Transcatheter aortic valve implantation (TAVI) has emerged as an effective option for the treatment of high-risk patients with native aortic stenosis.1 Furthermore, there has been a rapid expansion of TAVI toward a larger spectrum of patients, such as those with degenerative surgical bioprosthesis.2 While the procedure is successful in most cases, some life-threatening complications such as coronary obstruction still remain.3,4 Anatomical factors such as low lying coronary ostia and shallow sinus of Valsalva have been associated with a higher risk for coronary obstruction,3 but no specific preventive measure has been established to date for this life-threatening complication. To this effect, we describe the case of a patient considered at high surgical risk for conventional aortic valve replacement in whom TAVI was carried-out. Due to high-risk features for coronary obstruction we decided to protect the left main coronary artery with a coronary guidewire prior to valve implantation.
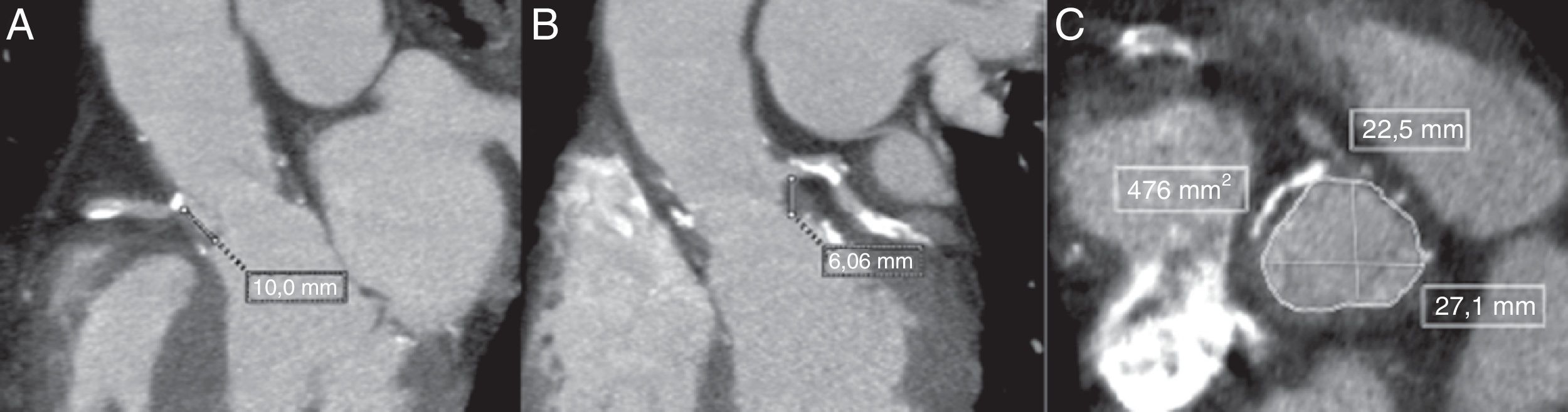
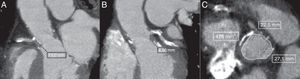
An 80-year-old frail male presented to the ER with rapidly progressive dyspnea (NYHA class III–IV) and chest-pain. He had surgical aortic valve replacement with a 23-mm Freestyle stentless bioprosthetic valve performed 14 years earlier with concomitant coronary artery bypass graft (CABG). An echocardiogram showed a mild stenosis of the bioprosthesis (peak gradient: 35mmHg; mean gradient: 15mmHg; aortic valve area: 0.96cm2), severe regurgitation due to leaflet rupture and reduced left ventricular ejection fraction (currently 35 vs. 50% 6 months earlier). A coronary angiography showed a severe lesion in the proximal left anterior descending artery (LAD). Due to the high-risk profile of the patient (logistic EuroSCORE: 20%; STS-PROM: 10%), the Heart Team opted for TAVI treatment, and the treatment of the LAD stenosis with a drug-eluting stent was successfully performed before the TAVI procedure. Angiographic computed tomography prior to TAVI showed a sinus of Valsalva diameter of 28mm and height of the RCA and left main (LM) of 10 and 8mm, respectively (Fig. 1). Taking into consideration these high-risk anatomical characteristics and the presence of a previous stentless bioprosthesis, we decided to perform the TAVI with left main guidewire protection.
Multi-detector computed tomography (MDCT) evaluation pre-TAVI showing the measurements in the long-axis view with a height of 10mm for the right coronary artery (A) and 8.06mm for the left coronary artery (B). Measurements of the sinus of Valsalva were obtained from the mean of the maximum and minimum cross-sectional diameters in the short-axis view (C).
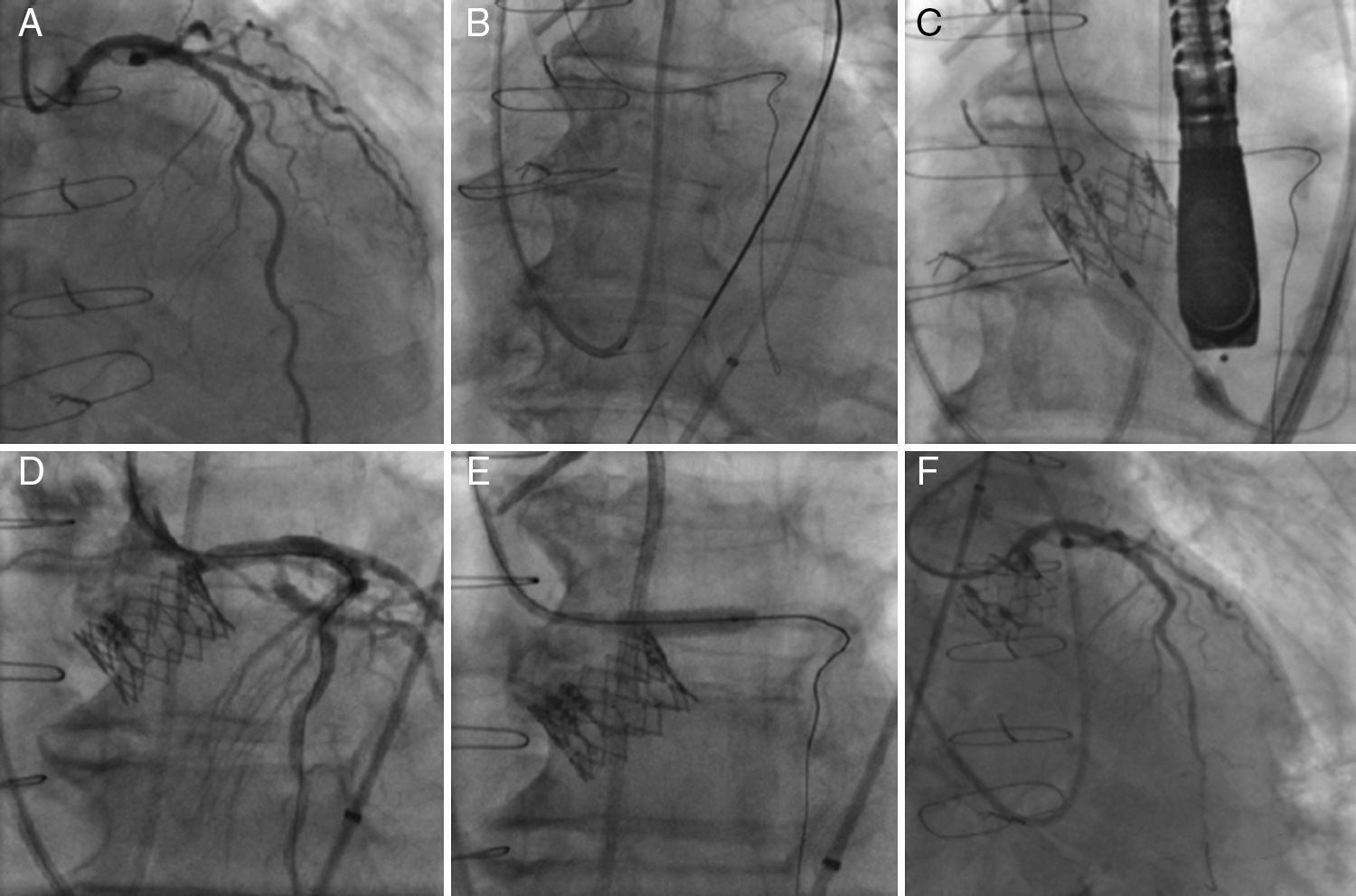
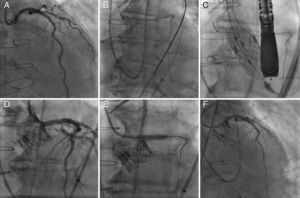
The TAVI procedure was performed through transfemoral approach, under general anesthesia, with fluoroscopy and echocardiographic guidance. Before any maneuver at the level of the aortic valve, an extra-support Wiggle guidewire (Abbott Vascular, Santa Clara, CA, USA) was placed in the distal LAD (Fig. 2). The deployment of a 23-mm SAPIEN XT valve (Edwards Lifesciences Inc., Irvine, CA, USA) valve was performed under rapid pacing with the slow inflation technique,5 and with the valve slightly more ventricular (Fig. 2). Immediately after valve deployment the patient presented ST-segment elevation and severe persistent hypotension. Using a DOC extension to the Wiggle guidewire, an Extra BackUP 6Fr guiding catheter was advanced and the contrast injection showed obstruction of the LM ostium (Fig. 2). A pre-dilatation with a 4.0×12mm balloon (Sprinter Legend RX-Medtronic, Minneapolis, MN, USA) restored the coronary flow and pressures, followed by a Promus Element stent (Boston Scientific, Natick, MA, USA) 4.0×12mm implantation in the LM, partially protruding into the aorta. A final angiogram showed (Fig. 2) no significant residual coronary stenosis and coronary flow TIMI 3. Following the procedure, the patient had an excellent recovery and was discharged four days later. At 6-month follow-up the patient was in NYHA class I, with a normofunctioning valve (mean gradient 20mmHg, valve area of 1.1cm2, and mild paravalvular leak), and left ventricle ejection fraction of 55%. Cardiac CT demonstrated the permeability of the coronary stent with a good position of the SAPIEN XT valve.
Left coronary artery (LCA) angiography before deployment of the transcatheter valve (A). LCA protection with an extra-support Wiggle guidewire™ (B). Image showing the deployment of the transcatheter valve (C). Angiography showing partial ostial obstruction of the LCA after the transcatheter valve implantation (D). Pre-dilatation of the left main coronary artery with a 4.0×12mm balloon (E). Final angiography after the successful deployment of a drug-eluting stent into the ostium of the left main coronary artery (F).
Coronary obstruction following TAVI presents a high mortality rate (∼50%),3,4 and is usually caused by the displacement of an aortic valve leaflet toward the coronary ostium, with an incidence of up to 3.5% in the context of TAVI in patients with prior surgical bioprosthesis (“valve-in-valve” – ViV-TAVI).2
The presence of low-lying coronary ostia and shallow sinus of Valsalva were identified as potential risk factors for this complication in our case.3 The cutoffs determined by computed tomography, as of increased risk, are a LM height<12mm and a sinus of Valsalva less<30mm.4 Severe persistent hypotension, which is present in ∼70% of patients, and ST-segment changes immediately post-TAVI may establish the diagnosis in some cases.3,4 It has been shown that either crossing the obstruction with the guidewire and/or advancing a stent through the guidewire may be challenging in such cases.3,4 It has been therefore suggested that leaving a preventive stent in the coronary, together with the guidewire, might potentially avoid the difficulty in crossing with the stent throughout the valve stent frame.6
In conclusion, coronary obstruction following TAVI, although rare, is a potential fatal complication. Some clinical and anatomical characteristics may determine a higher risk for its occurrence. In such patients, the preventive placement of a coronary guidewire may be advisable to promptly depict this complication and proceed with percutaneous coronary intervention. Future studies, with a larger number of patients at risk may confirm if this maneuver should be more widely recommended for patients undergoing TAVI with high-risk features for this complication.
FundingH.B.R. is supported by a research PhD grant from “CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico – Brasil (246860/2012-0)”. The other authors declare not receiving any funding for this study.
Conflict of interestDr. Josep Rodés-Cabau is a consultant for Edwards Lifesciences and St-Jude Medical. The rest of the authors have no conflict of interest to disclose.