To evaluate the efficacy of natriuretic peptide (NP)-guided therapy compared to clinically-guided therapy in reducing mortality and hospital admissions in chronic heart failure (HF) patients.
MethodsRandomised clinical trials (RCT) were selected through a systematic review. Four meta-analyses were conducted for the outcomes of overall mortality, HF-related mortality, overall hospital admissions, and HF-related hospital admissions. Heterogeneity between studies and publication bias were also assessed.
ResultsNine RCTs were found with a total of 1914 patients. NP-guided therapy significantly reduced overall mortality and HF-related hospital admissions. No significant results were found for HF-related mortality and overall hospital admissions. Some clinical heterogeneity regarding interventions performed was found between studies. Publication bias was found for HF-related and overall hospital admissions.
ConclusionsNP-guided therapy seems to improve outcomes compared to clinically-guided therapy. However, heterogeneity found between interventions might reduce the generalisation of these results. Specific interventions of the clinical trials should be examined when making recommendations regarding NP-guided therapy.
Evaluar la eficacia de la terapia guiada por el péptido natriurético (PN) en comparación con la terapia guiada clínicamente para reducir la mortalidad y la hospitalización de la insuficiencia cardiaca (IC) crónica.
MétodosLos ensayos clínicos aleatorizados fueron seleccionados a través de una revisión sistemática. Cuatro metaanálisis se realizaron para los resultados de mortalidad general, mortalidad por IC, hospitalización general y la hospitalización por IC. Se evaluó la heterogeneidad entre los estudios y el sesgo de publicación.
ResultadosNueve ensayos clínicos aleatorizados se encontraron con un total de 1,914 pacientes. La terapia guiada con el PN reduce significativamente la mortalidad general y la hospitalización por IC. No se encontraron resultados significativos para la mortalidad por IC y la hospitalización general. El sesgo de publicación se encontró para las hospitalizaciones por IC y globales.
ConclusionesLa terapia guiada por PN parece mejorar los resultados en comparación con la terapia guiada clínicamente. Sin embargo, la heterogeneidad encontrada entre las intervenciones podría reducir la generalización de estos resultados. Las intervenciones específicas de los ensayos clínicos deben ser analizadas al hacer recomendaciones con respecto a la terapia de guiada por PN.
Chronic heart failure (HF) is associated with high morbidity and mortality rates.1 The availability of a wide range of treatments (including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-adrenergic blockers, and spironolactone), is not still enough to improve outcomes.2 An incorrect dose titration can lead to a poor response to HF treatment.
Natriuretic peptides (NP) are biomarkers of endoventricular myocardial strain associated with the progression of HF.3–5 In the last decade, some studies have tried to show the potential of both B-type Natriuretic Peptides (BNP) and N-terminal pro-BNP (NT-pro BNP) plasma concentration as valid indicators to drive HF treatment.6,7 These biomarkers are proposed as parameters able to lead dose titration in HF patients. Multiple studies have compared whether NP-guided management could improve results compared with usual strategy based on assessment of clinical status.
NP biomarkers have well recognized applicability in diagnosis and prognosis of HF.2,7,8 However, their use to guide therapy titration is not as accepted. The ACCF/AHA guideline reports that the reduction of mortality and hospitalization of HF patients through NP monitoring is not well established. It considers reasonable to monitor NP in order to achieve optimal therapeutic doses only in select clinically euvolemic patients followed in well-structured management programs.8 NICE recommends that NP monitoring is considered in some patients; for example, those with problematic dose titration and patients admitted to hospital.7
The aim of this study was to evaluate whether NP-guided therapy, compared to clinically guided therapy, improves clinical results in HF patients. Meta-analyses of the available evidence were performed for four outcomes: overall mortality, HF-related mortality, overall hospitalization, and HF-related hospitalization; including the most recent clinical trials.
Materials and methodsData searchA computerized search was performed between 2000 and 2014 using biomedical databases Cochrane, Medline, EMBASE, with the keywords “Natriuretic Peptide, Brain” AND “Heart Failure”. Searches were performed using Mesh terms and free text in order to find the, non-indexed, most recent publications. Gray literature was searched on the database clinicaltrials.gov. A manual search was performed from the references of selected studies. Whenever possible, limits were used for language: Spanish and English; and type of study: randomized clinical trials (RCT).
Study selectionStudy selection was done by pairs, resolving disagreements by consensus. Included studies were those answering the question: “The modification of drug therapy in HF patients based on the NP monitoring, compared to clinically-guided therapy, is effective in reducing the frequency of mortality and hospitalization events?”.
The exclusion criteria for selecting articles were: language (other than English or Spanish); publication type (other than RCT); not answering the outlined question; and those studies which did not report any of the outcomes defined or which did not report the outcomes as absolute number of patients.
Data extractionData extraction was done by pairs, resolving disagreements by consensus. The outcomes extracted were overall mortality, HF-related mortality, overall hospitalization, and HF-related hospitalization provided as absolute numbers of patients.
Statistical analysisOdds ratios (OR) and 95% Confidence Intervals (CI) were calculated for each individual trial from the absolute numbers reported for the outcomes under evaluation: overall mortality, HF-related mortality, overall hospitalization, HF-related hospitalization.
Pooled OR and 95% CI were calculated for each of the four outcomes. The limit for statistical significance was set at p≤0.05. Heterogeneity was studied through the Cochran Q test and I2 statistic. For the Q test p-value of 0.10 was taken as the limit of significance.9I2 was classified into low (25–50%), medium (51–75%) and high (<75%) heterogeneity. When significant heterogeneity was not found, the studies were combined using fixed models using the Mantel-Haenszel. When heterogeneity was detected, random effects models (DerSimonian-Laird method) were used. Forest Plot graphs were used to represent the effectiveness of the intervention compared to the control group, in terms of OR.
Publication bias was assessed by Funnel plots and Begg and Egger methods.
Data were analyzed using STATA (version 11.0, Stata Corp., College Station, TX, USA).
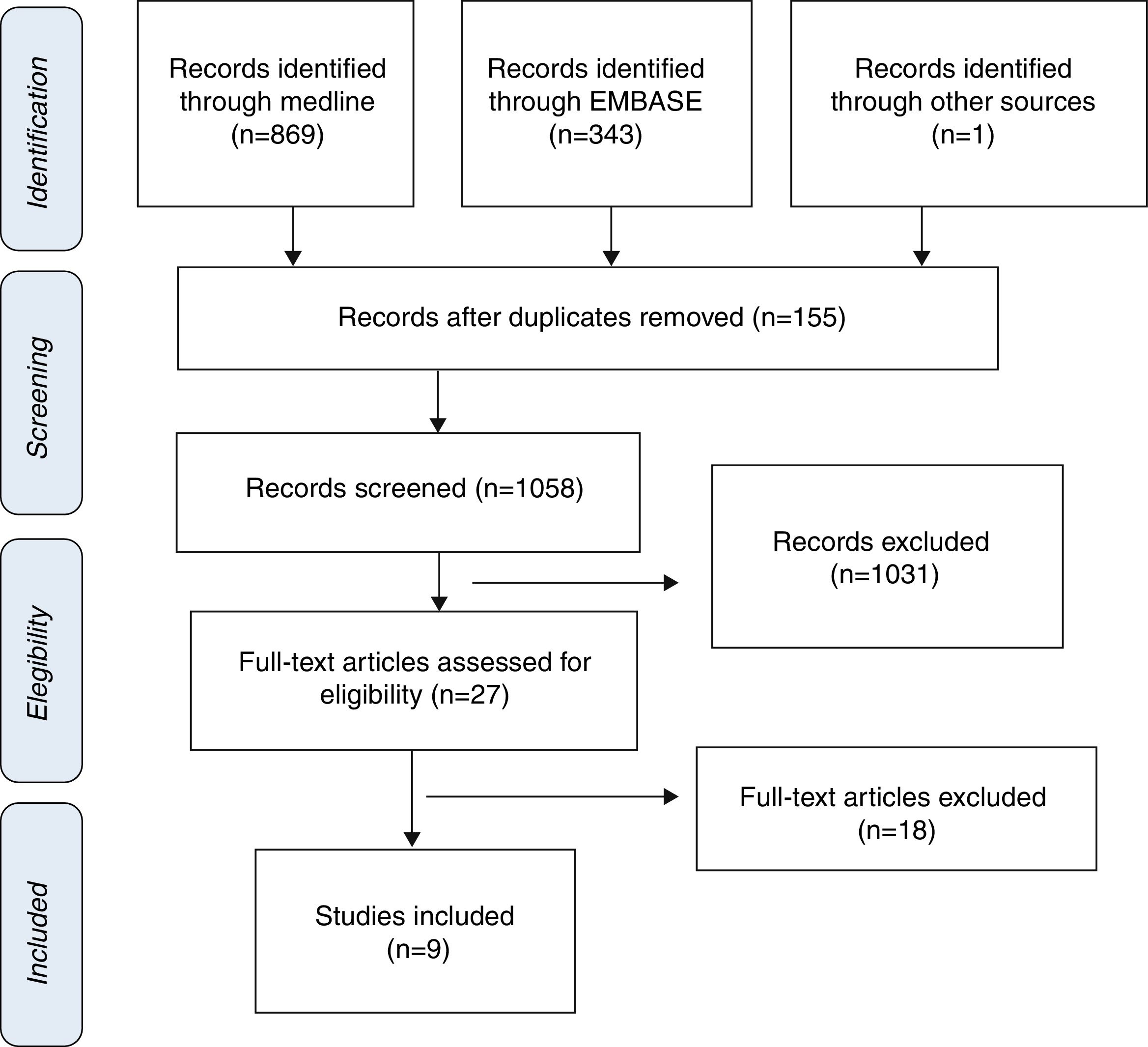
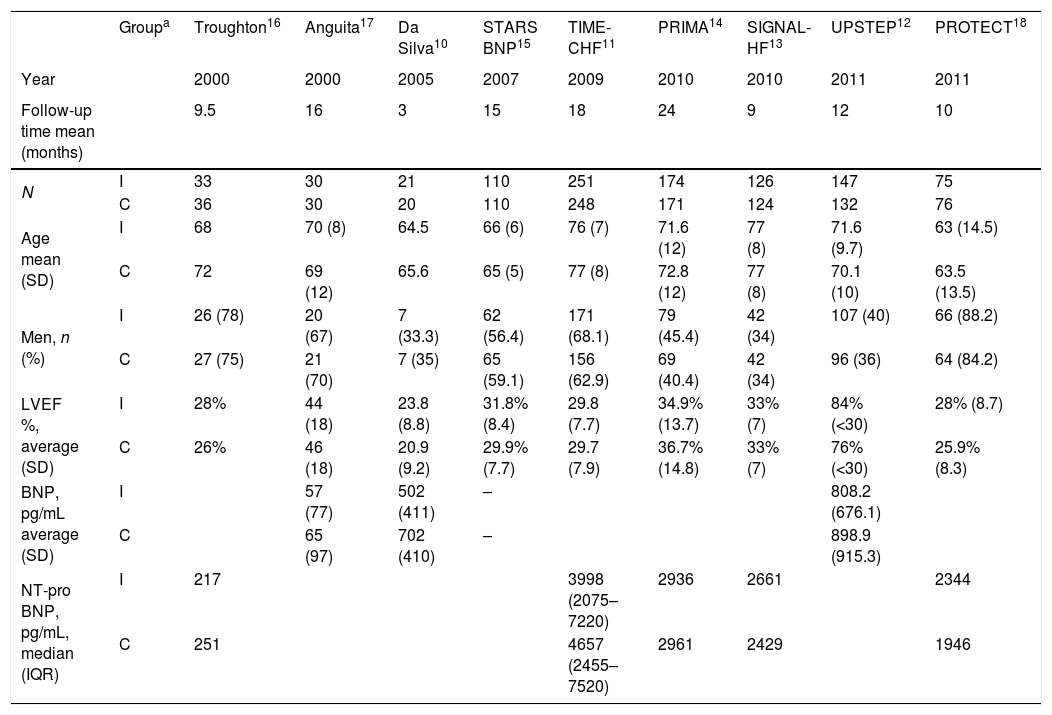
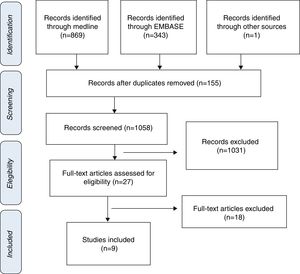
ResultsStudy selection and characteristics of included trialsInitially, 1213 articles were identified of possible relevance to the study, of which 27 were analyzed in full text to decide their eligibility. Finally, 9 articles were included in the systematic review and meta-analyses10–18 (Fig. 1). The mean follow up was 11.9±6.1. The total number of patients was 1914 with mean age of 70.0±4.8, being men 55.9% (Table 1).
Characteristics of clinical trials and patients included.
| Groupa | Troughton16 | Anguita17 | Da Silva10 | STARS BNP15 | TIME-CHF11 | PRIMA14 | SIGNAL-HF13 | UPSTEP12 | PROTECT18 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2000 | 2000 | 2005 | 2007 | 2009 | 2010 | 2010 | 2011 | 2011 | |
| Follow-up time mean (months) | 9.5 | 16 | 3 | 15 | 18 | 24 | 9 | 12 | 10 | |
| N | I | 33 | 30 | 21 | 110 | 251 | 174 | 126 | 147 | 75 |
| C | 36 | 30 | 20 | 110 | 248 | 171 | 124 | 132 | 76 | |
| Age mean (SD) | I | 68 | 70 (8) | 64.5 | 66 (6) | 76 (7) | 71.6 (12) | 77 (8) | 71.6 (9.7) | 63 (14.5) |
| C | 72 | 69 (12) | 65.6 | 65 (5) | 77 (8) | 72.8 (12) | 77 (8) | 70.1 (10) | 63.5 (13.5) | |
| Men, n (%) | I | 26 (78) | 20 (67) | 7 (33.3) | 62 (56.4) | 171 (68.1) | 79 (45.4) | 42 (34) | 107 (40) | 66 (88.2) |
| C | 27 (75) | 21 (70) | 7 (35) | 65 (59.1) | 156 (62.9) | 69 (40.4) | 42 (34) | 96 (36) | 64 (84.2) | |
| LVEF %, average (SD) | I | 28% | 44 (18) | 23.8 (8.8) | 31.8% (8.4) | 29.8 (7.7) | 34.9% (13.7) | 33% (7) | 84% (<30) | 28% (8.7) |
| C | 26% | 46 (18) | 20.9 (9.2) | 29.9% (7.7) | 29.7 (7.9) | 36.7% (14.8) | 33% (7) | 76% (<30) | 25.9% (8.3) | |
| BNP, pg/mL average (SD) | I | 57 (77) | 502 (411) | – | 808.2 (676.1) | |||||
| C | 65 (97) | 702 (410) | – | 898.9 (915.3) | ||||||
| NT-pro BNP, pg/mL, median (IQR) | I | 217 | 3998 (2075–7220) | 2936 | 2661 | 2344 | ||||
| C | 251 | 4657 (2455–7520) | 2961 | 2429 | 1946 |
The Cochran Q test and I2 statistic detected significant heterogeneity or the outcomes of HF-related mortality and HF-related hospitalization; therefore, random effects models were used. Meta-analyses for overall mortality and overall hospitalization were performed using fixed effects models.
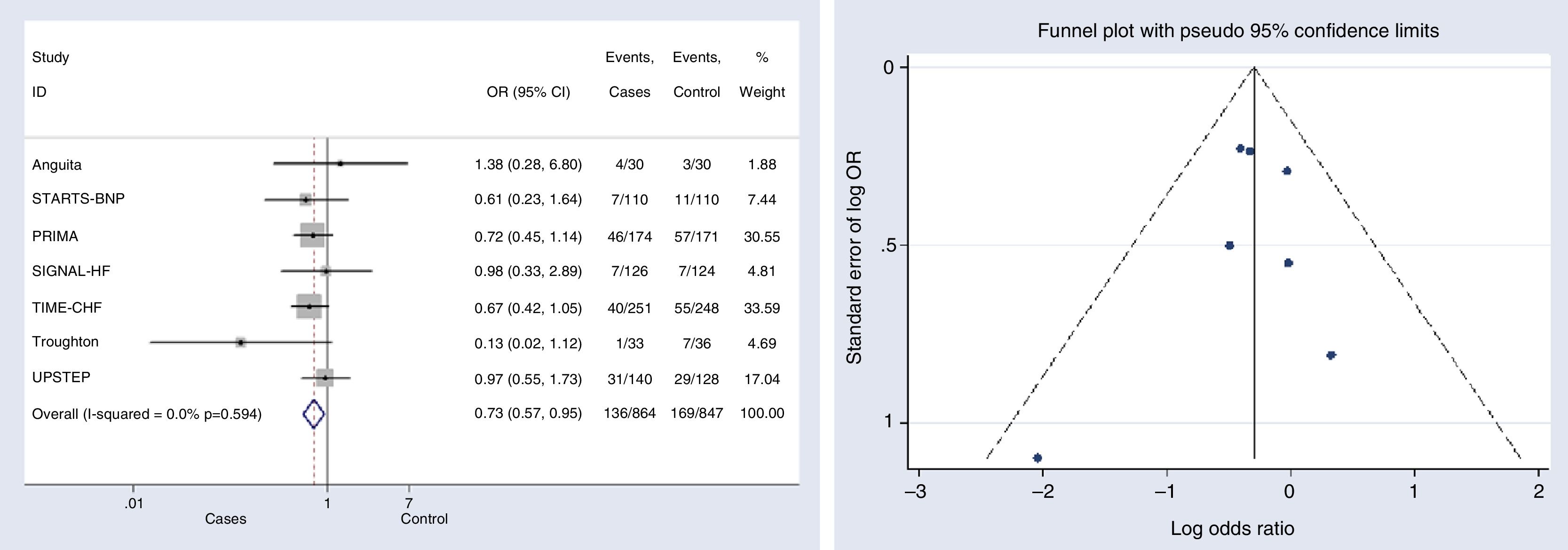
Outcomes analysesOverall mortalitySeven studies provided data on overall mortality.11–17 The mortality risk decreased significantly in patients under NP-guided therapy (OR, 0.733, 95% CI, 0.568 to 0.946, p=0.017, I2=0%). Only one study reported an OR above 1; however this effect was not statistically significant and the study represented a very small weight (1.88%)17 (Fig. 2).
Begg's (p-value=0.881) and Egger's (p-value=0.718) tests and funnel plot (Fig. 2) showed a low publication bias for this outcome.
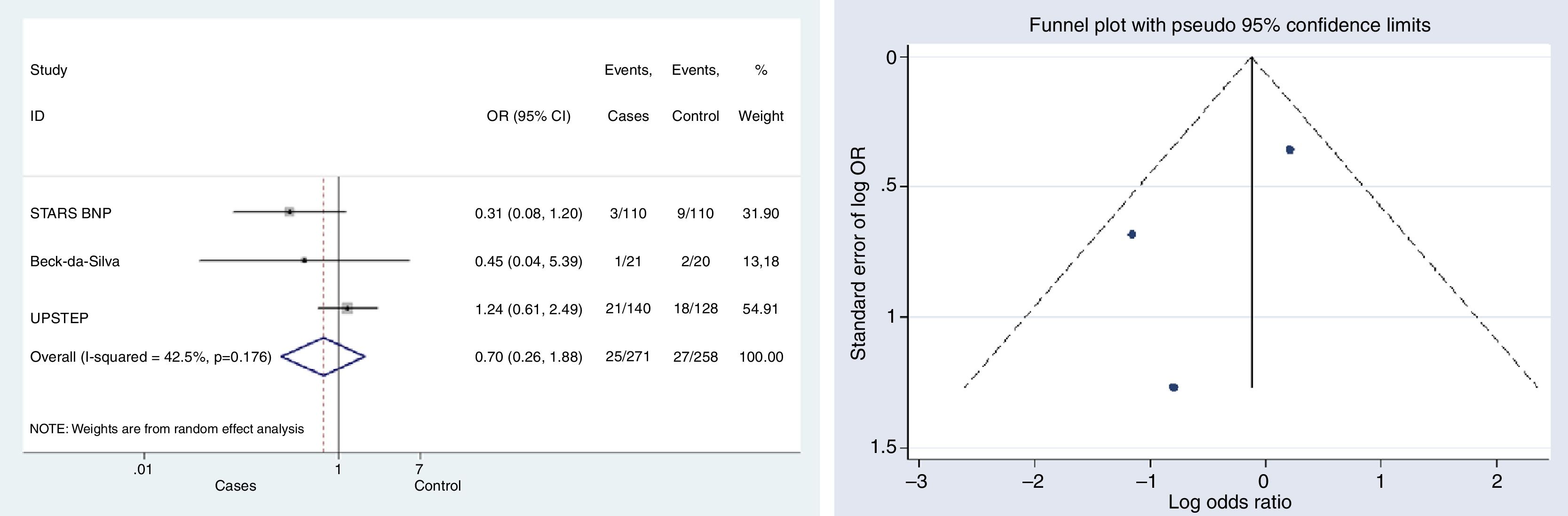
HF-related mortalityThree studies provided data on mortality associated with HF.10,12,15 The difference in mortality risk between groups was not statistically significant; neither globally (OR, 0.699, 95% CI, 0.26–1.89, p=0.479, I2=42.5%), nor for any of the studies individually (Fig. 3).
Begg's (p-value=0.602) and Egger's (p-value=0.441) tests and funnel plot (Fig. 3) showed a low publication bias for this outcome.
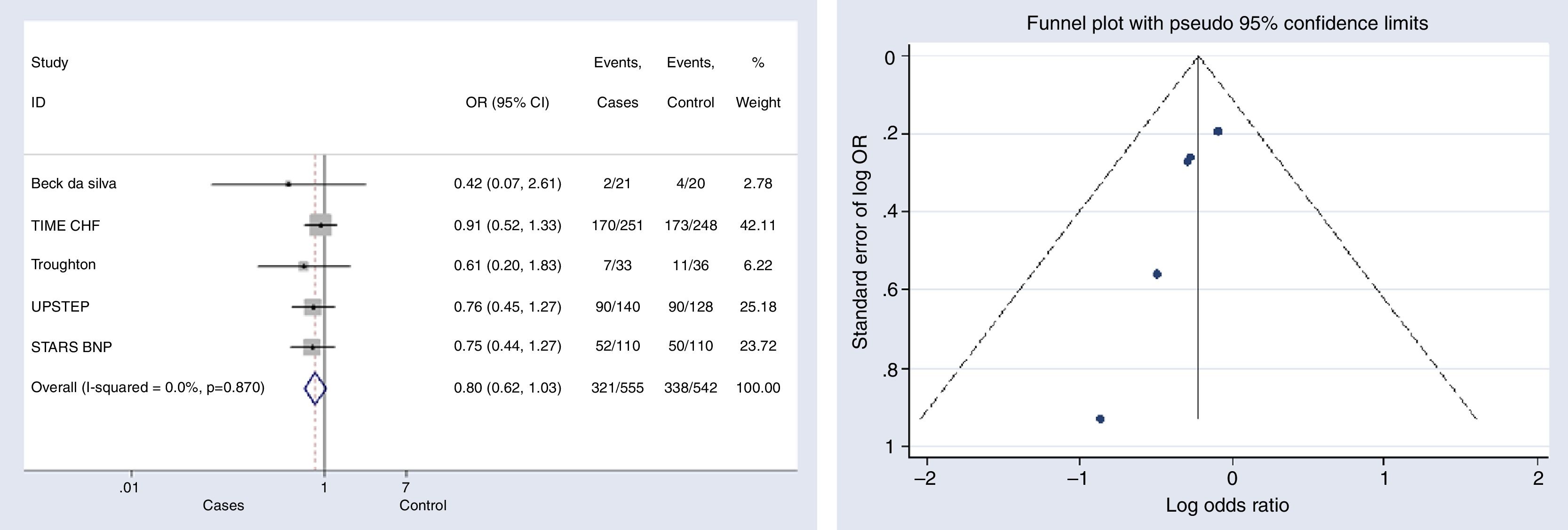
Overall hospitalizationsFive studies reported information on this outcome.6,10–12,15 The risk of hospitalization did not decreased significantly in the group with NP-guided therapy (OR, 0.801, 95% CI, 0.622–1.033, p=0.087, I2=0%). None of the studies reported statistically significant results either (Fig. 4).
Begg's (p-value=0.014) and Egger's (p-value=0.025) tests and funnel plot (Fig. 4) showed a significant publication bias for this outcome.
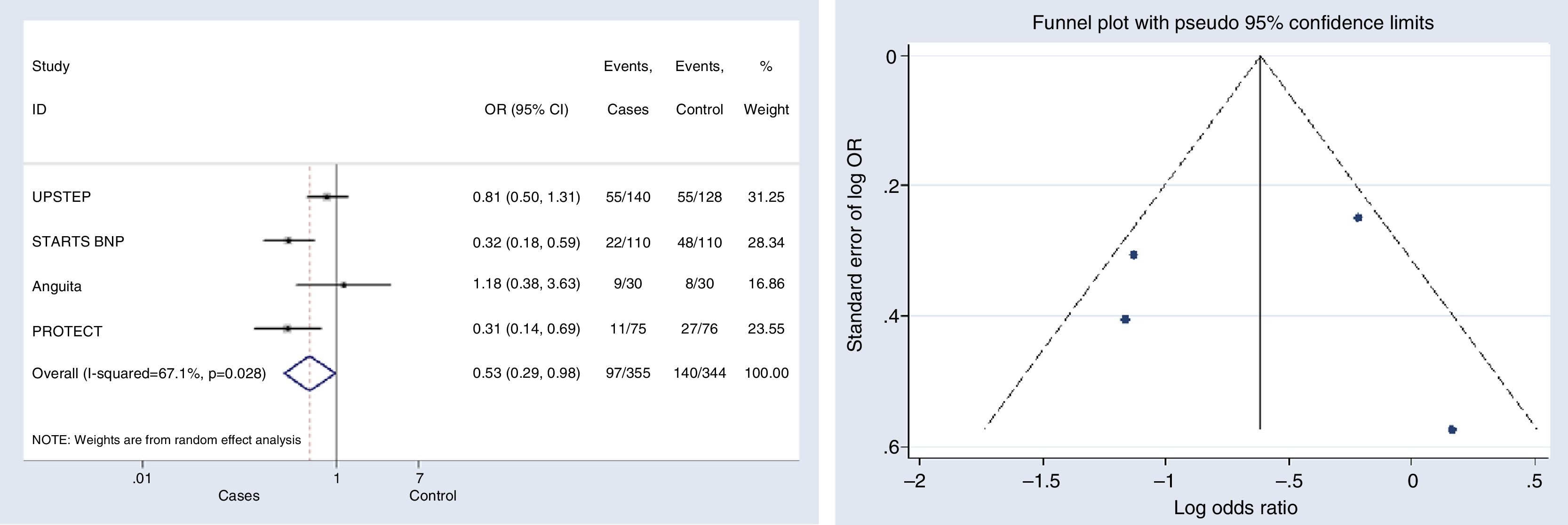
HF-related hospitalizationFour studies were included for this outcome.12,15,17,18 The risk of hospitalization associated with HF decreased significantly in the group with NP-guided therapy (OR, 0.530, 95% CI, 0.287–0.979, p=0.043; I2=67.1%). Two of the studies evaluated found statistically significant results for this outcome.15,18 Only one clinical trial reported an OR above 1; however this effect was not statistically significant and the study represented a very low (16.86%) weight17 (Fig. 5).
Begg's (p-value=1) and Egger's (p-value=0.957) tests and funnel plot (Fig. 5) showed a significant publication bias for this outcome.
DiscussionThe present study found that NP-guided therapy for HF management decreases overall mortality and HF-related hospitalization. These findings met results from previous meta-analyses.19–21 Only for overall mortality, one of the previous studies did not report differences between NP monitoring and conventional clinically guided management, although results were close to significance.21
This study did not show a significant effect of NP-guided management on HF-related mortality and all-cause hospitalization. Again, the same result was obtained by another meta-analysis evaluating all-cause hospitalization.19 None of the previous meta-analysis evaluated the outcome HF-related mortality.
Considering the primary evidence, only two of the clinical trials included in the meta-analyses showed a significant positive effect for NP-guided management, decreasing HF-related hospitalization.15,18 The rest of the studies did not have statistically significant results for any of the outcomes evaluated; although, there was a general trend favoring NP-guided management.
The main limitation of the present study comes from the clinical heterogeneity found in the primary evidence. Even when controlling for statistical heterogeneity, it might happen that similar results come from clinically different interventions. For example, some of the studies evaluated specifically NT-pro BNP biomarkers11,13,14,16,18; whilst, others looked at BNP-guided therapy.10,12,15,17 NT-pro BNP has been reported to be more effective than BNP-guided therapy.19 Another difference across interventions was that some study protocols established to intensify drug therapy to reach NP concentrations below a certain level (different for each study)11,12,15–17; whilst, others changed therapy according to clinical factors, NP levels, and physicians criteria; but, without establishing a threshold of this type.10,13,14
Another limitation arises from the difficulty of blinding physicians and patients for these interventions. This lack of blinding in most of the studies might overestimate the positive results found for NP-guided management. Also, taking into account that patients in the intervention group have more frequent visits to health care professionals, it is not straightforward to attribute the intervention effect to the NP monitoring rather than to a more stretch control of the patients.
This study did not include three of the clinical trials considered in previous meta-analyses because they not reported results as absolute number of patients for the outcomes under this assessment.22–24
Guided management of HF patients through NP monitoring seems to have a clinical benefit represented as mortality and hospitalization reductions. However, given the clinical heterogeneity found between the studies, these results should be examined cautiously and take into account the specific interventions perform for each study.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNone declared.
Conflict of interestThe authors declare no conflict of interest.