: Superior vena cava
SR: Sinus rhythm
VT: Ventricular tachycardia
LVT: Left ventricular tachycardia
FL: Atrial flutter
FIBR: Atrial fibrillation
AT: Atrial tachycardia
IN: Inosine
RBBB: Right Bundle Branch Block
MV: Mean Value
Corresponding author:
Dr. Alfredo de Micheli,
Instituto Nacional de Cardiología “Ignacio Chávez”, Juan Badiano No. 1, Col.
Sección XVI, México, D.F. CP 14080.
Received: March 31, 2009;
accepted: July 27, 2009.