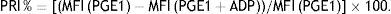Drug inhibition of platelet P2Y12 adenosine diphosphate receptor has reduced the incidence of adverse cardiovascular events after percutaneous coronary interventions. The analysis of the phosphorylation status of vasodilator-stimulated phosphoprotein by flow cytometry has shown a predictive value for adverse events and stent thrombosis. Polymorphisms of CYP2C19 in high risk patients may also relate to adverse cardiovascular events.
MethodsNinety patients were enrolled. Patients received a 600mg clopidogrel loading dose. Blood samples were obtained at the time of the procedure and 24h later, platelet reactivity was assessed by vasodilator-stimulated phosphoprotein phosphorylation measurement using flow cytometry. Low response to clopidogrel was defined as a platelet reactivity index≥50%. The presence of CYP2C19*2 was identified with the restriction enzyme SmaI.
ResultsMean platelet reactivity index: 53.45±22.48% in the baseline sample and 57.14±23.08% at 24h (p=0.183); 40% of patients behaved as good responders, the rest behaved as non-responders with 38% of patients showing platelet reactivity indexes between 50–70% and 22% showing indexes above 70%. The CYP2C19*2 polymorphism was found in 17% of patients, with a 3.9% AA homozygous genotype carriers.
ConclusionResponse to the clopidogrel loading dose showed a wide variability among patients with 40% responding to the drug according to previously established cut-off values. Our results showed that 3.9% of patients show the AA genotype. To our knowledge, this is the first study involving clopidogrel response by flow citometry and genotype typification in Mexican Mestizo population.
La inhibición del receptor plaquetario P2Y12 se ha asociado con reducción en incidencia de eventos cardiovasculares mayores en pacientes sometidos a intervenciones coronarias percutáneas. El estudio de la fosfoproteína estimulada por vasodilatadores mediante citometría de flujo tiene valor predictivo para desarrollo de eventos adversos y trombosis del stent. Los polimorfismos del CYP2C19 en pacientes de alto riesgo pueden también asociarse con eventos adversos.
Método90 pacientes, dosis de carga de clopidogrel: 600mg. Se obtuvieron muestras de sangre basales y post-24 horas. La reactividad plaquetaria se estudió mediante medición de fosfoproteína estimulada por vasodiatadores por citometría de flujo. Se consideró baja respuesta al clopidogrel un índice de reactividad plaquetaria ≥50%. La presencia del CYP2C19*2 se identificó con enzima de restricción SmaI.
ResultadosLa media del índice de reactividad plaquetaria fue: 53.45±22.48% en muestras basales y 57.14±23.08% a 24h (p=0.183); 40% de los pacientes repondieron a clopidogrel, el resto de comportó como no-respondedores, un 38%, mostró índices de reactividad plaquetaria entre 50 -70% y 22%, índices>70%. El polimorfismo CYP2C19*2 se encontró en 17% pacientes, con un 3.9% portadores de genotipo homozigótico AA.
ConclusionesLa respuesta a clopidogrel mostró amplia variabilidad entre pacientes, el 40% presentó respuesta de acuerdo con puntos de corte pre establecidos. Un 3.9% de los pacientes presentó genotipo AA. Consideramos que este es el primer estudio realizado en población mestizo-mexicana utilizado citometría de flujo para evaluar la respuesta a clopidogrel así como la tipificación genética de los pacientes.
Platelets play a central role in the pathogenesis of acute coronary syndromes. Plaque rupture precipitates both the activation and aggregation of platelets with the formation of a thrombus, but this is avoided in patients with acute coronary syndromes as well as in those undergoing percutaneous coronary intervention (PCI) by using antiplatelet drugs to prevent abrupt vessel occlusion. Current guidelines recommend dual therapy with aspirin and P2Y12 antagonists,1 and clopidogrel is the most widely used P2Y12 antagonist. Doses of up to 600mg clopidogrel prior to PCI result in lower platelet aggregation and P-selectin expression with reduced cardiovascular events in patients with non-ST elevation coronary syndromes.2 In recent years, however, resistance to aspirin or clopidogrel has gained importance,3 particularly the presence of a high inter-individual variability of response to the drug.4–9
The phosphorylation state of vasodilator-stimulated phosphoprotein (VASP) is a specific intracellular marker of residual P2Y12 receptor reactivity in patients treated with P2Y12 blockers, which is currently measured by flow cytometry and has also been correlated with ischemic risk.10,11 Unlike methods that include ADP-induced aggregation, the VASP phosphorylation assay does not include the contribution of the P2Y1 receptor to the overall response.10 The ratio of dephosphorylated and phosphorylated VASP is a specific measure of P2Y12 activity, which is expressed as the “platelet reactivity index” (PRI).
Genetic factors influence the absorption and/or the extent of metabolism of the prodrug clopidogrel to its active metabolite, and this contributes to the observed variability of response. To date, several polymorphisms have been related to high-dose treatment platelet reactivity, with the best established genetic factor being located within the CYP2C19 gene. A single nucleotide mutation (SNP) is associated with a reduction in clopidogrel metabolism and with a slow metabolism phenotype,12 although the frequency of slow metabolizers varies according to the study population. For example, in Asiatic populations, the frequency is as high as 13–23%, compared with 2–5% in Caucasians and 4–6% in African populations.13,14 The aim of this study was to evaluate platelet response to high-dose clopidogrel and to identify the presence of CYP2C19*2 carriers.
MethodsThis descriptive, cross-sectional, observational clinical study was approved by the Institutional Research and Ethics Committee, and all subjects were literate adults who signed an informed consent form. Ninety consecutive patients were enrolled in the study between 2009 and 2012. The cohort consisted of patients with a diagnosis of non-ST acute coronary syndromes, stable angina, or those with a positive ischemia detection test in whom coronary angiography was performed. Exclusion criteria were ST elevation coronary syndromes, use of proton pump inhibitors, hepatic disease, a contraindication to antiplatelet use, and hemorrhagic diathesis. All patients received a 100mg dose of aspirin at the time of the study; a 600mg clopidogrel loading dose was administered 6–8h prior to angiography in all cases. Blood samples were obtained from fasting patients. Basal samples were drawn once arterial access was obtained and a 6F sheath was placed via radial or femoral approach 6–8h after the clopidogrel loading dose. A second sample was obtained by venipuncture 24h after angiography. In all cases, 5mL of blood were discarded from each sample drawn to avoid platelet activation.
Angiography and PCIAll patients underwent a diagnostic angiography. Patients undergoing PCI received unfractionated heparin (70UI/kg). Stent placement was performed according to international guidelines,15 and drug-eluted stents or bare metal stents were implanted according to patient requirements. The sheath was removed and anticoagulation was stopped immediately at the end of the procedure in all cases. No GPIIb/IIIa inhibitors were used. A failed PCI was defined as a failure to obtain a residual stenosis <30% after stent placement, or a post-PCI anterograde TIMI 3 flow. All study subjects were followed up after 6 months.
VASP phosphorylation analysisThe VASP phosphorylation state was evaluated in all patients within 4h after blood sampling using a standardized flow cytometric assay. Blood samples were collected using 3.8% sodium citrate as an anticoagulant, then incubated with prostaglandin E1 (PGE1) alone or PGE1 plus ADP for 10min, and then fixed in paraformaldehyde for 5min. Cells were permeabilized and labeled with a specific primary monoclonal anti-VASP antibody (clone 16C2) followed by a secondary fluorescein isothiocyanate-labeled polyclonal goat anti-mouse antibody and a platelet reactive marked with PE (anti-CD61PE) according to the manufacturer's instructions of the VASP assay (PLATELET VASP/P2Y12 Biocytex, Marseille, France. The platelet mean fluorescence intensity (MFI) was determined using a flow cytometer (Beckman Coulter Epics Altra). The platelet population was identified by its distribution and at least 20,000 platelet events were analyzed. The platelet reactivity index (PRI) was then calculated from the MFI obtained in the presence of PGE1 alone (PGE1) or PGE1 and ADP simultaneously (PGE1+ADP):
The ratio is expressed as the percentage of mean platelet reactivity and is inversely proportional to the platelet inhibition obtained with clopidogrel.
Determination of polymorphismsGenomic DNA from anticoagulated blood samples was extracted using the phenol-chloroform method, the quality of the obtained DNA was assessed according to standard procedures. The reaction mixture to amplify the sequences of interest containing the CYP2C19*2 polymorphism consists of 25ng of DNA, 10mH pH 9.0 Tris–HCl, 1.0mH MgCl2, 0.2mM of each tryphosphate deoxynucleoside (dATP, dCTP, dGTP, dTTP), 0.5U of Platinum Taq DNA polymerase high fidelity (Gibco-BRL, Life Technologies, Carlsbsad, CA, USA) and 1μM of 5′-CAGAGCTTGGCATATTGTATC-3′ and 5′-GTAAACACACAAAACTAGTCAATG-3′ primers specific for the CYP2C19*2 polymorphism in a final 25μl volume.16 The amplification program was performed as follows: an initial denaturation at 94°C for 5min followed by 35 cycles (consisting of 45s at 94°C, 1min at 60°, and 1min at 72° by cycle) with a final extension of 72°C for 7min. The presence of CYP2C19*2 polymorphism was identified by digesting the amplification product of 316bp with the restriction enzyme Sma I followed by standard 2% agarose gel electrophoresis. We assayed all samples by duplicate. Negative controls were included with every set of amplifications. The presence of an intact 316-bp fragment revealed the AA polymorphism, while a 109- and 207-bp fragment together represented no such polymorphism (GG). In cases where all three fragments were obtained, the patients were classified as heterozygotes (GA). The first amplicons of the CYP2C19 gene positive for GG, AA or GA polymorphism obtained from the samples, were sequenced by Macrogen USA in a Life Tech's AB 3730XL DNA Sequencing analyzer. The sequences were compared in the Gen Bank by the Blast algorithm to found the presence of the SNP and were included as positive controls in subsequent set of amplifications and digestions.
Statistical analysisDescriptive statistical analyses were conducted using means and standard deviations for continuous variables. Percentages were applied to categorical variables. Variable distributions were analyzed using the Kolmogorov-Smirnov test, and discrete variables were analyzed with the χ2 test. A two-tailed Student's t-test was used for non-paired continuous variables. Significance was set at an alpha level of 0.05. Analysis was conducted using the statistical package SPSS 18 for Windows (SPSS Inc., Chicago, IL, USA).
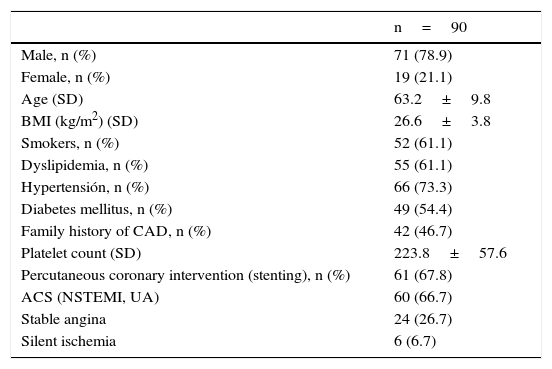
ResultsNinety patients were included in the study and their demographic, biological, and angiographic characteristics are summarized in Table 1. The mean age was 63.2 years, and 78.9% were male. As expected, there was a high incidence of cardiovascular risk factors, with 73.3% having hypertension and 54.4% having diabetes mellitus. The diagnosis on admission was non-ST elevation acute coronary syndromes in 60 individuals (66.7%), stable angina in 24 (26.7%), and silent ischemia in six cases (6.6%). PCI with stent placement was performed in 61 (67.8%) patients and drug-eluted stents were implanted in 36 (60%).
Baseline characteristics.
| n=90 | |
|---|---|
| Male, n (%) | 71 (78.9) |
| Female, n (%) | 19 (21.1) |
| Age (SD) | 63.2±9.8 |
| BMI (kg/m2) (SD) | 26.6±3.8 |
| Smokers, n (%) | 52 (61.1) |
| Dyslipidemia, n (%) | 55 (61.1) |
| Hypertensión, n (%) | 66 (73.3) |
| Diabetes mellitus, n (%) | 49 (54.4) |
| Family history of CAD, n (%) | 42 (46.7) |
| Platelet count (SD) | 223.8±57.6 |
| Percutaneous coronary intervention (stenting), n (%) | 61 (67.8) |
| ACS (NSTEMI, UA) | 60 (66.7) |
| Stable angina | 24 (26.7) |
| Silent ischemia | 6 (6.7) |
ACS=acute coronary syndromes, NSTEMI=non ST elevation myocardial infarction, UA=unstable angina.
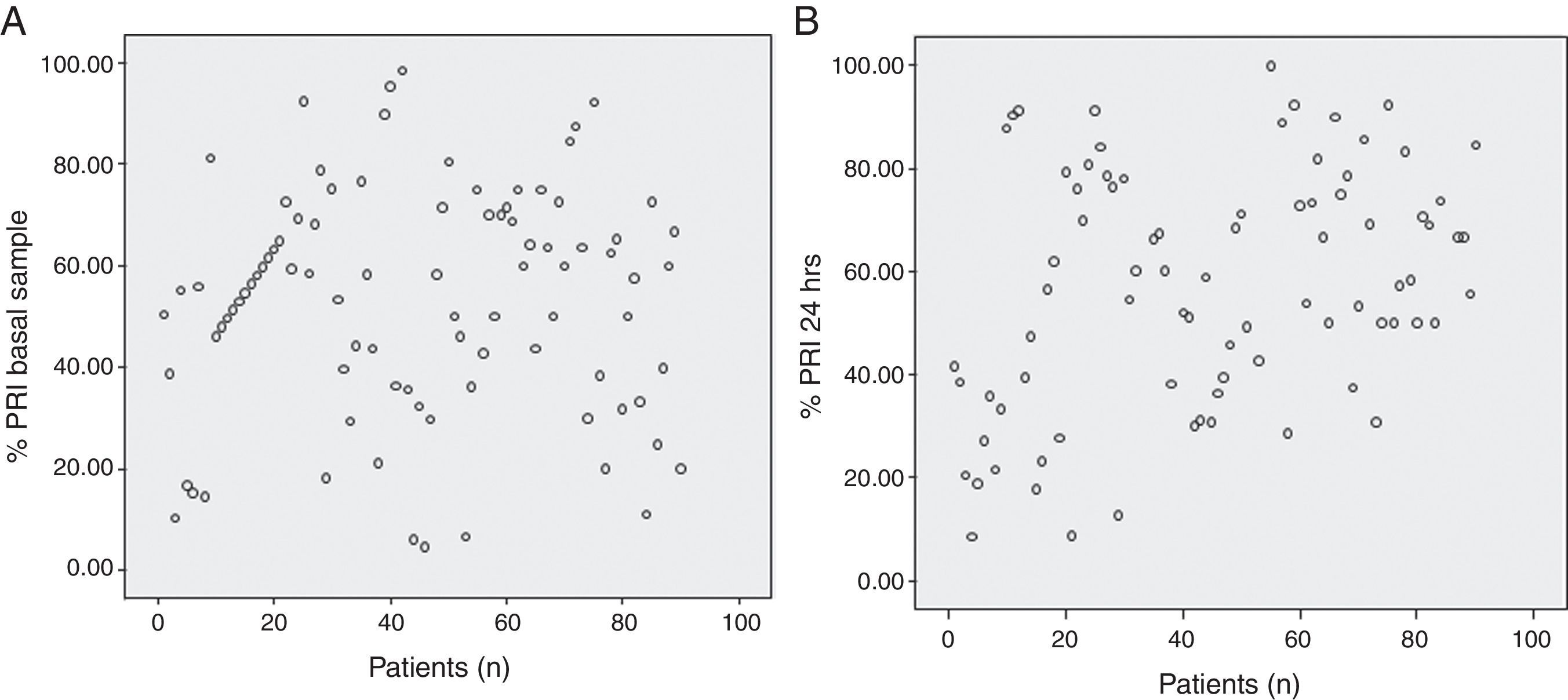
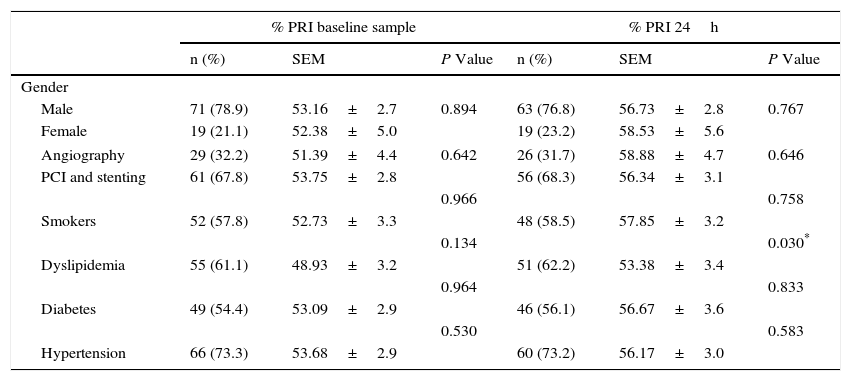
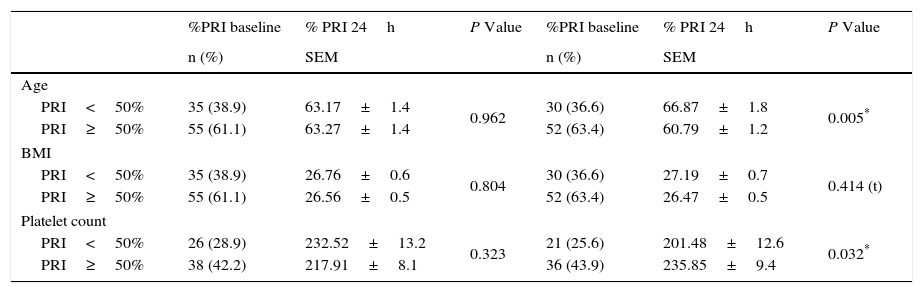
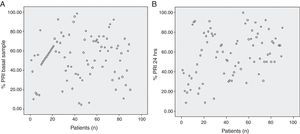
We analyzed platelet function using flow cytometric analysis of VASP phosphorylation in samples 6–8h after a 600-mg bolus of clopidogrel was administered and 24h after the angiographic procedure. The mean PRI value was 53.45±22.48% in the baseline sample and 57.14±23.08% at 24h (p=0.183), with a large inter-individual variability observed in the biological response to clopidogrel (Fig. 1, Panels A and B). At 24h, patients with dyslipidemia had a significantly better response to clopidogrel (p=0.030), this was also found in older patients (p=0.050). Lower platelet counts were also associated with a better response to the drug at 24h (p=0.032). No significant results were associated with other variables including body mass index and diabetes (Tables 2 and 3). No hemorrhagic complications were reported.
Patient distribution according to VASP-PRI results (<50% and >50%) baseline and 24h samples.
| % PRI baseline sample | % PRI 24h | |||||
|---|---|---|---|---|---|---|
| n (%) | SEM | P Value | n (%) | SEM | P Value | |
| Gender | ||||||
| Male | 71 (78.9) | 53.16±2.7 | 0.894 | 63 (76.8) | 56.73±2.8 | 0.767 |
| Female | 19 (21.1) | 52.38±5.0 | 19 (23.2) | 58.53±5.6 | ||
| Angiography | 29 (32.2) | 51.39±4.4 | 0.642 | 26 (31.7) | 58.88±4.7 | 0.646 |
| PCI and stenting | 61 (67.8) | 53.75±2.8 | 56 (68.3) | 56.34±3.1 | ||
| 0.966 | 0.758 | |||||
| Smokers | 52 (57.8) | 52.73±3.3 | 48 (58.5) | 57.85±3.2 | ||
| 0.134 | 0.030* | |||||
| Dyslipidemia | 55 (61.1) | 48.93±3.2 | 51 (62.2) | 53.38±3.4 | ||
| 0.964 | 0.833 | |||||
| Diabetes | 49 (54.4) | 53.09±2.9 | 46 (56.1) | 56.67±3.6 | ||
| 0.530 | 0.583 | |||||
| Hypertension | 66 (73.3) | 53.68±2.9 | 60 (73.2) | 56.17±3.0 | ||
SEM=standard error.
VASP-PRI and mean values, age, bodie mass index and platelet count baseline and 24h samples.
| %PRI baseline | % PRI 24h | P Value | %PRI baseline | % PRI 24h | P Value | |
|---|---|---|---|---|---|---|
| n (%) | SEM | n (%) | SEM | |||
| Age | ||||||
| PRI<50% | 35 (38.9) | 63.17±1.4 | 0.962 | 30 (36.6) | 66.87±1.8 | 0.005* |
| PRI≥50% | 55 (61.1) | 63.27±1.4 | 52 (63.4) | 60.79±1.2 | ||
| BMI | ||||||
| PRI<50% | 35 (38.9) | 26.76±0.6 | 0.804 | 30 (36.6) | 27.19±0.7 | 0.414 (t) |
| PRI≥50% | 55 (61.1) | 26.56±0.5 | 52 (63.4) | 26.47±0.5 | ||
| Platelet count | ||||||
| PRI<50% | 26 (28.9) | 232.52±13.2 | 0.323 | 21 (25.6) | 201.48±12.6 | 0.032* |
| PRI≥50% | 38 (42.2) | 217.91±8.1 | 36 (43.9) | 235.85±9.4 | ||
SEM=standard error.
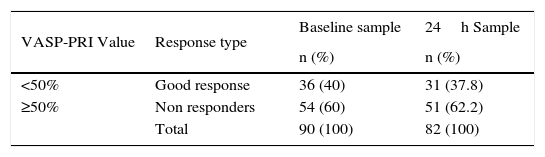
We found that 36 patients (40%) were good responders, with a VASP-PRI<50% in the baseline sample and 31 (37.8%) at 24h (p=NS); a VASP-PRI greater than 50% (non-responders) was observed in the remaining patients (Table 4). VAPS-PRI results were further divided into three groups as follows: group 1, VASP-PRI<50%; group 2, VASP-PRI 50–70%; group 3, VASP-PRI>70%. According to this as stated above, 40% of patients were good responders (group 1), the rest of the patients behaved as non-responders, 38% of them had a VASP-PRI 50–70% and 22% had VASP-PRI values greater than 70% (group 3). Dyslipidemia was associated with a significantly better response to the drug at 24h (p=0.017).
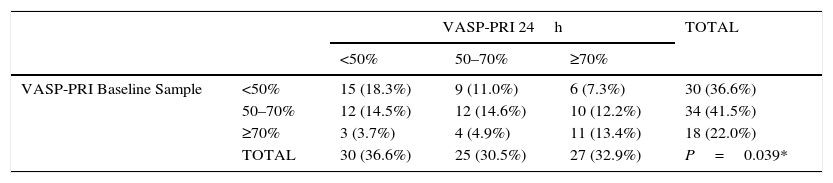
A change of status of clopidogrel response among the three groups was observed at 24h; nine patients (11%) considered to have a good response in the first sample changed to group 2 (VASP-PRI 50–70%) and six (7.3%) moved to group 3 (VASP-PRI>70%). In group 2 (VASP-PRI 50–70%), 12 patients (14.5%) were good responders at 24h and nearly half of these (12.2%) had VASP-PRI values greater than 70%. Finally, in group 3 (VASP-PRI >70%), only three individuals (3.7%) migrated into group 1 (VASP-PRI <50%) and four (4.9%) into group 2 (VASP-PRI 50–70%). These findings did not follow a normal variation and were statistically significant (p=0.0039; Table 5). Of all patients included in the present study, major adverse cardiac events (MACE) were only observed in four.
Patient distribution on three groups baseline and 24h samples.
| VASP-PRI 24h | TOTAL | ||||
|---|---|---|---|---|---|
| <50% | 50–70% | ≥70% | |||
| VASP-PRI Baseline Sample | <50% | 15 (18.3%) | 9 (11.0%) | 6 (7.3%) | 30 (36.6%) |
| 50–70% | 12 (14.5%) | 12 (14.6%) | 10 (12.2%) | 34 (41.5%) | |
| ≥70% | 3 (3.7%) | 4 (4.9%) | 11 (13.4%) | 18 (22.0%) | |
| TOTAL | 30 (36.6%) | 25 (30.5%) | 27 (32.9%) | P=0.039* | |
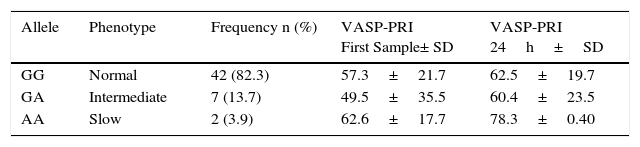
DNA was obtained from 51 of the 90 patients, nine (17%) of whom were found to carry the CYP2C19*2 polymorphism. Of these, seven (13.7%) were heterozygotes (GA) and two (3.9%) were homozygous (AA); the remaining 42 patients (82.4%) had the wild-type genotype (GG).
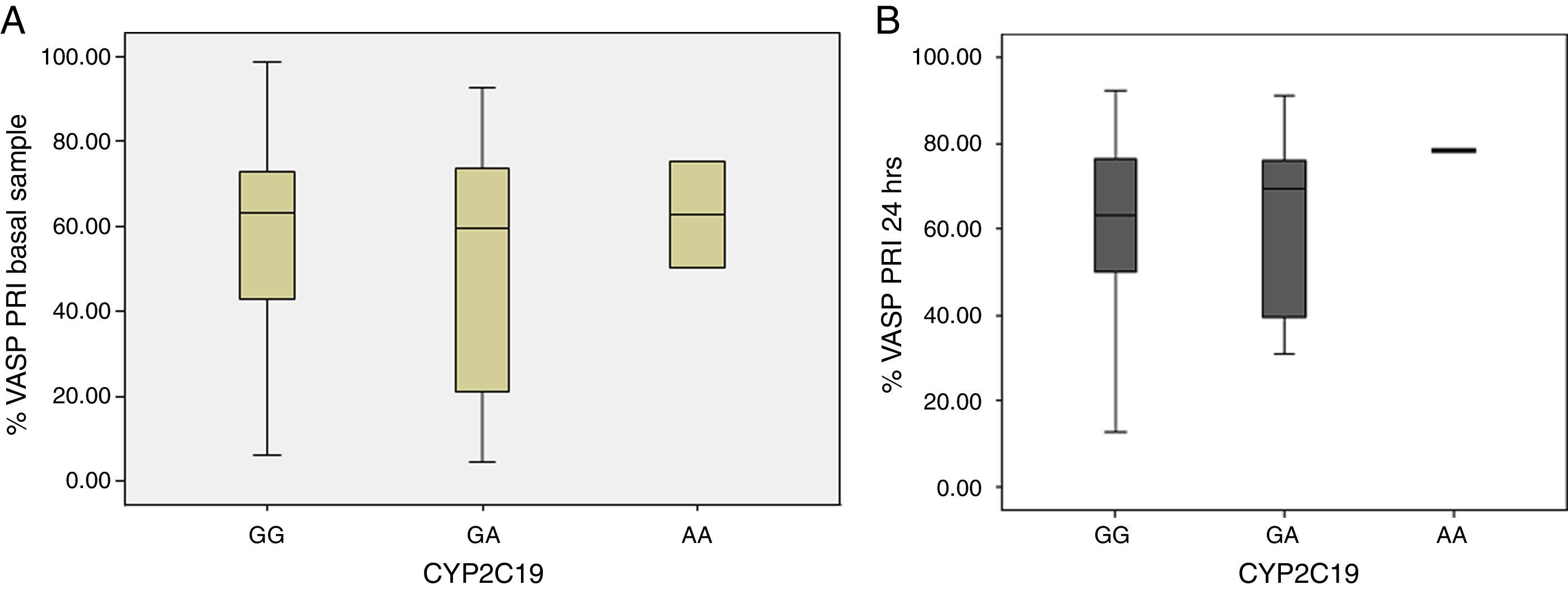
When VASP-PRI results were analyzed in this group of patients, we found that the homozygous genotype had a mean VASP-PRI of 62.6% in the baseline sample and a VASP-PRI of 78.3% at 24h (non-responders); these patients did not present MACE at follow-up (Table 6). Heterozygote patients (AG) had a mean VASP-PRI of 49.5% in the baseline sample and 60.4% at 24h; finally, patients with the GG genotype showed VASP-PRI mean values of 57.3% and 62.5% in the baseline sample and 24h, respectively (p=NS) (Fig. 2, Panels A and B).
The present study was conducted in Mexican Mestizo patients in the central region of Mexico. This population is widely distributed across the country and represents about ninety three percent of the total population, and it is also present in the United States of America, where about the 66 percent of the Hispanic population is from Mexican descent. In Mexico, coronary artery disease is the second cause of death according to national statistics. To date, in this country clopidogrel is the antiplatelet drug must widely used in spite of the newer and more potent antiplatelet drugs. Our results are consistent with several publications that showed a large inter-individual variability. In a previous prospective study of patients undergoing successful coronary artery stenting, a persistent increase in platelet reactivity measured by conventional aggregometry following a 300mg clopidogrel loading dose was demonstrated in some patients, thus a higher loading dose was suggested.7 Similarly, enhanced platelet inhibition was observed during the post-stent period after a 300mg loading dose was given 3–24h prior to stenting, compared with a 75mg dose given at the time of the procedure.8 Studies have also shown that doses of clopidogrel up to 600mg enable a more potent antiplatelet effect to be achieved.17
Moreover, several studies have suggested a link between a low clopidogrel response or persistence of high platelet reactivity after treatment, as assessed by platelet assays, and post-PCI thrombotic events.18,19 These findings have determined a threshold of platelet reactivity that can be used to predict thrombotic events.20,21 Prospective studies reported that platelet reactivity above 50% according to the VASP index is associated with MACE after PCI and with stent thrombosis.22 Furthermore, recent trials demonstrated that increased platelet reactivity inhibition results in reduced MACE.23 Bonello et al.24 investigated the impact of a tailored clopidogrel loading dose according to platelet reactivity monitoring for stent thrombosis in patients undergoing nonemergency PCI, and observed a decrease in the primary endpoint without major bleeding complications. Although 60% of our patients presented a VASP-PRI value>50%, we found no significant association with MACE, this could be related to the small sample size and also the percentage of PCI and stenting performed.
It is important to note that within groups of responders and non-responders, some patients demonstrated an appropriate response to clopidogrel in the first sample but showed an increased reactivity at 24h in the present study. Similarly, Gurbel et al.25 found that ∼30% of patients were resistant to clopidogrel on days 1 and 5 post-stenting, and 15% were resistant at day 30. Based on these observations, they hypothesized that clopidogrel resistance was related to insufficient active metabolite generation following a 300mg load and a 75mg maintenance dose in selected patients. Further studies were conducted using a 600mg loading dose, and higher platelet inhibition was observed at 24h compared with patients receiving 300mg. However, in the present study, we found that nearly 30% of patients had a VASP-PRI >50% at 24h despite receiving a 600mg loading dose; moreover, patients considered to be good responders at the first sample changed their status to non-responders. This could be related to factors involving clopidogrel pharmacokinetics and deserve further study.
Our results showed that 3.9% of patients had the AA genotype, which is higher than the previously reported frequency of 0–1.45% in the Mexican Mestizo groups.26 Although AA frequencies of 3.2% and 1.1% were reported in Mexican-Americans and in Colombian Mestizo individuals, respectively, neither of these studies investigated clopidogrel response.27,28 The absence of the polymorphism in the present study was not related to lower PRI values; indeed, high platelet reactivity was also found in such individuals, suggesting that the inter-individual response to clopidogrel must be influenced by other factors.
Hulot29 previously showed that the presence of the CYP2C19*2 polymorphism was associated with a reduction in clopidogrel metabolism leading to a reduction in its antiplatelet effect. In the present study, homozygous (AA) patients showed higher, albeit non-significant, PRI values compared with heterozygote and wild-type patients. The presence of the CYP2C19*2 SNP in both alleles strongly suggests a poor metabolizer phenotype with reduced clopidogrel response. Heterozygote patients showed a variable behavior, which could be related to the expression of the alleles involved. Some studies not only observed the negative biological effects of the polymorphism but also its association with MACE in patients with PCI and stenting.30 However, the presence of the CYP2C19*2 allele accounts for only 5–15%31 of clopidogrel response heterogeneity; moreover, the meta-analysis by Holmes32 concluded that there is no clinically significant association of the CYP2C19*2 genotype with cardiovascular events. The patients that presented with the AA polymorphism in our study did not develop MACE at the follow-up despite higher levels of VASP-PRI.
There are several limitations to our study. First, the sample size is small, which reflects the limited number of patients who agreed to participate, however previous studies have been conducted with a similar number of patients.7,17 Second, the patient sample is a heterogeneous mix of those with coronary artery disease, even though we aimed to evaluate the response to a 600mg dose of clopidogrel in all clinical scenarios. Not every patient in the study received a stent and 40% were treated with bare metal stents; such patients may show an improved response to clopidogrel. Third, although VASP analysis is widely accepted, it is an expensive test requiring trained personnel and a flow cytometer, which may limit its widespread use in clinical practice. In conclusion, the response to a 600mg loading dose of clopidogrel showed a wide variability in patients, with 40% responding to the drug according to previously established VASP-PRI cut-off values. Patients that initially responded to this loading dose showed a worsening of such response in the next 24h, although no adverse cardiovascular events were related to this behavior. Of the 51 patients in whom genetic testing was performed, CYP2C19*2 was present in 17%. These data are similar to frequencies found in other populations and ethnic groups, but higher than the one reported in a similar population in Mexico. To our knowledge, this is the first study to evaluate the clopidogrel response using VASP-PRI analysis and correlation with CYP2C19 in Mexican mestizo population.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingAll of the authors acknowledge that this project was financed by the CONACYT grant: CONACYT.SSA.IMSS.ISSSTE S008-2010-142034.
Conflict of interestDr. Carlos Areán has served as a consultant and/or speaker for Bristol Myers Squibb, MSD, Elly-Lilly and Bayer receiving less than 3000 USD honorarium. The rest of the authors declare having no competing interests.
We would like to thank the “Hospital General Dr. Miguel Silva” Cath Lab personnel for their support.