A 47-year-old man with no medical history presented to the emergency department complaining of severe sharp chest pain 3h lasting, exacerbated with deep inspiration and accompanied by increasing dyspnea.
At first examination, asymmetric thoracic respiratory movements were observed with a diminished expansion of the left side, where inaudible breath sounds were present.
Blood samples were obtained and arterial gases reported pH 7.41, PaO2 63mmHg, PaCO2 21.9mmHg, HCO3− 16.2mmol/L, oxygen saturation 93%. Red cell count reported hemoglobin of 7.7g/dl and hematocrit of 24%.
A chest X-ray showed an almost fully opacified left hemithorax suggesting a massive left pleural effusion with the ipsilateral lung collapsed and displacement of the trachea toward the right side. Blood was obtained from the thoracentesis.
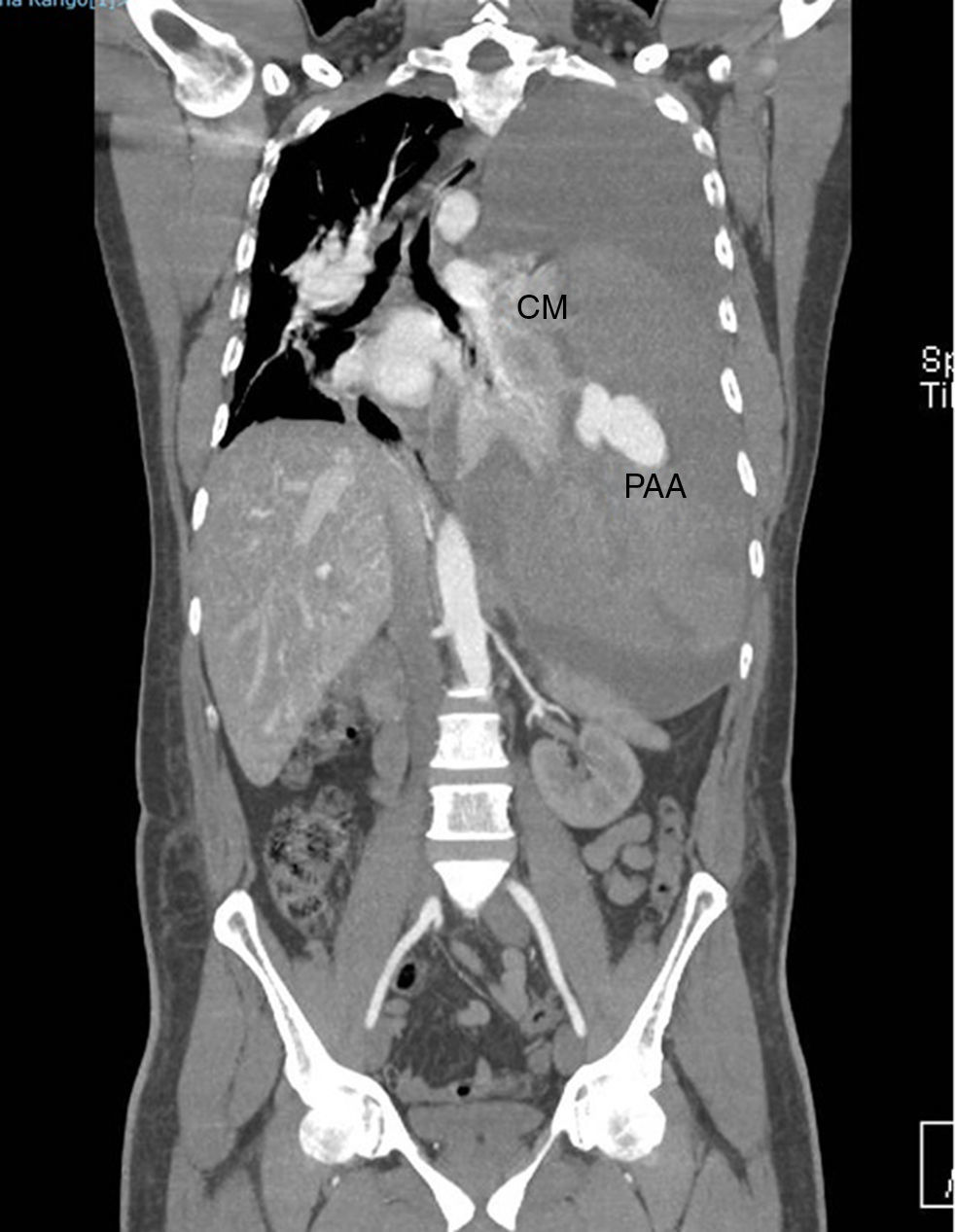
A CT-angiography (Fig. 1) was mandatory for a better assessment of the pulmonary vasculature. It showed an image suggesting an aneurysmatic lesion of the left pulmonary artery (Fig. 2).
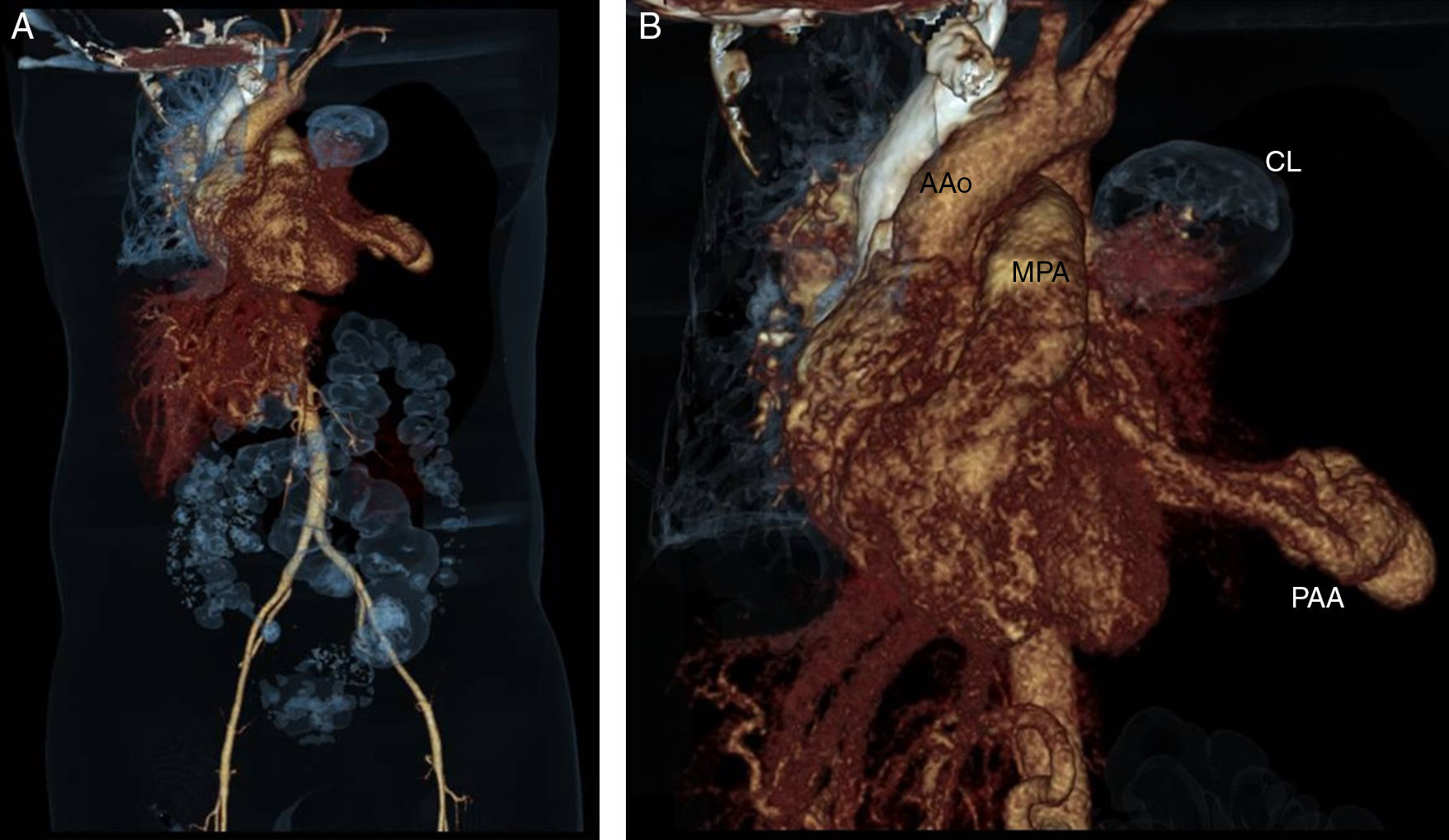
CT angiography 3-D reconstruction (A) and close up image (B) in which the left pulmonary aneurysm and its relationship with other cardiovascular structures are observed. The left pulmonary parenchyma is almost totally collapsed. AAo, ascending aorta; CL, collapsed lung; MPA, main pulmonary artery; PAA, pulmonary arterial aneurysm.
An invasive approach was decided by means of a posterolateral thoracotomy, where 5000ml of coagulated blood were obtained. A ruptured aneurysmal lesion of 2cm×3cm of diameter in the lingular branch of the left pulmonary artery was identified and resected. Lingular lobe resection was also performed.
The patient was admitted to the intensive care unit for further observation, requiring the administration of vasopressors and mechanical ventilatory support. Two weeks after his admission he died because of a nosocomial pulmonary infection.
Pulmonary artery aneurysms (PAAs) are rare and infrequently diagnosed,1–4 the best part we know is derived from autopsy findings.5,6 They were first described by Bristowe in 1860 at a necropsy5 and subsequently by Deterling and Claggett in 1947 who reported eight cases in 109,571 necropsies.2,5 However the true incidence is unknown.5
An aneurysm is defined as a focal dilation of a blood vessel involving all three layers of the wall.1,3,7 Pseudoaneurysms do not involve all layers of the arterial wall but they pose a higher risk of rupture.7 In computed tomography (CT) the upper limit of the main pulmonary artery (PA) diameter in adults is 29mm and for interlobar PAs 17mm1; PAAs are defined as a dilatation greater than 4cm in the main PA.8
PAAs can be congenital or acquired,1,5,8 in 50% of the postmortem cases were associated with congenital heart disease, in decreasing order, patent ductus arteriosus, ventricular septal defects and atrial septal defects.1 It is presumed that increased flow caused by left-to-right shunt results in hemodynamic shear stress of the vascular wall and promotes aneurysm formation in the PAs.1,8
This entity generally affects younger people than aortic aneurysms and there is no sex predilection.1 Most of these anomalies affect the main pulmonary artery,7 but when they affect the pulmonary artery PA branches, the left PA affection is more common than the right one.1
The most frequent association with the formation of PAAs has been pulmonary arterial hypertension,1 present in 66% of cases, which favors the formation of giant aneurysms.4 Low-pressure aneurysms seem to have a better prognosis than hypertensive aneurysms.4 Chronic pulmonary embolism is a relatively common cause of PAAs,3 such aneurysms present mural thickening, webs, and intramural thrombi that can calcify.1
Pathophysiological mechanisms involved in PAA formation are limited. Structural changes in elastin and collagen secondary to increased PA pressure that leads to PA dilatation has been proposed.1 An abnormal opening of the pulmonary valve or shear stress from a right-to-left shunt may induce apoptosis, remodeling, and aneurysmal transformation of the vessel wall.1,4
Among acquired causes the insertion of Swan-Ganz catheters has been a cause of iatrogenic pseudoaneurysms with a 0.2% incidence of rupture and hemorrhage.1 Other iatrogenic causes include chest tube insertion, conventional angiography, surgical resection, biopsy and after penetrating trauma.1 Idiopathic PAAs are rare.1,7
The natural history of the PAAs is poorly understood because of the limited number of cases diagnosed ante-mortem; however, not all aneurysms progress to the rupture stage.2
Most patients with PAAs are asymptomatic or have non-specific symptoms.9 Clinical symptoms include dyspnea, chest pain, hoarseness, palpitations, and syncope. Bronchus compression may produce cyanosis, cough, fever, pneumonia and bronchiectasis. There is an increased risk of pulmonary embolism.1
Hemoptysis has been the cause of death in one third of the reported cases and, when present, it should be considered as an indicator of imminent aneurysm rupture,1 massive hemoptysis, considered as a loss of more than 300ml of blood expectorated from the bronchial tree within 24h,9 is an emergency condition that can cause asphyxiation, exsanguination, cardiogenic shock and sudden death.1
Pulmonary angiography is the gold standard for establishing the diagnosis of PAAs1,3 but, owing to its high spatial resolution, contrast-enhanced multi-detector CT is considered the primary technique for diagnosing PAA,5,7 since it allows the evaluation of the size, shape and exact location of the aneurysm, and concomitant structural cardiovascular abnormalities.7
The optimal treatment for PAAs remains unclear since there is limited experience because of the low incidence of the disease.1,3 Due to the lack of guidelines for its management, patients should be managed individually.6
Treatment of PAAs used to be predominantly surgical, however nowadays endovascular techniques like coil embolization, balloon embolization or stent graft placement have been increasingly used, since they are less invasive and produce less damage to the lung parenchyma.10
When the main pulmonary trunk is involved, surgical intervention consists of aneurysmectomy or aneurysmorrhaphy.1 With peripheral lesions, embolic therapy has been the treatment of choice, instead of lobectomy.10
In case of rupture, surgery is the only possible life-saving treatment option. In addition, dissection is an indication for surgery in case of reasonable preoperative morbidity.1,9 Surgical outcome is unknown.1
In the case of our patient, the first ruptured pulmonary aneurysm related massive hemothorax reported in our country as far as we know, no etiology was identified. The management of the patient, as mentioned before, was individually handled, relying on the information provided by the few reported cases in the literature, since apart of being already recognized as a rare disease, the location of the aneurysm at a peripheral branch of the pulmonary artery is even less frequent. Successful resection of the pulmonary aneurysm and drainage of the massive hemothorax, that required multiple transfusions, were life-saving measures in the acute setting and back the indication of an emergency surgical approach in such patients.