To evaluate intra-procedural imaging with transesophageal echocardiography and angiography during left atrial appendage occlusion using the Amplatzer™ Cardiac Plug with regard to sizing and final device shape.
MethodsLeft atrial appendage ostium dimensions and diameter at a depth of 10mm from the ostium were measured by transesophageal echocardiography (0–180°) and angiography (RAO 30° – Cranial 20°) in consecutive patients undergoing left atrial appendage occlusion using the ACP with an oversizing strategy of 10–20% relative to the baseline measurements. After delivery, ACP dimensions were measured and device shape was assessed.
ResultsTwenty-seven consecutive patients underwent successful uncomplicated left atrial appendage closure with Amplatzer™ Cardiac Plug. We found a significant difference between the largest and smallest left atrial appendage diameter measured with transesophageal echocardiography (22.3±4.2 vs. 18.1±4.1mm, p<0.001). By the end of the procedure (by angiography), ACP had an optimal shape in 17 patients (63%), a strawberry-like shape in 7 patients (26%), and a square-like shape in 3 patients (11%). ACP was oversized on average by 1.5±2.7 and 3.3±2.3mm compared to transesophageal echocardiography and angiography, respectively. The final shape of the device was not significantly associated with the degree of oversizing.
ConclusionsWe found a considerable variability in the assessment of the left atrial appendage, using transesophageal echocardiography and angiography. The degree of Amplatzer™ Cardiac Plug expansion within the left atrial appendage and the final shape of the device were not associated with the degree of oversizing.
Evaluar las dimensiones de la orejuela izquierda antes del cierre percutáneo y la correlación de sus dimensiones finales y la forma del dispositivo Amplatzer™ cardiac plug con ecocardiografía transesofágica y angiografía.
MétodosSe midieron las dimensiones de la orejuela izquierda a una distancia de 10mm a partir del ostium con ecocardiografía transesofágica (0 a 180°) y angiografía (RAO 30° Craneal 20°). Se utilizó una estrategia para sobre dimensionar el tamaño del dispositivo del 10 al 20% con respecto a las mediciones iniciales. Se evaluaron las dimensiones y la forma final del dispositivo.
ResultadosSe realizó el procedimiento en 27 pacientes. Se encontró una diferencia significativa entre el diámetro mayor y menor de orejuela izquierda medido por ecocardiografía transesofágica (22.3±4.2 vs 18.1±4.1mm, p<0.001). Una vez liberado el dispositivo, se encontró que en 17 pacientes (63%) adoptó una forma óptima, de “fresa” en 7 (26%) y cuadrada en 3 (11%). El tamaño del dispositivo seleccionado se sobre dimensionó en promedio 1.5±2.7mm con la ecocardiografía transesofágica y 3.3±2.3mm con la angiografía. La forma final del dispositivo no se asoció de manera significativa con el grado de sobre dimensionamiento del mismo.
ConclusionesExiste variabilidad considerable en la evaluación de la orejuela izquierda entre la ecocardiografía transesofágica y la angiografía. No se encontró asociación entre el grado de expansión del dispositivo dentro de la orejuela izquierda ni de su forma final con el grado de sobre dimensionamiento del mismo.
Cardiac embolism due to atrial fibrillation (AF) is responsible for 25% of ischemic strokes.1,2 More than 90% of cardiac emboli originate from the left atrial appendage (LAA).3 Oral anticoagulation therapy is the standard of care for AF-related stroke prevention.4 However, this therapy is commonly underused and poorly controlled and it carries a significant risk for bleeding complications.4,5 An alternative to anticoagulation is surgical ligation of the LAA,6 but it is highly invasive and not associated with predictable results and clinical outcome.7 Percutaneous LAA occlusion by device implantation has recently been developed, showing promising results using the WATCHMAN system (Atritech-Boston Scientific, Plymouth, MN) in a randomized multicentre trial.8 Technical feasibility and procedural safety have been shown with the Amplatzer™ Cardiac Plug (AGA-St-Jude, Minneapolis, MN) (ACP).9
Percutaneous LAA occlusion can be performed under fluoroscopic and transesophageal echocardiographic (TEE) guidance. Anatomical studies have demonstrated considerable variability in the shape and size of the LAA.10,11 The assessment of LAA dimensions and particularly, proper sizing of the LAA occlusion device can be challenging. With respect to the ACP, there are 2 important anatomical landmarks to consider; the proximal “ostium” (i.e. the orifice of the LAA where the occluding disk of the ACP deploys) and the distal “neck” located at a depth of 10mm from the LAA “ostium” (i.e. the landing zone of the lobe of the ACP).
The implanted ACP can adopt 3 different shapes9 based on its degree of expansion: (1) “strawberry”, (2) “tire-like”, and (3) “square” shape. The “tire-like” shape is considered as optimal as the ACP lobe is only slightly deformed (i.e. slightly under-expanded) by the LAA walls. The “strawberry” shape corresponds to excessive under-expansion (i.e. an oversized ACP), whereas the “square” shape corresponds to over-expansion of the ACP lobe (minimal deformation; i.e. undersized). Intuitively, adequate assessment of the LAA dimensions, correct sizing of the device and objective evaluation of the device's expansion within the LAA are expected to be important factors in achieving a favorable, uneventful percutaneous LAA occlusion procedure. Therefore, we sought to evaluate and compare intra-procedural imaging with TEE and angiography during LAA occlusion procedures using the ACP device with respect to sizing and final shape of the device.
MethodsPatients who underwent percutaneous LAA closure with the ACP at our institution between November 2009 and November 2011 were prospectively included in this study. All patients were more than 18 years of age, with persistent or permanent AF with high risk for stroke (CHADS2 score ≥2), and at least one contraindication to long-term anticoagulant therapy. Patients with LAA thrombus, mobile aortic atheroma or symptomatic carotid artery disease were excluded. All patients provided written informed consent before the procedure. The study was approved by the institutional internal review board.
Procedures were performed under general anesthesia, with simultaneous fluoroscopic and TEE guidance (iE33 ultrasound system and X7-2t matrix array transducer, Philips Healthcare, Andover, MA, USA and Vivid 7 ultrasound system, 5.0MHz transducer, General Electric, Horten, Norway). In order to achieve optimal LAA expansion during the procedure, a bolus of 500cm3N/S 0.9% was given aiming for a left atrial pressure of at least 12mmHg.
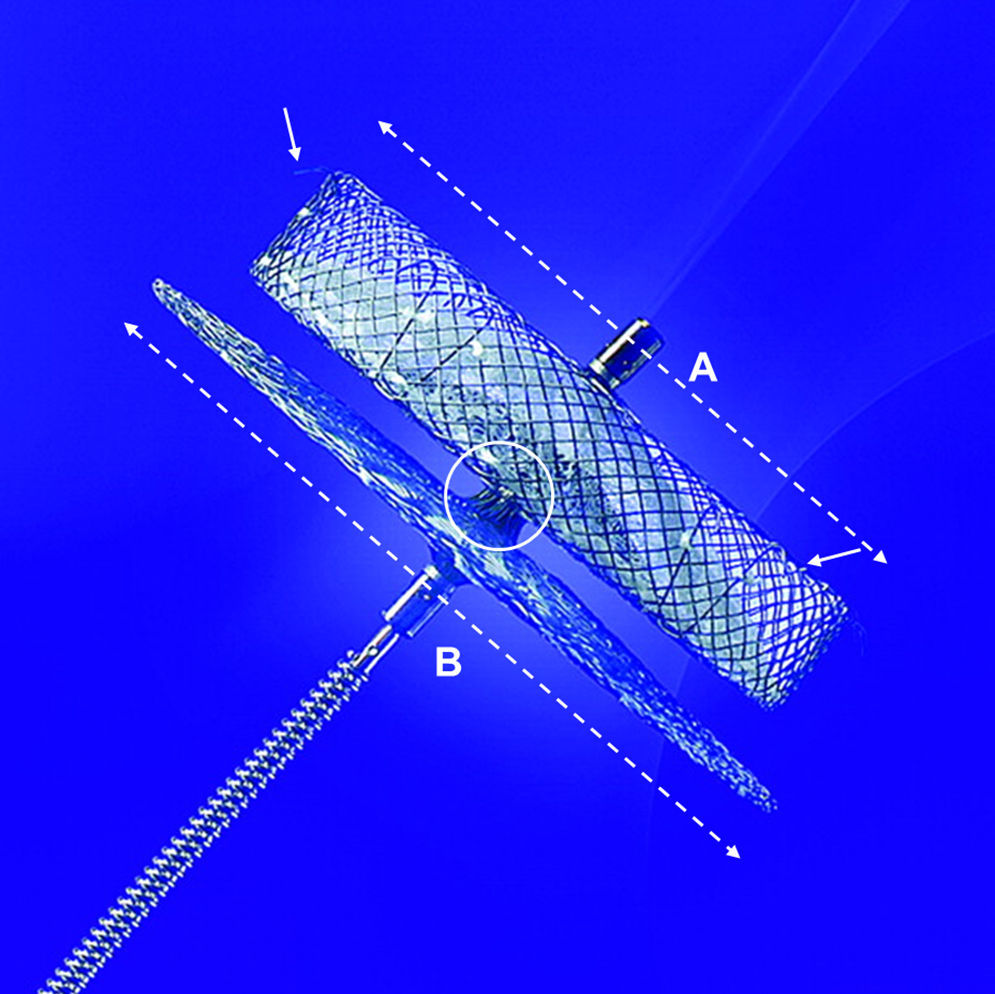
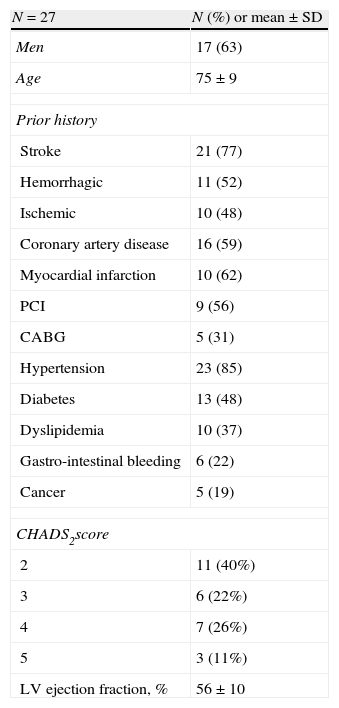
Amplatzer™ cardiac plug (ACP) designThe ACP is a self-expanding device, specifically designed for LAA closure (Fig. 1). It consists of a distal lobe and a proximal disk connected by a short waist, made of a nitinol mesh with two polyester patches sewn on the lobe and the disk. The lobe has up to six additional stabilizing wires (i.e. miniature teeth) to assure retention. The ACP is available in lobe diameter sizes ranging from 16 to 30mm (in 2mm size increments), with a 6.5mm fixed lobe length irrespective of the lobe width. The disk is 4 and 6mm larger than the lobe for sizes ranging between 16–22mm and 24–30mm, respectively. The total device length is 10mm. The lobe adapts to the inner LAA walls at a depth of approximately 10mm from the ostium, and the disk seals the LAA orifice in a way that has been termed the “pacifier effect”, which is self-explanatory.9 The articulating waist allows self-orientation of the device, adjusting to the LAA anatomy. The ACP is implanted using a trans-septal approach via the femoral vein,9–13 and is fully retrievable and repositionable. Radio-opaque markers at each end of the device facilitate fluoroscopic positioning.
The Amplatzer™ Cardiac Plug. The Amplatzer™ Cardiac Plug consists of a distal lobe (broken white line A) and a proximal disk (broken white line B) which are connected by a short articulating waist (white circle). The lobe has up to six additional stabilizing wires (white arrows) to assure device retention. The A/B ratio is unique for each device (e.g. for a 20mm lobe, the A/B ratio is 20/24=0.833, and for a 26mm lobe, the A/B ratio is 26/32=0.8125).
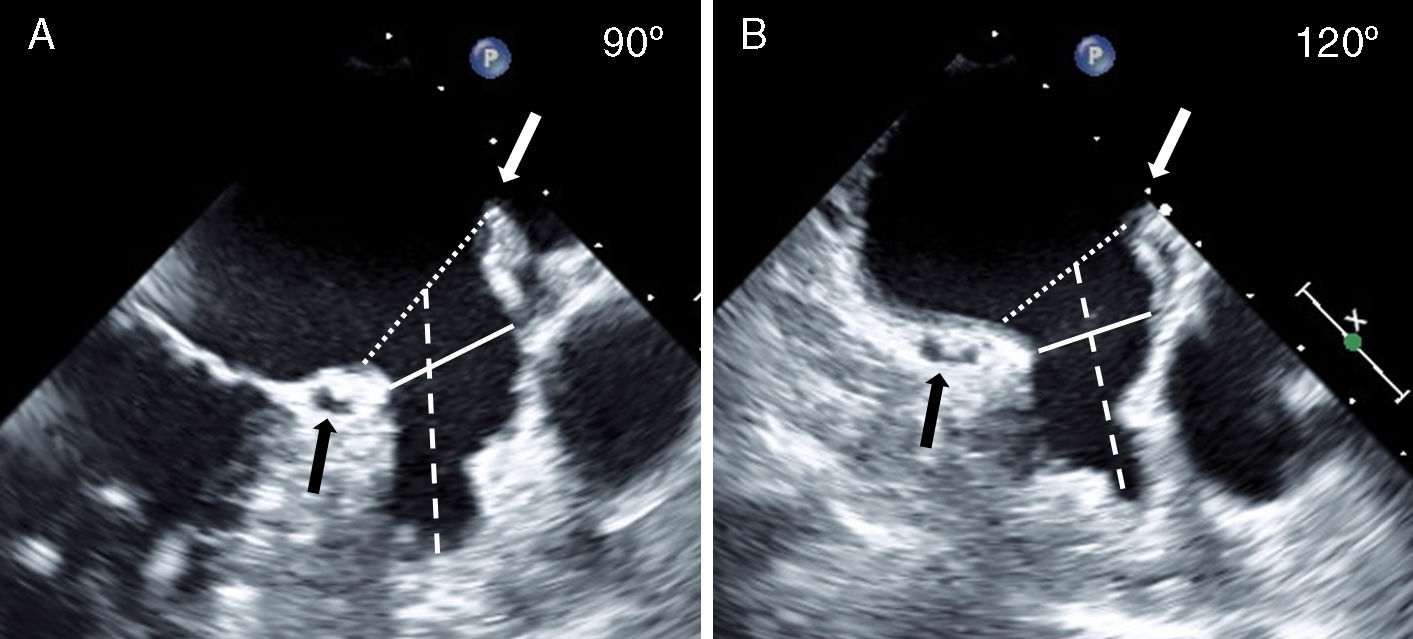
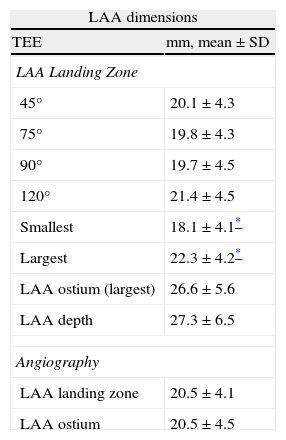
The LAA was assessed from the mid-esophageal and high-esophageal views from 0° to 120° (at 0°, 45°, 75°, 90°, and 120°). Because of width variability during the cardiac cycle, the largest LAA diameters were measured (Fig. 2). Measurements were made at three levels: (1) the LAA ostium (LAA-O), measured from the most proximal part of the LAA, between the proximal left circumflex artery to the roof of the LAA at the level of the ligament of Marshall or to the left upper pulmonary vein; (2) the landing site of the ACP lobe (LAA-L), measured 10mm distal to the LAA ostium and, (3) the depth of the LAA (LAA-D), measured by drawing a perpendicular 90° line, from the ostium to the apex of the LAA. During and after the procedure, TEE was also used to look for possible complications such as thrombus formation and pericardial effusion.
Transesophageal Evaluation of the LAA. The LAA is assessed in multiple TEE views (examples, panel A: 90°, panel B: 120°; see Methods section for details). The diameter (broken white line) of the LAA-O is measured from the proximal aspect of the origin of the circumflex artery (black arrow) to the tip of the ligament of Marshall (white arrow). The diameter (solid white line) of the LAA at a depth of 10mm from the ostium representing the lobe landing zone (LAA-L), and the LAA depth (broken white line) are also measured. LAA, left atrial appendage; LAA-L, left atrial appendage at 10mm depth from ostium; LAA-O, left atrial appendage at ostium; TEE, transesophageal echocardiography.
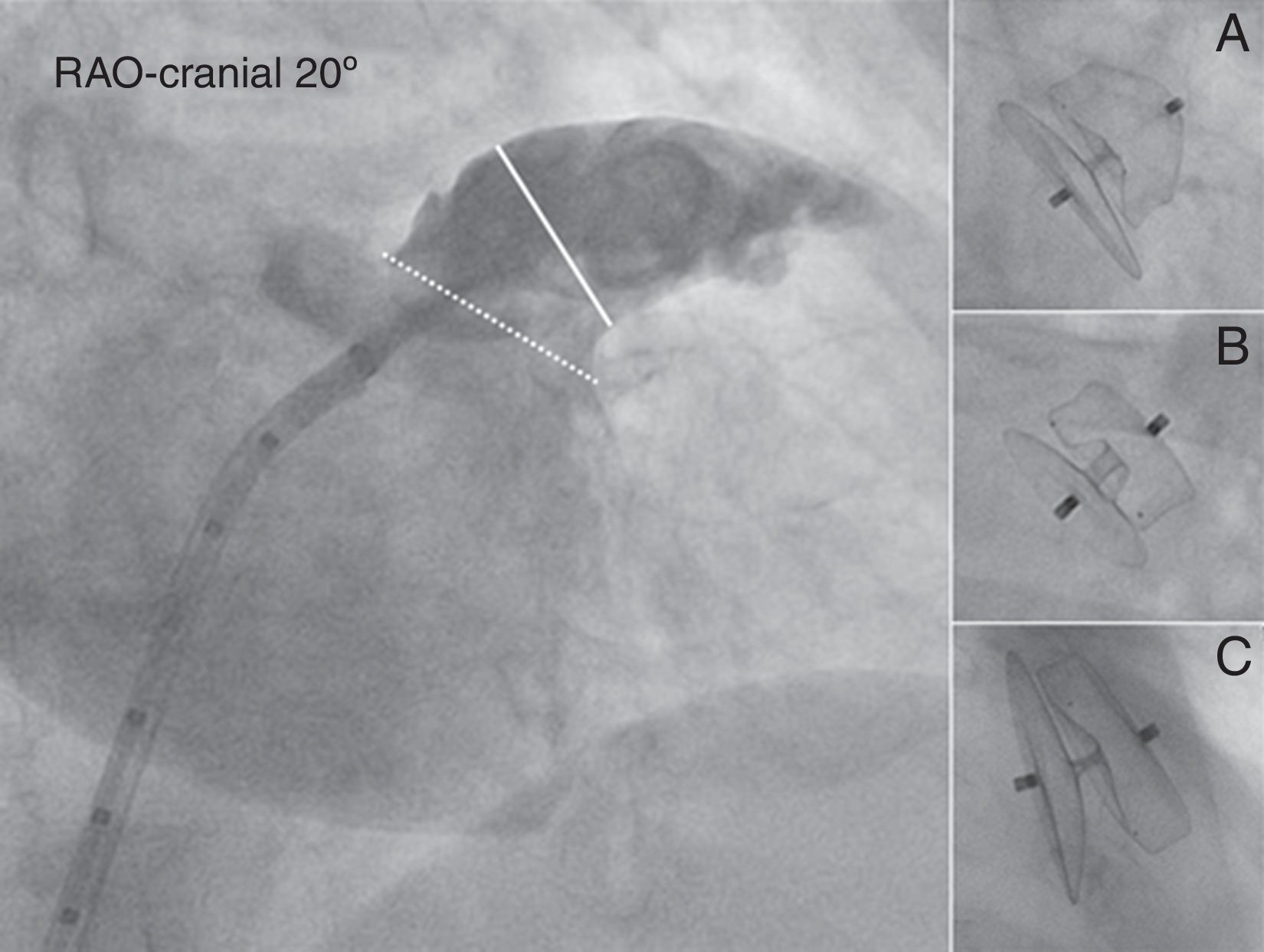
With the delivery sheath positioned in the LAA, a power injection of 15cm3 of contrast (20cm3/s) was performed in the RAO 30° – Cranial 20° projection (Fig. 3). Two methods were used for calibration: either a 0.032in. straight wire with radio-opaque markers was placed in the LAA during contrast injection or the injection was made using a 5 French marked pigtail catheter which was advanced in the LAA through the delivery sheath. In a similar fashion to measurements made by TEE, the dimensions of the LAA ostium, and the LAA diameter at a depth of 10mm from the ostium were measured during the procedure using AXIOM sensis software (Siemens). After ACP release a final contrast injection was given. For the purpose of this study, all angiograms were analyzed off-line by two interventional cardiologists blinded to each other's results.
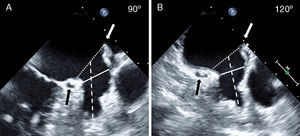
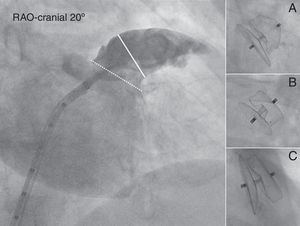
Angiographic evaluation of the LAA. Angiography of the LAA is performed in the RAO 30° – Cranial 20° projection (large panel). The diameter of the LAA-O (broken white line) and the LAA-L size at a depth of 10mm from the ostium (solid white line) are measured. Contrast injection and calibration are performed using the markers of the pigtail catheter. After release, the ACP can be either under-expanded adopting a “strawberry” shape (panel A), optimally expanded adopting a “tire-like” shape (panel B) or over-expanded adopting a “square” shape (panelC).
ACP, Amplatzer™ Cardiac Plug; LAA, left atrial appendage; LAA-L, left atrial appendage at 10mm depth from ostium; LAA-O, left atrial appendage at ostium; RAO, right anterior oblique.
The implantation strategy was based on manufacturer's recommendations; i.e. a mild degree of oversizing by 10–20%, which is considered necessary for better sealing and an adequate stability of the device. The final shape of the ACP after release was assessed by fluoroscopy. More specifically, the dimensions of the ACP lobe's width, of the disc's width, and the angulations between the disk and the lobe were measured. In order to avoid variability during the cardiac cycle, all analyses were made in mid diastole and calibration was performed using the delivery sheath diameter. In order to avoid calibration errors and achieve accurate measurements, the assessment of the ACP expansion was done by comparing ratios. The ratios of the lobe's width were compared with those of the disc's (A/B ratio, Fig. 1). The nominal lobe-disk ratio for each ACP size could be calculated using the dimensions provided by the manufacturer. This nominal ratio is unique for each device and used as a reference (fully expanded ACP). It should be noted that after release of the ACP, the disk is not compressed by the LAA walls; therefore its diameter remains unchanged. The relation between the lobe-disk ratio measured in vivo and the nominal lobe-disk ratio was expressed as a percentage and served to assess the degree of ACP expansion within the LAA. Intra- and inter-observer variability were assessed for all measurements. Two observers made all measurements twice (blinded to each other and to the TEE results). For each observer, there was a 2-week time interval between the first and second analysis of the same study.
Statistical analysisVariables are presented as mean±standard deviation (SD) or, in case of a non-Gaussian distribution, as median and inter-quartile range (IQ range). Comparisons of different measurements in the same patient were done using the Student's t-test for paired data. When considered appropriate, a one-way ANOVA test was used with a Bonferoni correction to define statistical difference between groups. Agreement between TEE and angiography, and intra- and inter-observer variability were assessed by linear regression analyses and Bland–Altman methodology.12 With respect to logistic regression, a correlation between variables was defined as good (r≥0.80), moderate (r<0.80, >0.60) or poor (r≤0.60). Two-tailed tests were used for all the analyses. Statistical analysis was done using SPSS 16.0. Statistical significance was defined as p<0.05.
ResultsTwenty-seven consecutive patients underwent LAA occlusion with the ACP between November 2009 and November 2011. In 19 patients (70%), the ACP was delivered at the first attempt. A second, third or fourth attempt was needed during the same session in 4 (15%), 2 (7%), and 2 (7%) patients, respectively. Five patients (19%) required a second ACP with a different size than the initial operator's choice, two patients (1 with PFO, 1 with ASD) underwent occlusion of their septal defect during the same procedure, after successful delivery of the ACP. Procedural success rate was 100%. No device embolization was detected, neither air embolism nor procedure-related stroke. Significant vascular access-related peri-procedural bleeding was observed in 3 patients (11%), and these patients were treated with blood transfusion. No new pericardial effusion was observed during the peri-procedural period. One patient had cardiac tamponade 2 weeks after the index procedure. The patient was successfully treated with pericardial drainage. Contrast echocardiography did not show leakage from the LAA.
Table 1 shows the baseline patient characteristics. All patients had a contraindication for chronic long-term anticoagulation, the majority of which was due to high risk of bleeding or bleeding from an incorrigible cause. Table 2 shows intra-procedural assessment of the LAA dimensions by TEE and angiography.
Baseline patient characteristics.
| N=27 | N (%) or mean±SD |
| Men | 17 (63) |
| Age | 75±9 |
| Prior history | |
| Stroke | 21 (77) |
| Hemorrhagic | 11 (52) |
| Ischemic | 10 (48) |
| Coronary artery disease | 16 (59) |
| Myocardial infarction | 10 (62) |
| PCI | 9 (56) |
| CABG | 5 (31) |
| Hypertension | 23 (85) |
| Diabetes | 13 (48) |
| Dyslipidemia | 10 (37) |
| Gastro-intestinal bleeding | 6 (22) |
| Cancer | 5 (19) |
| CHADS2score | |
| 2 | 11 (40%) |
| 3 | 6 (22%) |
| 4 | 7 (26%) |
| 5 | 3 (11%) |
| LV ejection fraction, % | 56±10 |
Intra-procedural assessment of the LAA dimensions by TEE and angiography.
The diameter of the LAA-L (at a depth of 10mm from the ostium) was measured in pre-specified high and mid-esophageal TEE views (Table 2; Fig. 2). TEE image quality was sub-optimal to accomplish the required measurements at the 0° view; therefore this view was not used for analysis. Mean LAA-L diameter varied depending on the TEE probe angulations. Overall, the largest mean diameter was observed in the TEE 120° view. We found a significant difference between the largest and smallest mean LAA-L diameters (22.3±4.2 vs. 18.1±4.1mm, paired sample t-test, p<0.001), demonstrating the elliptical shape of the LAA at a 10mm depth from the ostium. Logistic regression analysis revealed a good correlation between the largest and the smallest LAA-L diameters (r=0.84), showing consistency of the measurements. The mean depth of LAA was 27.3±6.5mm. LAA depth was not a limitation for ACP implantation since it was deployed in the proximal part of the LAA. With TEE, maximal LAA-O (at ostium) was significantly larger than maximal LAA-L (26.6±5.6 vs. 22.3±4.2mm, paired sample t-test, p<0.001). The logistic regression analysis showed a moderate correlation between LAA-O and LAA-L (r=0.79), i.e. some degree of variability. The echocardiographic view that best corresponded to the working fluoroscopic projection (i.e. the angulations of the C arm) during the implantation was the mid-esophageal 75° view.
AngiographyAngiographic analyses were based on the RAO 30° – Cranial 20° view (Table 2; Fig. 3). Angiographic mean LAA ostium (LAA-O) diameter was similar to angiographic mean LAA-L (20.5±4.5 vs. 20.5±4.1mm; p=ns). However, logistic regression analysis showed only moderate correlation between the two variables (r=0.74). Mean LAA-L by angiography was 1.8mm smaller than the corresponding largest measurement by TEE.
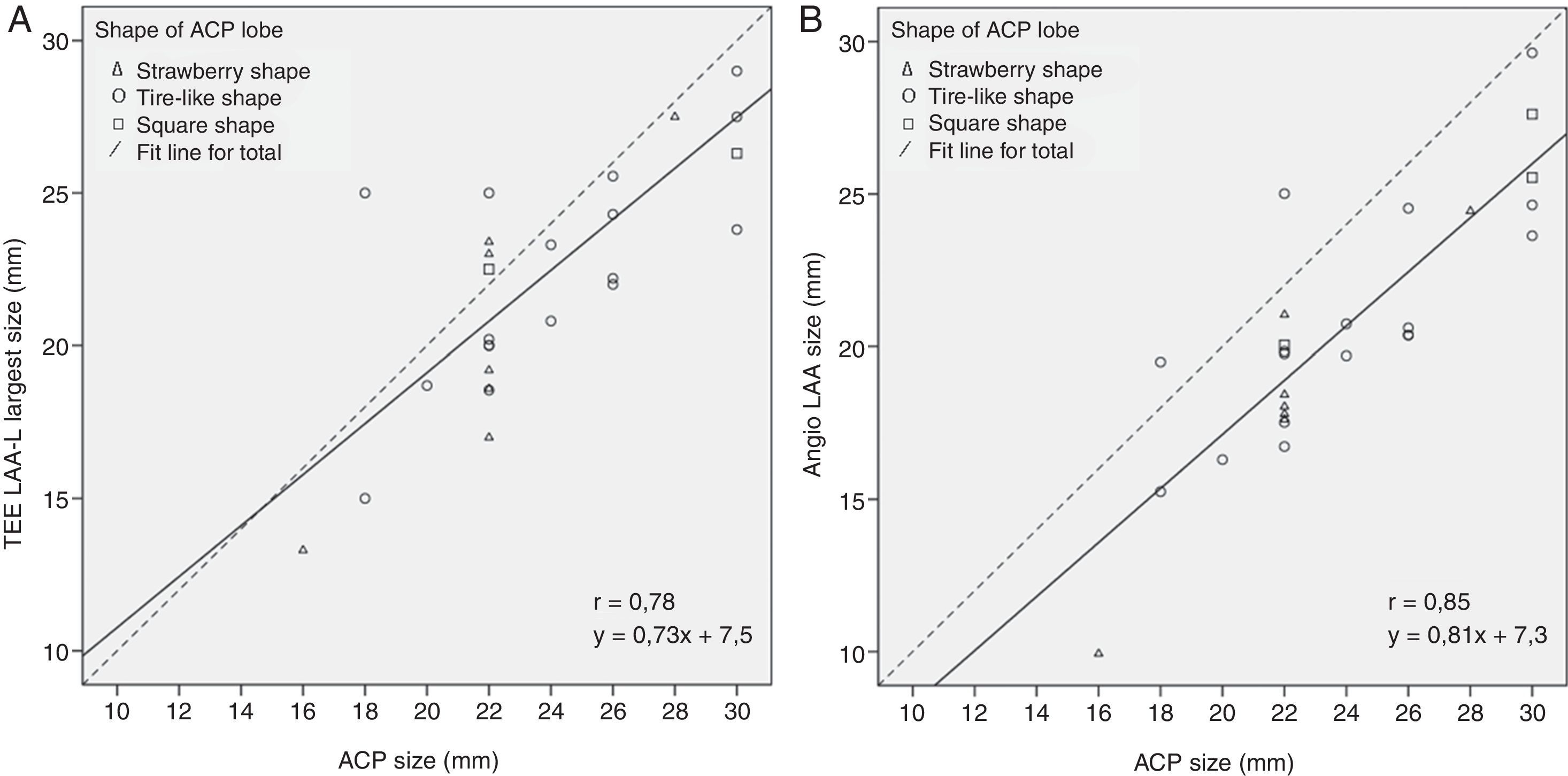
ACP shape analysisThe most commonly used ACP size was 22mm (9 patients, 33%). At the end of the procedure, the ACP had an optimal “tire-like” shape in 17 patients (63%), a “strawberry” shape in 7 patients (26%), and a “square” shape in 3 patients (11%) with a degree of ACP expansion of 93% (88–96) [median % (IQ range)], 84% (77–84), and 100% (99–102), respectively (Table 3). The mean angle (mean±SD) between the ACP disk and lobe was 3.1±5.9°. Overall, the ACP was oversized by 1.5±2.7 and 3.3±2.3mm compared to the largest LAA-L TEE and RAO 30° – Cranial 20° angiographic measurements, respectively. Fig. 4 shows the relationship between TEE and angiography and the operator's choice, i.e. the size of the implanted ACP. With angiography, over-sizing was higher (more right-shifted curve) and the correlation between variables was slightly better (r=0.85 vs. 0.78 with TEE). Neither the size of the implanted ACP nor the size of LAA by TEE or angiography was significantly associated with the final shape of the device. However, Table 3 shows a logical trend; i.e. that ACP with “strawberry” shape had greater oversizing and those with a square shape, were less oversized (i.e. undersized).
Device analysis.
| Mean±SD, mean (IQ range), n (%) | |
| Lobe width, mm | 28.9±5.1 |
| Disk width, mm | 28.9±5.1 |
| Disk-lobe angle,° | 3.1±5.9 |
| Size of ACP implanted | |
| Lobe, mm | 23.8±4.0 |
| 22 (22–26) | |
| Disk, mm | 28.7±4.8 |
| 26 (26–32) | |
| ACP Shape | |
| “Strawberry” shape | 7/27 (26) |
| “Tire-like” shape | 17/27 (63) |
| “Square” shape | 3/27 (11) |
| Expansion of ACP Lobe | |
| All shapes, % | 92 (84–96) |
| “Strawberry” shape, % | 84 (77–84) |
| “Tire-like” shape, % | 93 (88–96) |
| “Square” shape, % | 100 (99–102) |
Logistic regression analysis. Logistic regression analysis of the ACP sizing by TEE (chart A), and angiography (chart B) in relation to the operator's choice (the broken black line represents y=1×). LAA-L diameter was measured at a depth of 10mm from the LAA-O (see Methods section). The majority of cases are in the right lower part of the chart. It reflects a certain degree of oversizing by the operator as recommended by the manufacturer. The correlation was moderate with TEE (r=0.78) and slightly better with angiography (r=0.85). There was no association between the degree of oversizing and the final shape of the device. ACP, Amplatzer™ Cardiac Plug; LAA, left atrial appendage; LAA-L, left atrial appendage at 10mm depth from ostium; LAA-O, left atrial appendage at ostium; TEE, transoesophageal echocardiography.
In regard to the effect of procedure related experience, an optimal (“tire-like”) device shape was seen in 7 (50%) of the first 14 patients treated in this series, whereas an optimal device shape was seen in 10 (77%) of the last 13 patients. The patient who presented late cardiac tamponade had an optimally expanded ACP.
Intra-observer variability (mean difference±SD) for measurements of ACP lobe diameter, disk diameter, and disk-lobe angle were 0.1±0.3mm, 0.2±0.2mm, and 0.2±0.8°, respectively. Inter-observer variability (mean difference±SD) for measurements of ACP lobe diameter, disk diameter, and disk-lobe angle were 0.2±0.4mm, 0.3±0.4mm, and 0.3±1.0°, respectively.
DiscussionThe main finding of this study is that there is considerable variability in the assessment of the LAA, by using TEE and angiography. The degree of ACP expansion within the LAA and the final shape of the device were not associated with the degree of oversizing.
The wide range of LAA ostium and “landing zone” diameters reported in this study are in accordance with previous observations and confirms that the LAA orifice and “body” have an elliptical shape.10,11 TEE has the advantage of providing multiple views of the LAA without radiation exposure and contrast use. Conversely, full anatomical detailed analysis is not always optimal as it may depend on the patient's LAA anatomy and the experience of the echocardiographer. On the other hand, angiography provides a more consistent and less-operator related imaging, although the number of views is limited, and small calibration errors can influence measurement accuracy. In LAA device occlusion, similar to other percutaneous procedures, the operator has to decide whether the patient's LAA anatomy is suitable for device closure and, which size of device should be used. The findings of our study show that both TEE and angiography can provide useful information for this purpose, therefore we would support combining the information acquired by the two imaging modalities for decision making. Moreover, TEE should not be omitted because it is the best modality to provide information regarding presence of thrombus in the LA or LAA and can rapidly assess for potential periprocedural complications.
The ACP is specifically designed for LAA occlusion. With respect to LAA depth, implantation is rarely problematic because the device has a relatively short length. The landing zone of the ACP is in the proximal part of the LAA, therefore the issue of bifid or peculiarly shaped appendages did not represent a limitation to ACP device implantation. However, it should be highlighted that the ACP lobe should be ideally implanted in an area where the LAA walls are more straight and parallel to each other. The ACP lobe has 6 hooks or teeth, which increases device stability. In case of extreme lobe under-expansion, these hooks may not function properly, resulting in device instability. The ACP disk plays a pivotal role in LAA occlusion by sealing the LAA from the left atrial side. In this study, the mean ACP disk diameter was 1–2mm larger than the largest LAA ostium diameter; which is important in order to assure that the disk remains deployed in the left atrium and does not slip inside the LAA. Thus, there is mild tension maintained at the level of the articulating waist that connects the lobe and the disk, which assures tight LAA sealing and device stability within the LAA.
The angiographic working view used in this series was RAO 30° – Cranial 20°. Other useful projections are RAO 30° – Cranial 10° or 30°, and RAO 30° – Caudal 20°. Although many operators believe that measured LAA diameters are usually slightly larger in more caudal views, our group prefers to use the RAO 30° – Cranial 20° because it provides a better view of the proximal part of the LAA allowing deeper and safer positioning of the delivery catheter and easier assessment of the profile of the ACP lobe during deployment.
In order to increase stability and successful closure, it has been recommended to oversize the ACP by 3±1mm or 10–20%.13 However, recommendations are less clear-cut in cases of discrepancy between TEE and angiography, which although small, are not uncommon as shown in this study. In this series, oversizing by an average of 3mm using angiography (based on RAO 30° – Cranial 20° view), and 1.5mm using TEE (based on 45–120° views) resulted in successful implantations in all patients. This observation should be confirmed in future larger series with long-term clinical and echocardiographic follow-up.
Based on our clinical experience with the ACP, we now use the following sizing strategy: LAA diameter is measured with angiography and TEE at the LAA-L and we oversize by 3mm by angiography and 1.5mm by echocardiography. In case of discrepancy greater than 2mm between TEE and angiography, measurements are repeated and device sizing is based on the largest measurement made by either imaging method. It should be emphasized that communication and collaboration between interventionalist and echocardiographer are essential.
The degree of oversizing was not significantly associated, in this series, with the degree of expansion and the final shape of the ACP device; many reasons can account for this. LAA anatomy and compliance are highly variable. Variability of the thickness and rigidity of the LAA walls may influence the degree of device expansion. ACP deployment can also occur at a level slightly different from the site of measurement. The difference in the degree of oversizing was possibly too small in the study sample to be able to show a statistically significant association with device shape. There was in fact, as shown in Table 3, a logical trend of ACP final shape in regard to sizing; i.e. patients with strawberry-like shaped ACP had on average a greater degree of oversizing, and those with a square shape were less oversized (i.e. undersized).
Unfortunately, the “response” of the LAA walls to the tension applied by the device can actually be evaluated only after deployment. This highlights the importance of the repositioning capabilities of the ACP. Also of note, the LAA being a relatively fragile structure and the ACP lobe having 6 stabilizing hooks, as opposed to other closure devices (e.g. PFO or ASD occluders), the so-called “Minnesota wiggle” should be performed very gently if at all, as it appears to be less helpful.9
As shown by our results, there was a learning curve in order to observe more optimal deployment and final shape of the ACP. Percutaneous LAA closure is a technically demanding procedure which requires sufficient training and operator experience as well as close collaboration between the interventionalist and the echocardiographer.
LimitationsThe present study is based on a single center experience with a relatively small number of patients. Device sizing was chosen by the operator and may have been influenced by several unmeasured or unaccountable factors. This is a prospective observational study, therefore suggestions can be made but should be confirmed in larger series with mid- to long-term clinical and echocardiographic follow-up. The lack of statistical association between the degree of oversizing and final shape of ACP device can be due to sample size and a tight sizing policy relative to measured LAA size. However, the very good immediate results and safety data are in favor of the sizing approach suggested by this study. Finally, all device implantations were performed by a single operator experienced in structural heart disease intervention.
ConclusionsWe found considerable variability in the assessment of the LAA by using TEE and angiography. The degree of ACP expansion within the LAA and the final shape of the device were not associated with the degree of oversizing.
FundingDr. Sobrino was supported by grants provided by the Juárez Autonomous University of Tabasco. Dr. Tzikas was supported by grants provided by the Hellenic Cardiology Foundation and the Hellenic Society of Cardiology.
Conflict of interestAuthors have no conflict of interest to declare.