Informamos cinco casos de miocardiopatías de estrés (en una edad entre 49 y 90 años), inter-nadas en nuestro Servicio durante el último año. El contexto clínico en el cual se manifestaba el evento agudo, se diferenciaba en cada caso por: angina pectoris típico, endoarterectomía carotídea, edema pulmonar con evolución a choque cardiogénico, astenia grave.
En tres pacientes, el ecocardiograma evidenció grave compromiso del ápex del ventrículo izquierdo (punta del ventrículo), asociado a signos electrocardiográficos de leve supradesnivel del segmento ST en las derivaciones precordiales anteriores, de idéntica presentación que en la de infarto miocárdico agudo anterior. En dos pacientes se evidenciaron durante el ecocardiograma, una disfunción medio ventricular asociada a signos electrocardiográficos de infradesnivel del segmento ST, en las derivaciones precordiales anteriores.
La evolución electrocardiográfica consistió en una inversión de la onda T en cuatro casos, y en una normalización de la repolarización en un caso. En todos los casos, los exámenes de química sanguínea mostraron un leve aumento de la troponina. Las alteraciones de la cinética parietal se redujeron en cuatro pacientes, durante el tiempo de la interacción. Para completar el diagnóstico, en dos pacientes fueron efectuados ecocardiograma de estrés (con dobutamina en la paciente con persistencia de hipocinesia medio ventricular, ecocardiograma de esfuerzo a la otra). En ambos casos, el eco-estrés fue suficientemente útil para predecir correctamente la ausencia de enfermedades de las arterias coronarias principales.
En conclusión, un análisis exhaustivo de la motilidad parietal mediante ecocardiograma transtorácico, en conjunto con las modificaciones del segmento ST observados en el electrocardiograma, y de los principales marcadores de necrosis miocárdica, permiten reconocer la presencia de miocardiopatía de estrés en diversos contextos clínicos. El ecocardiograma de estrés puede ser propuesto a las pacientes en las cuales permanece la sospecha de enfermedades coronarias.
We report five cases of stress related cardiomyopahies that occurred in post-menopausal women (age range from 49 to 90) consecutively admitted to our Department in the last year in different clinical settings: typical anginal pain, carotid endarterectomy, pulmonary edema, cardiogenic shock, and severe asthenia. Apical left ventricular involvement was observed in three patients in conjunction with ECG mild ST segment elevation in anterior precordial leads resembling acute anterior myocardial infarction; isolated mid ventricular dysfunction was present in two patients in conjunction with ST segment depression in the anterior precordial leads. The ECG evolved showing T wave inversion in four cases and normalized in one. In all cases, blood chemistry showed mild elevation of CK-MB and TN. The observed wall motion abnormalities were reversible in four of five cases during hospital stay. Stress echocardiography was performed in two patients (dobutamine in the patient with persistent mid-ventricular hypokinesis, exercise in another case) and correctly predicted the absence of coronary artery disease. We conclude that wall motion analysis at echocardiography combined with ECG ST segment changes and serum markers of myocardial necrosis (CK-MB and TN) may allow recognition of stress cardiomyopathies in different clinical settings. Echo stress may be proposed in those patients in whom some suspicion of coronary artery disease persists.
Introduction
Stress cardiomyopathies include a different set of clinical conditions in which anginal pain or other symptoms occur in conjunction with ST segment electrocardiographic changes and transient echocardiographic wall motion abnormalities, without coronary stenosis. In classical Takotsubo cardiomyopathy, the ECG findings resemble those found in acute anterior myocardial infarction although the reversible extension of apical akinesis at echocardio-gram (mid-apical ballooning) is out of proportion to the ECG ST segment elevation; atypical apical sparing Takotsubo cardiomyopathy has also been described more often involving the mid left ventricular segments. These forms of cardiomyopathies may be highly suspected in postmenopausal women who experience an either physical or emotional stressful event that, however, is not always observed. Similar echocardiographic and electrocardiographic patterns have also been reported in other clinical settings ranging over diverse medical illnesses, surgical procedures, and acute intracranial events suggesting a common pathophysiological link. Differential diagnosis between stress cardiomyopathies and acute coronary syndromes is mandatory, even though not yet possible without the support of coronary angiography. However, we believe that careful echocardiographic wall motion analysis together with electrocardiogram and serum markers of myocardial necrosis at presentation might facilitate the correct identification of patients with stress related cardiomyopathies.
Written informed consent was obtained from all the patients for publication of these case reports and accompanying images. A copy of the written consents is available for review by the Editor-in-Chief of this journal
Case reports
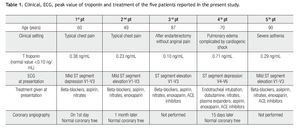
In all case reports, for the assessment of regional wall motion by echocardiography, the heart was divided into 17 segments as recommended by the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart association, according to the following definitions: basal, mid-cavity, and apical to define the location along the long axis of the left ventricle from the apex to the base, and mid anterior, mid anterolateral, mid inferolateral, mid inferior, mid inferoseptal, mid anteroseptal to define circumferential locations of the left ventricle (Table 1).1
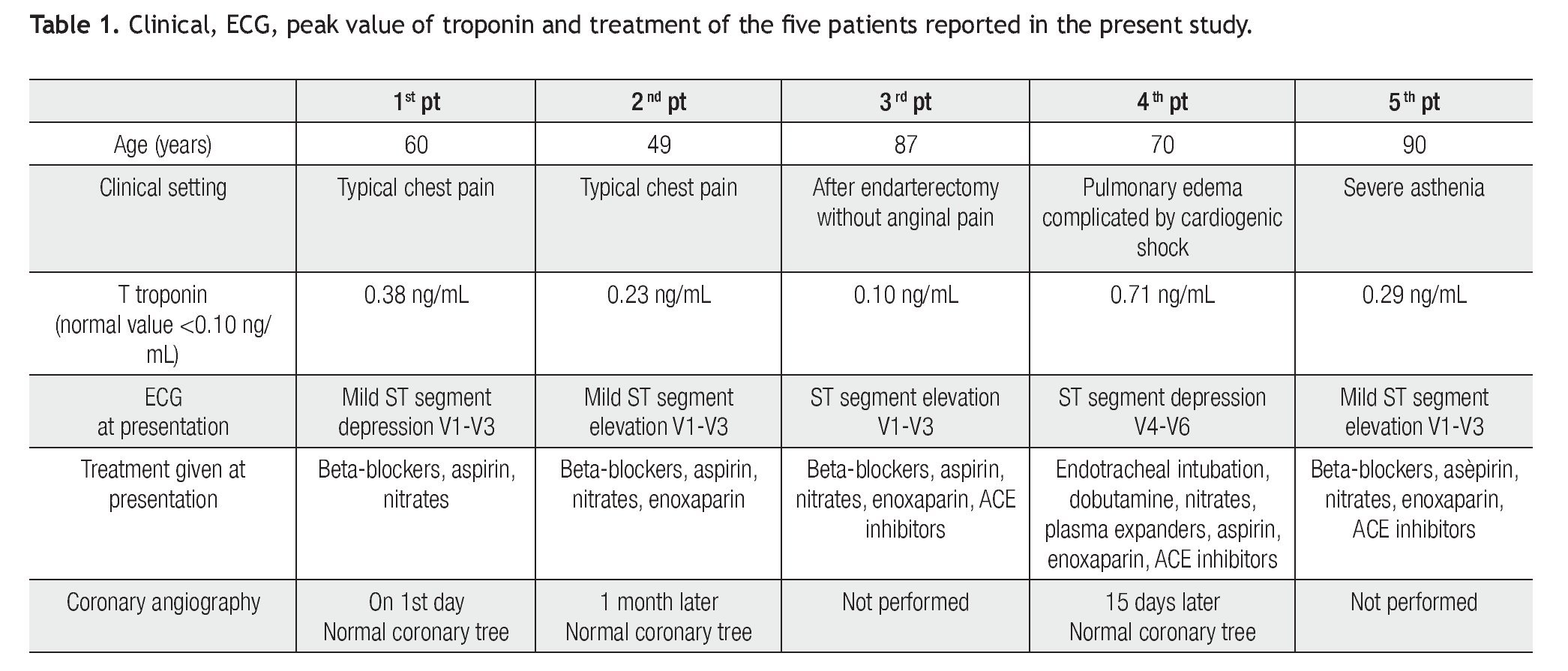
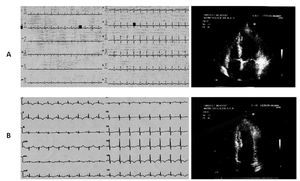
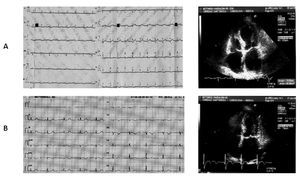
Case 1 (Figure 1)
Figure 1. A) A 60-year-old woman. During anginal pain, mild ST segment depression in the precordial leads from V1 to V3 at the ECG is associated with akinesia of mid segments of the left ventricle at the echocardiogram. B) Two days later ECG: T wave inversion in the inferior leads and in the precordial leads from V4 to V6. The echocardiogram repeated on the same day revealed restoring of normal motion in all left ventricular segments.
A 60-year-old Caucasian woman was admitted to our Cardiology department for typical chest pain during gardening without any recent stressful event. The ECG showed mild ST segment depression in the precordial leads from V1 to V3. Blood markers of cardiac ischemia were in the normal range. The echocardiographic investigation revealed akinesia limited to mid inferolateral, mid anterior, and mid anterolateral walls with coexisting reduced thickening of the mid anterior septum; no apical ballooning was observed. The left venticular ejection fraction was in the normal range (53%). The patient underwent diagnostic coronary angiography, which showed absence of obstructive atheromatous disease. The left ventriculography in the right anterior oblique view showed mid ventricular ballooning with hyperkinesia of the basal segments, hypokinesia of the mid anterior wall, and akinesia of the mid infero-postero-lateral wall.
Control blood chemistry on the second day showed a peak CK-MB level of 14 ng/mL (normal range < 3 ng/ mL) and a T-troponin level of 0,38 ng/mL (normal range < 0,10 ng/mL). The ECG registered two days later showed T wave inversion in the inferior leads and in the precordial leads from V4 to V6. The echocardiogram, repeated on the same day, revealed restoring of normal motion in all left ventricular segments.
The patient was discharged fully asymptomatic six days after admission, on pharmacological treatment with ACE inhibitors, beta-blockers, and Ca-blocking agents.
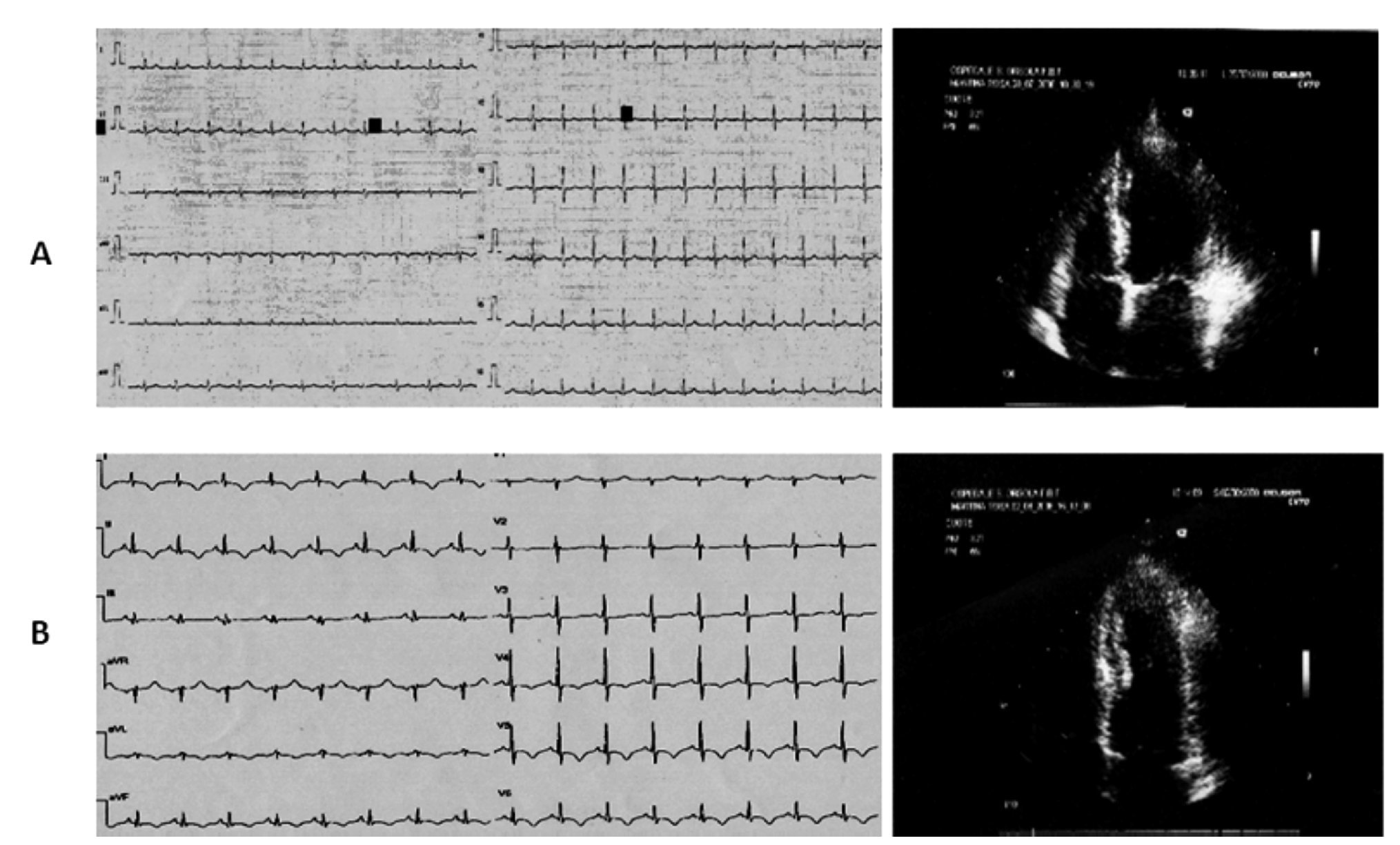
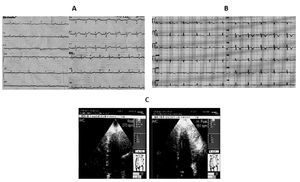
Case 2 (Figure 2)
Figure 2. A) A 49-year-old woman. Mild ST segment elevation in anterior precordial leads at the ECG during anginal pain (The echocardio-graphic investigation showed hypo-akinesia of the apical, mid inferolateral and anterolateral segments of the left ventricle. B) On the second day the resting ECG revealed an ischemic evolving pattern with inverted T waves in the precordial leads contrasting with complete restoring of normal wall motion at the echocardiographic examination. C) Exercise echocardiography: left ventricular hyperdynamic response during exercise predicted the absence of coronary artery stenosis at coronarography.
A 49-year-old woman was admitted to our emergency room for recurrent attacks of typical anginal pain occurring at rest after emotional event and radiating to the left arm. The first electrocardiogram revealed mild ST segment elevation in anterior precordial leads, without any increase in blood myocardial ischemic markers. The echo-cardiographic investigation during anginal pain showed hypo-akinesia of the apical segments and of the mid inferolateral and mid anterolateral segments of the left ventricle with preserved systolic function (EF >60%). Administration of antianginal therapy promptly resolved symptoms and stabilized the patient. On the second day, the resting ECG revealed an ischemic evolving pattern with inverted T waves in the precordial leads whereas the echocardiographic examination showed complete restoring of normal wall motion. There was mild increase in T-troponin and CK-MB levels with peak values of 0.23 ng/ mL (normal range <0.10 ng/mL) and 16.6 ng/mL (normal range <3 ng/mL), respectively.
One week later the patient underwent exercise echo-cardiogram, which showed hyperdynamic response of the left ventricle without asynergic areas. One month later the coronary angiography revealed a normal coronary tree.
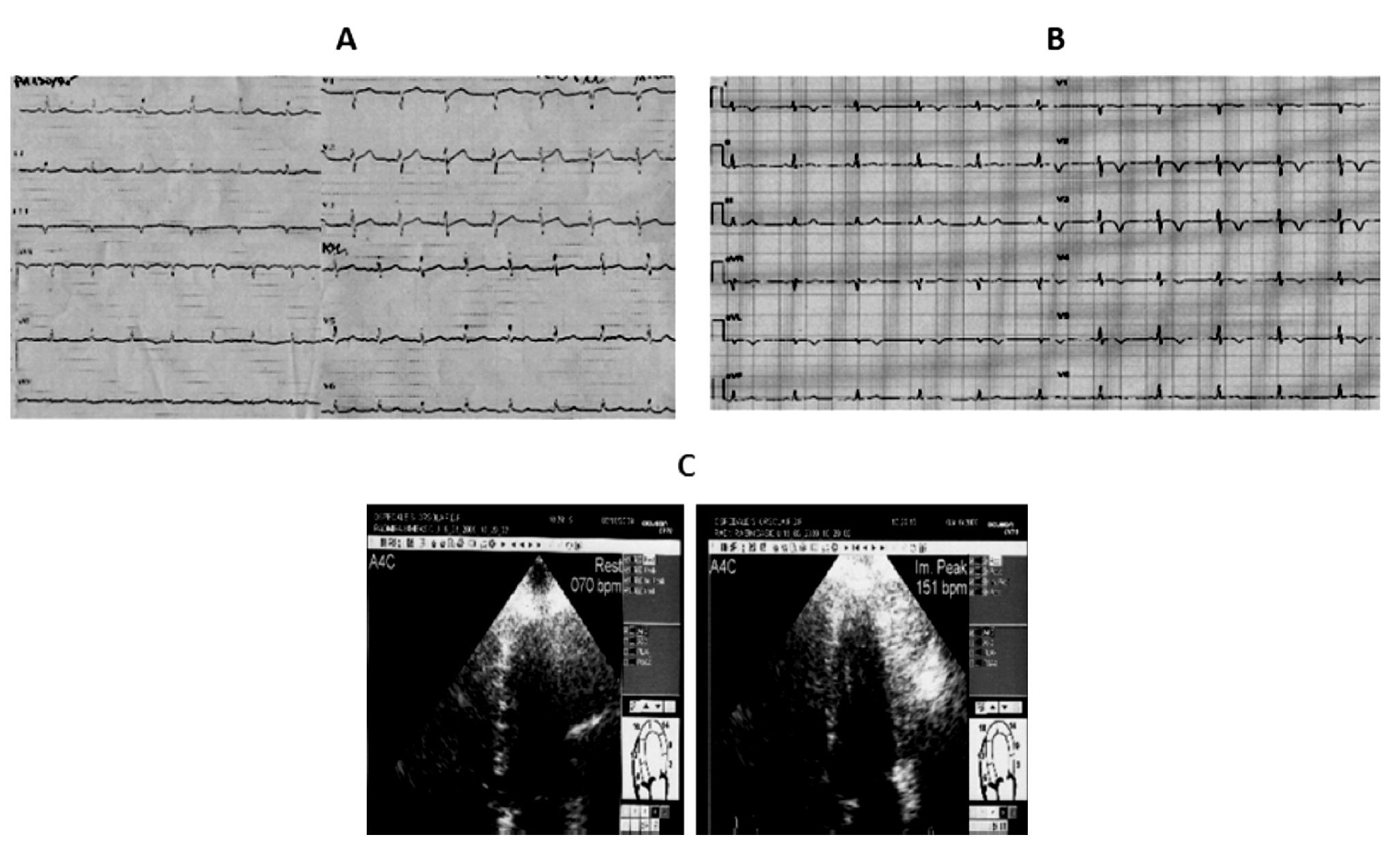
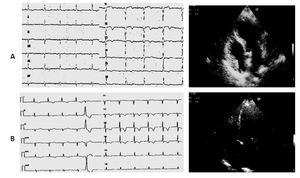
Case 3 (Figure 3)
Figure 3. A) A 87-year-old woman. ECG: subepicardial ischemic lesion in the anterior precordial leads after carotid endarterectomy. Echo examination: akinesia of the apical segments of the left ventricle with extension to mid anterior septum and mid infero-lateral wall. B) Five days later the ECG ischemic negative giant T waves in all precordial leads contrast with complete restoring of normal wall motion at Echo examination.
An 87-year-old woman was admitted to the Neurology department after a brain transient ischemic attack (TIA) that occurred three days before, with residual hemiparesis of the right arm and cephalalgia. Computed tomography of the brain did not reveal any recent cerebral ischemic area. Since the carotid Doppler examination showed significant narrowing of the left internal carotid artery, the patient underwent surgical endarterectomy. At cardiological evaluation before surgery no electrocardiographic repolarization changes, neither echocardiographic wall motion abnormalities were detected.
In the absence of clear anginal pain, postsurgical electrocardiogram revealed a subepicardial ischemic lesion in the anterior precordial leads whereas the echocardiographic examination showed akinesia of the mid-cavity and apical segments on the long axis of the left ventricle involving all the mid circumferential locations, with preserved ejection fraction (EF >50%). T-troponin slightly peaked to 0.10 ng/mL (normal values <0.10 ng/mL) and CK-MB peaked to 7.3 ng/mL (normal range <3 ng/mL). Pharmacological treatment during and after surgery consisted in daily administration of subcutaneous enoxaparin 4000 U, ASA, ACEI, nitrates, and beta-blocking agents at low dosage. The echocardiographic examination was repeated on the fifth day and showed complete restoring of normal wall motion with persistent ECG ischemic negative giant T waves in all precordial leads. Coronary angiography was not performed and the patient was discharged after one month of rehabilitation with consistent improvement of her neurological condition.
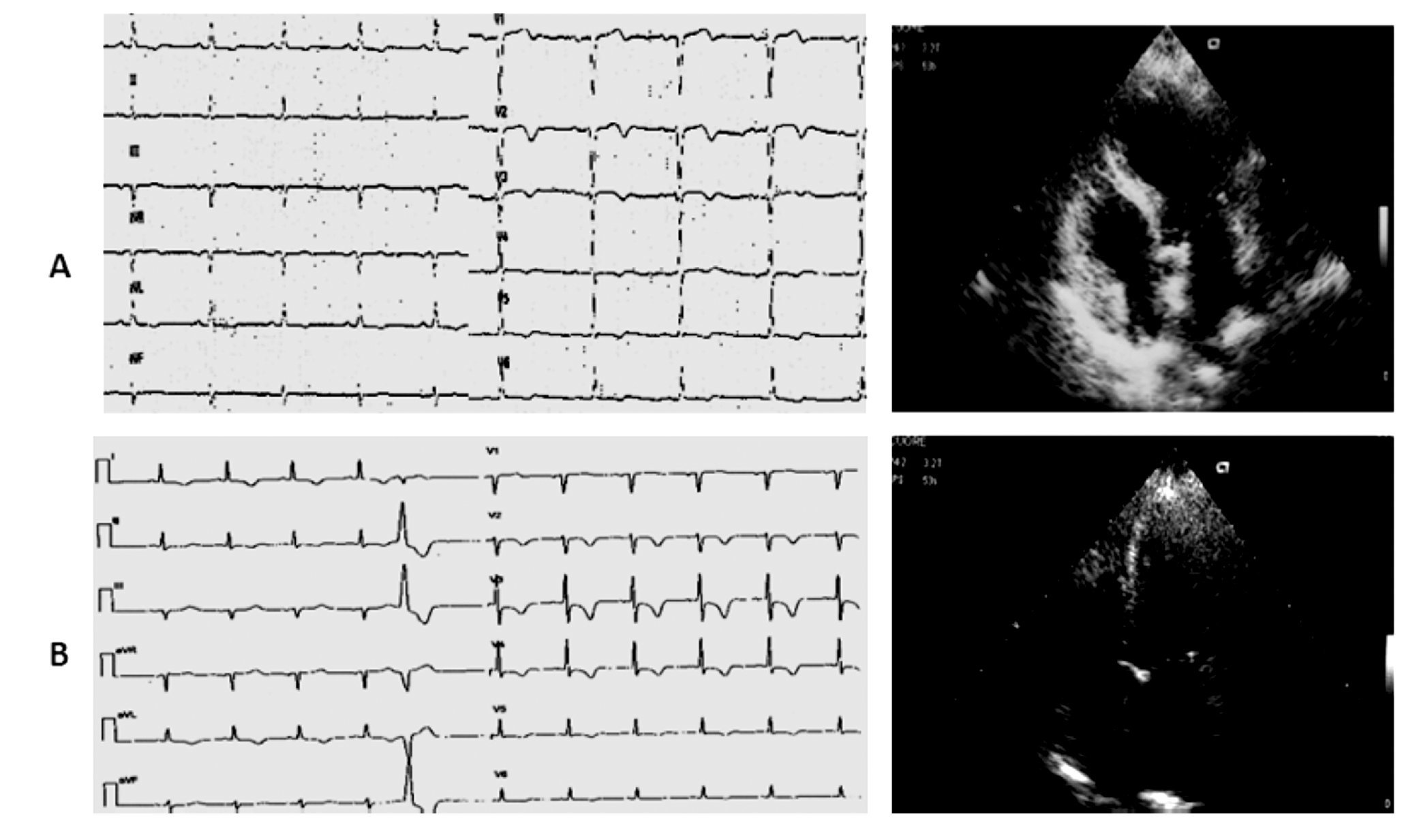
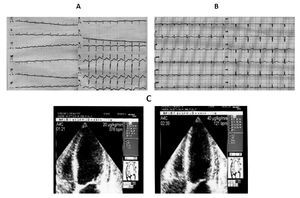
Case 4 (Figure 4)
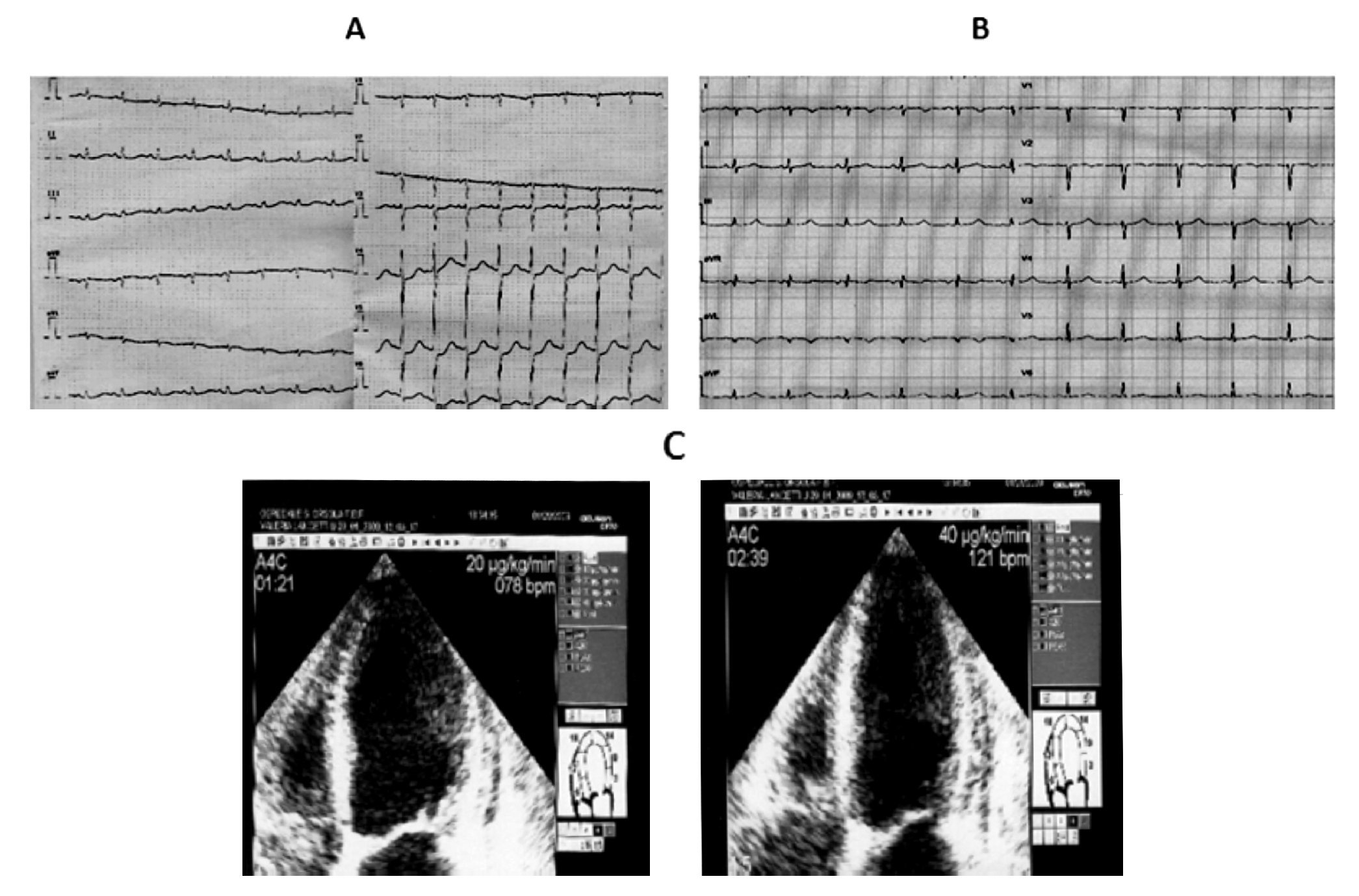
Figure 4. A) A 70-year-old woman. The ECG on admission shows sinus tachycardia (130 bpm) with 1 mm ST segment depression from lead V4 to V6 (the Echo investigation showed akinesia of the mid segments of the left ventricle and moderate LV dysfunction). B) Five hours later, ECG revealed restoring of normal repolarization in all leads (Echo confirmed the presence of asynergic areas in the mid segments of the left ventricle that persisted unchanged during the remaining hospital stay). C) After thirteen days, dubutamine infusion at peak dose of 40 μg/kg resulted in significant improvement of wall motion in all the asynergic segments of the left ventricle with increase in EF. The coronary angiography was normal.
A 70-year-old woman affected by bowel carcinoma was admitted to our Intensive Care Unit for pulmonary edema and cardiogenic shock, being successfully treated with endotracheal intubation, inotropic agents infusion, diuretics, and plasma expanders.
On admission to the emergency department, the electrocardiogram showed sinus tachycardia (130 bpm) with 1 mm shift of the ST segment from lead V4 to V6; the echocardiographic examination showed akinesia of all the mid segments of the left ventricle without apical involvement and a moderate reduction of the systolic function (LVEF 40%). The electrocardiogram recorded one hour later showed mild ST segment depression with prolonged QT interval (HR 100 bpm, QTc 0,628 sec), whereas five hours later it showed restoring of normal repolarization in all leads with lower value of QTc (HR 96 bpm, QTc 0,53). Peak values of T-troponin and CK-MB were, respectively, of 0.71 ng/mL (normal range < 0.10 ng/mL) and 10.83 ng/mL (normal range <3 ng/mL). Paroxismal atrial fibrillation was observed on the second day as the only complication. The patient was treated with ACE inhibithors, carvedilol, and enoxaparin at standard dosage. Thirteen days after the acute event, an echocardiographic examination was repeated at rest and during pharmacological stress with dobutamine: the rest echocardiogram revealed akinesia limited to mid inferior and mid inferolateral walls, hypokinesia of mid anterior and mid anterolateral walls, reduced thickening of the mid anterior septum with sparing of basal and apical segments, mild reduction of left ventricular function (EDV 120 mL, ESV 70 mL, EF 45%). Dobutamine infusion starting from 10 μg/kg and increased up to 40 μg/kg resulted in significant improvement of wall motion in all the asynergic segments with decrease in left ventricular volumes and increase in EF (EDV 99 mL, ESV 43 mL, EF 56%).
The coronary angiography showed absence of atheromatous disease in epicardial vessels. The patient was eventually discharged on carvedilol and ACE inhibitor.
Case 5 (Figure 5)
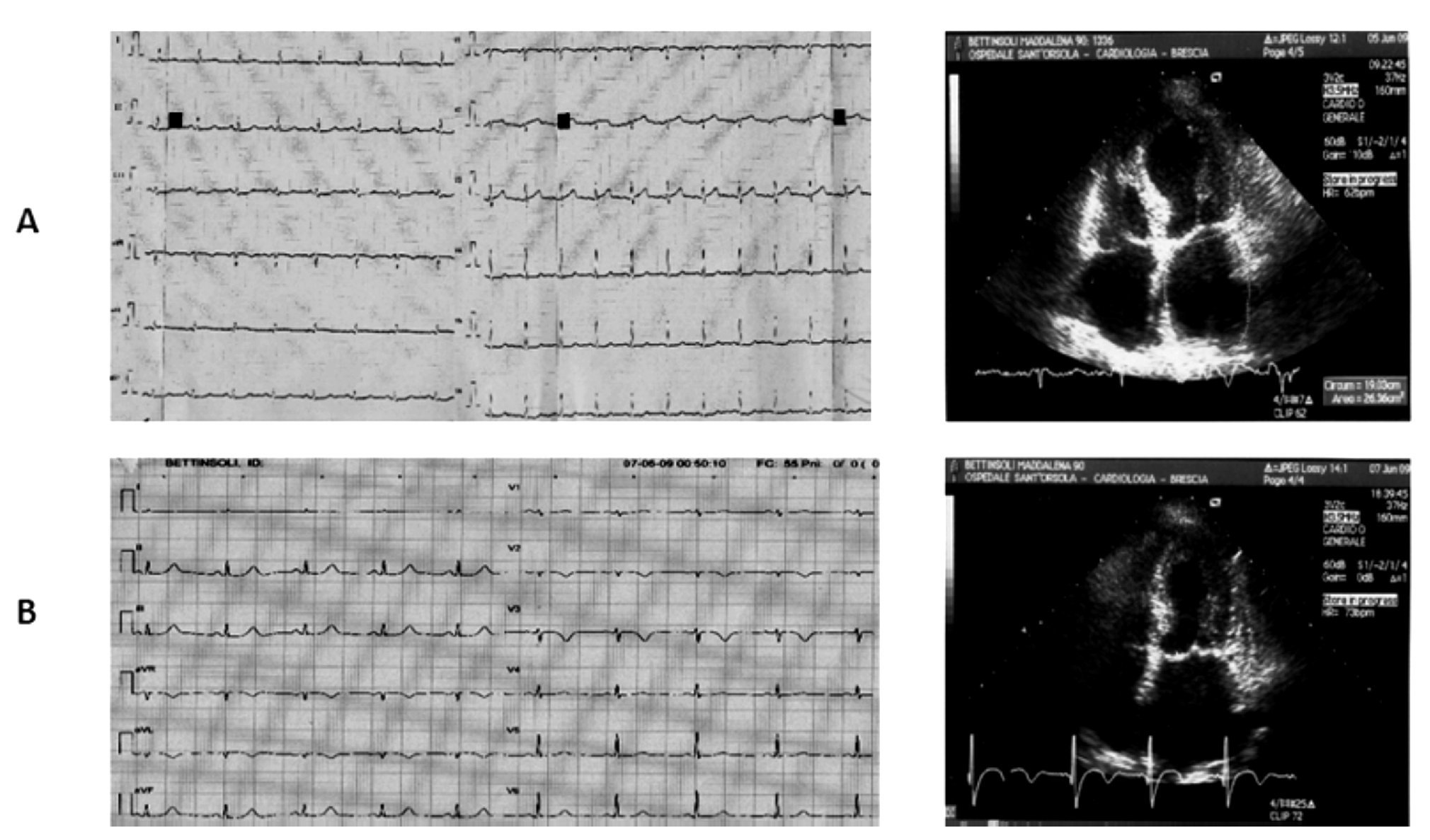
Figure 5. A) A 90-year-old woman. ECG reveals mild ST segment elevation limited to V1-V3 and Echo examination shows akinesia of the apical segments with extension to the mid anterolateral and mid anterior septum. Left atrial enlargement, moderate mitral regurgitaregurgitation and mild to moderate left ventricular dysfunction are also present. B) Three days later ECG typical ischemic changes with T wave inversion in leads V1 to V3 are seen together with echocardiographic complete restoring of normal wall motion in the apical segments and normalization of the left ventricular systolic function (EF >60%).
A 90-year-old woman affected by hypertension, hyper-colesterolemia, ischemic encephalopathy, and venous incompetence of lower limbs was admitted to the Internal Medicine Unit for severe asthenia and possible syncopal events without typical anginal pain. The electrocardiogram showed mild ST segment elevation limited to V1-V3. An echocardiographic examination performed on the same day showed akinesia of all the apical segments with extension to the mid anterolateral, mid inferolateral walls and mid anterior septum, left atrial enlargement, moderate mitral regurgitation, and mild to moderate left ventricular dysfunction (EF 37% in four-chamber view, 49% in the two-chamber view).
The patient was then admitted to the Coronary Unit and treated with a standard therapy scheme for acute coronary syndromes with enoxaparin, beta-blockers, aspirin, and transdermal nitrates.
The electrocardiogram showed typical ischemic changes with T wave inversion in leads V1 to V3. The T-troponin and CK-MB peaked respectively to 0.29 ng/mL (normal range <0.10 ng/mL) and 12 ng/mL (normal range <3 ng/ mL). On the third day, she underwent a new echocardio-graphic examination that showed complete restoring of normal wall motion in the apical segments with persistence of only mild hypokinesia limited to mid anterior septum and apical anterior wall, and normalization of the left ventricular systolic function (EF >60%). Moderate mitral regurgitation was also observed. The patient was transferred to the Cardiac Rehabilitation Unit without undergoing coronary angiography and with a therapeutic scheme consisting in beta-blockers, ACE inhibitor, amLodipine, and nitrates.
Discussion
Stress-related cardiomyopathies refer to transient left ventricular systolic dysfunction that can occur in different clinical settings.2-5 Precipitating factors include acute emotional stress,6,7 acute medical illness,8 surgical procedures and acute intracranial events.3,9-11 Diagnostic criteria have been proposed consisting in reversible left ventricular wall motion abnormalities together with transient electrocardiographic ST segment changes, in absence of obstructive coronary artery disease.12,13 Besides the typical apical and midventricular ballooning (Tako-Tsubo cardiomyopathy),2 morphological LV variants of stress cardiomyopathies have been described involving the midventricular or basal segments of the left ventricle (apical-sparing Tako-Tsubo cardiomyopathy).14-20
Since stress-related cardiomyopathy syndromes share symptoms and electrocardiographic changes with acute coronary syndromes,12,13,21 differential diagnosis is crucial. We believe that careful analysis of echocardiogram combined with electrocardiogram and serum biomarkers of myocardial injury (CK-MB and T-troponin) in a specific clinical setting may help in distinguishing the two forms, eventually facilitating the diagnosis and management.
In our report, typical anginal pain occurred in two patients (cases 1 and 2); in the remaining cases reversible left ventricular wall motion abnormalities were associated with carotid endarterectomy (case 3), pulmonary edema and cardiogenic shock (case 4) and severe asthenia (case 5). Apical left ventricular dysfunction was observed in three patients (cases 2, 3, 5), and was associated with mild ST segment elevation in anterior precordial leads mimicking anterior myocardial infarction. In all these cases, evolving electrocardiographic changes were recorded, showing T wave inversion in the precordial leads with later normalization; at this time the echocardiographic examination showed complete restoring of normal wall motion.
Isolated mid ventricular dysfunction was present in two patients (cases 1 and 4), in whom presentation ECG showed ST segment depression in the anterior precordial leads. Later ECG tracings showed typical T wave inversion in one case (case 1) and normalization in the other (case 4).
In four of the five patients (cases 1, 2, 3 and 5), the observed wall motion abnormalities found at echocardiographic examination were reversible during hospital stay; in the remaining case with persistent mid ventricular asynergic areas, high dose dobutamine echo-stress abolished wall motion abnormalities (case 4). One patient, after restoring normal wall motion, performed exercise echocardiography not revealing any worsening of the left ventricular kinesia (case 2). In both the patients subjected to stress echocardiography the coronary angiography was normal, confirming a high negative predictive value of the stress echocardiogram. Prolonged QT interval was observed in one patient (case 4) and elevation of cardiac T-troponin of mild degree was present in all patients.
Similar characteristics could be observed in all cases, that is: reversible left ventricular dysfunction at standard echo, ST segment transient abnormalities at ECG, mild increases of cardiac troponin. Thus, stress cardiomyopathies might share a common pathophysiological mechanism that is not necessarily related to a specific clinical setting. Proposed mechanisms for stress cardiomyopathies include ischemic myocardial stunning due to coronary spasm22,23 or acute microvascular dysfunction24,25 and/or cathecolamine-mediated direct myocardial injury.26-30 Coronary implication has not been documented during coronary angiography performed even in the presence of ST segment elevation; furthermore the unusual distribution of multiple asynergic areas at echocardiogram does not correspond with distribution of single or multiple coronary territories.31
However, as suggested by Angelini in his editorial comment on "Takotsubo behind the Octopus Trap"32 a credible cause of transient (and spontaneously resolving) segmental myocardial stunning remains coronary spasm; in the author's opinion not only the most typical apical form, but also the midventricular variety of Takotsubo is a manifestation of transient endothelial dysfunction.
Another proposed hypothesis considers the causative role of cathecolamine stimulation that could be responsible of direct myocardial injury through pathological calcium overload, which prolongs actin-myosin interaction resulting in reduced ATP generation:33 this injury may be confined to the subendocardial layers and since the endocardium is responsible for most of the wall thickening, akinesia will result in the damaged myocardial territory not necessarily corresponding to a specific coronary vessel distribution. To this regard, isolated mid ventricular dysfunction might reflect injury confined to the subendocardium. Apical sparing left ventricular dysfunction in our case report was associated with ST segment depression (cases 1 and 4).
Transmural extension of myocardial injury to the epicardial half of the myocardium may be associated with ST segment elevation; this pattern has been observed in classic Takotsubo cardiomyopathy with apical ballooning and was seen in three of the patients reported in this study (cases 2, 3 and 5). In stress cardiomyopathies, the ST segment elevation is of lesser magnitude than in anterior myocardial infarction perhaps reflecting only limited impairment of oxygen supply.
Contrary to stress cardiomyopathies, coronary occlusion in the context of an acute anterior myocardial infarction results in failure of oxygen supply, which increases end diastolic pressure: diastolic and systolic dys-function will increase resistance to coronary flow further compromising perfusion from the endocardium to the epicardium. The final result will be ST segment elevation of greater degree and irreversible apical left ventricular dysfunction if pharmacological or interventional therapy is not promptly administered.
Conclusions
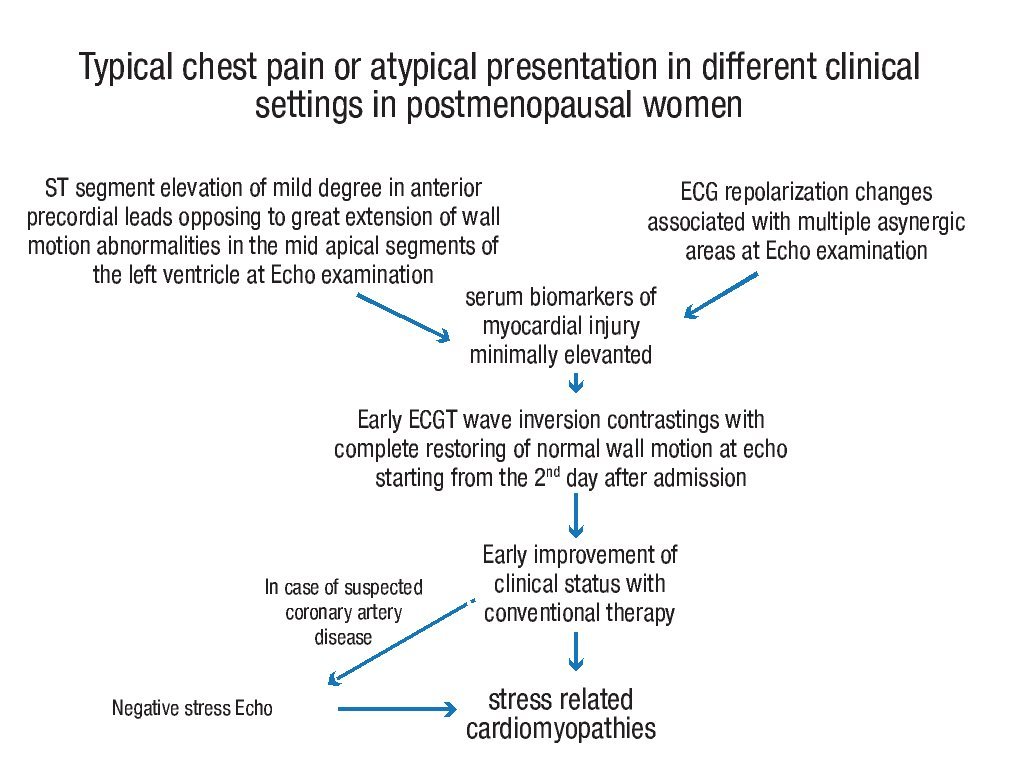
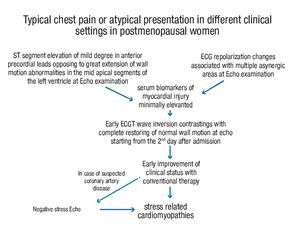
Although no ECG criteria have been identified discriminating stress cardiomyopathies from acute coronary syndromes (both STEMI and NSTEMI), careful echocardiographic wall motion analysis combined with electrocardiographic ST segment changes and serum markers of myocardial necrosis may help make early diagnosis of stress cardiomyopathies in different clinical settings, as follows: 1) electrocardiographic abnormalities at the time of presentation may be similar to those found in acute anterior myocardial infarction. In these cases, stress cardiomyopathies should be highly suspected in all postmenopausal women with ST segment elevation of mild degree in anterior precordial leads contrasting with the extent of wall motion abnormalities; serum biomarkers of cardiac injury will be usually minimally elevated; 2) in all the remaining atypical cases, the diagnosis should be suspected when mild ECG repolarization changes are associated with multiple asynergic areas at echo examination; 3) discrepancy between ST segment changes and wall motion analysis is further accentuated when time has elapsed since the acute presentation: T wave inversion is frequently seen together with restoring or great improvement of normal wall motion; 4) at this point, the echo stress may be proposed in those patients in whom some suspicion of coronary artery disease persists and may be an important tool for differential diagnosis from acute coronary syndromes (flow-chart for the diagnostic evaluation in stress related cardiomyopthies is reported in Figure 6).
Figure 6. Flowchart for the diagnosis of stress related cardiomyopathy in post-menopausal women.
In full agreement with the final considerations made by Angelini in his editorial comment,32 we also think that diagnosis of Takotsubo syndrome might be established on the basis of echocardiography and the clinical presentation; in doubtful cases, Angelini suggests computed axial tomo-graphic angiography that can rule out significant coronary occlusive disease. In our opinion, stress echocardiography may add information about the hemodynamic significance of coronary artery occlusive disease.
Study limitations
In the two oldest patients, coronary angiography was not performed (cases 3 and 5), so that uncertainty persists about real exclusion of atheromatous coronary disease; however both the patients had fast resolution of wall motion abnormalities that involved the mid apical segments of the left ventricle with an extension that was out of proportion to the ECG changes and to the slight increase of troponin and CKMB. In acute myocardial infarction, we do not expect resolution of wall motion abnormalities without coronary reperfusion by trombolysis or coronary angioplasty.
In the 49-year-old patient (case 2), coronary angiography should have been better performed immediately after the acute presentation since the ECG showed ST segment upsloping. Although we usually discourage postponing coronarography in similar cases, in our patient the ECG changes were of mild degree, no increase of troponin was observed at acute presentation and symptoms were promptly relieved by conventional antianginal therapy without further recurrence.
Lastly, some concerns might arise from the use of dobutamine in stress cardiomyopathies since a high cathecolaminergic state may be reproduced eventually bringing to a potentially harmful recurrence. Although
Mosley reported two cases displaying the typical features of Takotsubo cardiomyopathy during dobutamine infusion,34 other authors recently published their experience on dobutamine stress echocardiography for assessing the role of dynamic left ventricular obstruction in left ventricular ballooning syndrome in 22 patients with no major complication.35 In our study, echo stress with dobutamine was performed in a 70-year-old woman (case
4) with not complete recovery of regional function in basal conditions. The test was preferred to exercise for two reasons: patient inability to perform exercise and intention to demonstrate a biphasic response; the test showed persistent improvement of regional function without the development of a biphasic response (improvement at low doses followed by impairment in the mid segments at high doses). No obstruction of the left ventricular outflow tract was observed. The test was considered to be safe also because the patient was on beta blocker therapy.
Corresponding author: Marco Berti.
Via C. Poma,1. 20129 Milano, Italy.
E-mail:mao.marco@alice.it
Received on March 15, 2011;
accepted on September 1, 2011.