To evaluate efficacy and safety of 60mg and 120mg Fimasartan (FMS) alone or combined with 12.5mg hydrochlorothiazide (HCTZ) in a Mexican population.
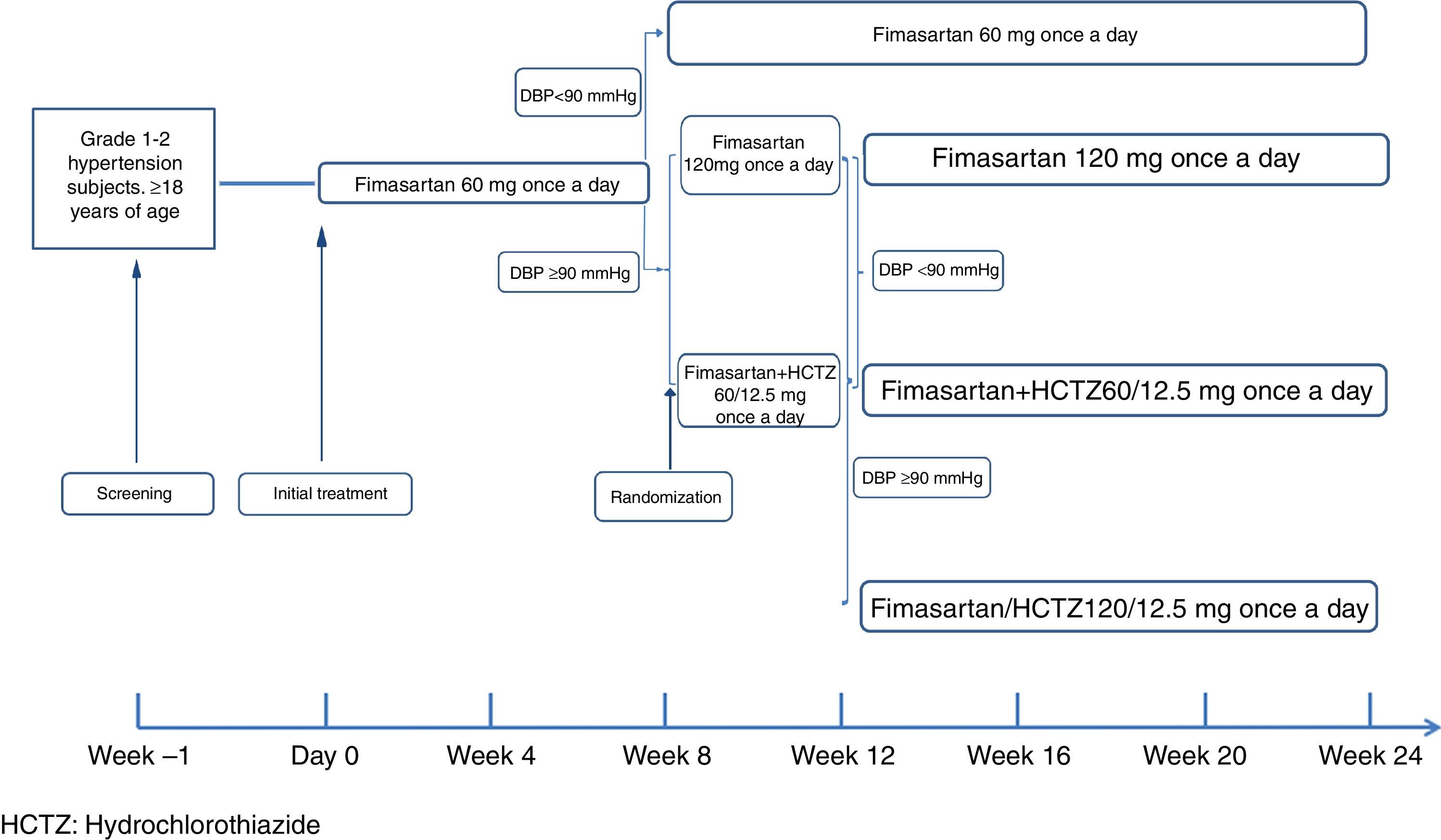
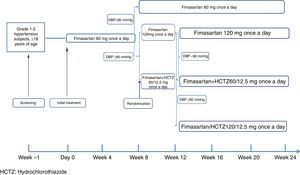
MethodsA six month, treat-to-target, open study was conducted on subjects with grade 1-2 hypertension. The subjects were initially treated with 60mg FMS once daily. In week 8, those with Diastolic Blood Pressure (DBP) <90mmHg continued on the same FMS dose during the rest of the study, while those with DBP ≥90mmHg were randomised to either 120mg FMS or 60mg FMS + 12.5mg HCTZ once daily. In week 12, randomised subjects with DBP ≥90mmHg received 120mg FMS+12.5mg HCTZ, while those achieving target continued with their assigned treatment until the end of the study.
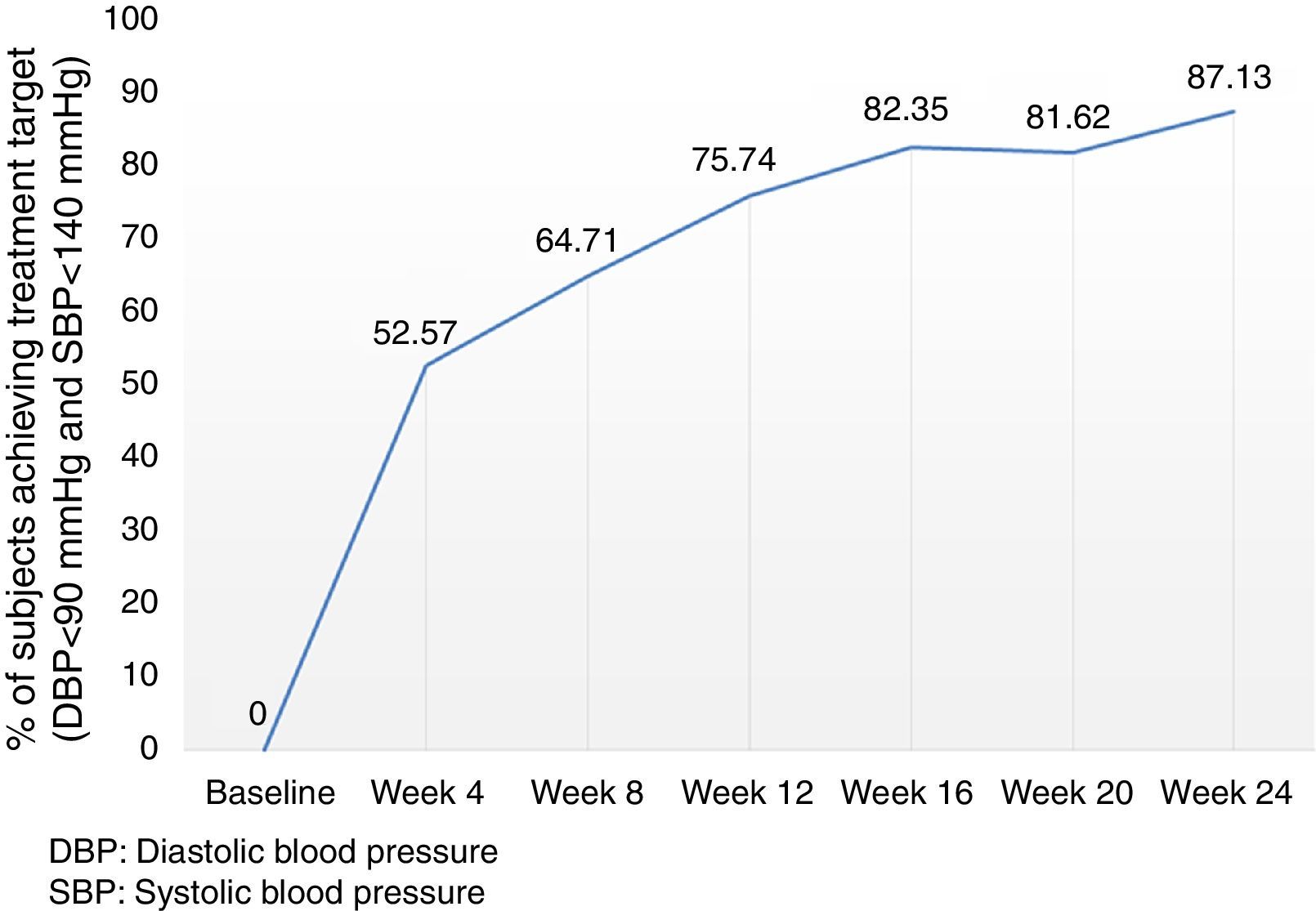
ResultsFMS 60mg (n=272) decreased both DBP and Systolic Blood Pressure (SBP) by 11.3±8.9 (p<.0001) and 16.0±14.1 (p<.0001)mmHg, respectively, with 75.4% of subjects reaching the treatment target. Subjects assigned to FMS 120mg, FMS 60mg+HCTZ 12.5mg, or FMS 120mg+HCTZ 12.5mg once daily, showed significant reductions in DBP and SBP with their assigned treatment. At the end of the study, 237/272 subjects (87.1%) achieved a DBP<90mmHg and an SBP<140mmHg. The most frequently reported adverse reactions included headache (3.7%), dry mouth (1.1%), transient liver enzyme increase (1.1%), and dizziness (0.7%).
ConclusionFimasartan is safe and effective in Mexican subjects with grade 1-2 essential hypertension.
Evaluar la eficacia y la seguridad de 60 y 120mg de fimasartán (FMS) solo o combinado con 12.5mg de hidroclorotiazida (HCTZ) en población mexicana.
MétodosEstudio abierto, de 24 semanas, con tratamiento escalado hasta el objetivo terapéutico en sujetos hipertensos grados 1–2. Tratamiento inicial: FMS 60mg una vez al día; en la semana 8, los sujetos con presión arterial diastólica (PAD) <90mmHg mantuvieron su tratamiento inicial durante el estudio, mientras que los sujetos con PAD ≥90mmHg fueron aleatorizados a 120mg de FMS o a 60mg de FMS+12.5mg de HCTZ. En la semana 12, los sujetos aleatorizados con PAD ≥90mmHg recibieron 120mg de FMS+12.5mg de HCTZ; quienes alcanzaron el objetivo terapéutico mantuvieron su tratamiento asignado hasta finalizar el estudio.
ResultadosFMS 60mg (n=272) disminuyó la PAD y la presión arterial sistólica (PAS) en 11.3±8.9 (p<0.0001) y 16.0±14.1 (p<0.0001)mmHg, respectivamente, con logro del objetivo de tratamiento en el 75.4% de los sujetos. Los sujetos asignados a 120mg de FMS, a 60mg de FMS+12.5mg de HCTZ 12.5 y a 120mg de FMS+12.5mg de HCTZ mostraron reducciones significativas de PAD y PAS; al final del estudio, 237/272 sujetos (87.1%) lograron PAD <90 y PAS <140mmHg. Las reacciones adversas más frecuentemente reportadas fueron: cefalea (3.7%), boca seca (1.1%), incremento de enzimas hepáticas (1.1%) y mareo (0.7%).
ConclusiónFMS es seguro y eficaz en sujetos mexicanos con hipertensión esencial de grados 1–2.
The Mexican Encuesta Nacional de Salud (ENSA, National Health and Nutrition Survey),1 reported a prevalence of arterial hypertension in the adult population of 31.5%, with 47.3% of these hypertensive subjects being unaware of their condition.1 In spite of increasing levels of awareness, an alarming proportion (49.8%) of known hypertensive patients remains uncontrolled.1 To effectively address this public health problem, significant improvements on the diagnosis and management of this condition as well as safe and effective treatment options are needed.
Fimasartan (FMS) is a newly developed angiotensin II receptor antagonist resulting from a bioisosteric replacement of losartan's imidazole moiety with a pyrimidin-4(3H)-one part that confers this agent with the highest affinity to the AT1 receptor among drugs from this class (IC50=0.42nM; Ki=6.3E−11M).2 Results from clinical studies in the Eastern-Asian population have shown that Fimasartan is not inferior and may be superior to losartan and valsartan,3,4 and that its antihypertensive effect is evident after two treatment weeks.5 FMS undergoes minimal hepatic metabolism, mainly by CYP3A4, which is well known for showing a marked genetic variability that may significantly influence drug disposition and therapeutic effect in different ethnic groups.6,7
The safety and efficacy of FMS have only been systematically explored in Eastern-Asian subjects, therefore, the above described potential for differences in drug disposition and the possibility for this agent to show different efficacy and safety profiles in the Mexican population8,9 require further assessment.
The purpose of this study was to evaluate the efficacy and safety of daily doses of 60mg and 120mg fimasartan, with or without 12.5mg hydrochlorothiazide in Mexican subjects with essential hypertension grades 1–2.
Method and patientsInclusion criteriaSubjects eligible to participate in the study were hypertension treatment naïve consenting adults, 18 years of age or older, with a baseline sitting diastolic blood pressure consistent with the diagnosis of grade 1 or 2 essential hypertension (diastolic blood pressure [DBP] ≥90 ≤109mmHg). Not adequately controlled subjects already on monotherapy – provided that their treating physician considered them as suitable for a two-week washout period, were also eligible for the study.
Exclusion criteriaSubjects with grade ≥3 hypertension (systolic blood pressure [SBP] ≥180mmHg and/or DBP ≥110mmHg); secondary hypertension; uncontrolled type II diabetes mellitus (HbA1c>9%); morbid obesity (BMI ≥40kg/m2); renal dysfunction (creatinine ≥1.5 times above the upper limit of the normal range), liver dysfunction (aspartate aminotransferase and/or alanine aminotransferase ≥1.5 times the upper limit of the normal range), gastrointestinal or hematological diseases or conditions with a potential to affect the absorption, distribution, metabolism and excretion of the study drugs; a history of myocardial infarction, severe coronary artery disease or clinically significant congestive heart failure within 6 months prior to the screening visit; auto-immune or connective tissue disease and clinically significant laboratory test abnormalities according to the investigator's judgment were not considered eligible to participate; additional exclusion criteria were the use of concomitant treatments which could affect blood pressure, known allergies or contraindication to the use of angiotensin II receptor antagonists, pregnant or breastfeeding women or in the case of women with childbearing potential, the rejection to use an effective contraceptive method (in accordance with the investigator's judgment), a history of alcohol or drug abuse, clinical trial participation within 6 months prior to screening and any other reason which in the investigator's opinion might contraindicate the participation of a subject in the study.
MethodEligible patients spontaneously attending to 13 participating sites in Mexico were enrolled to participate in the 24-week study treatment period. Starting on Day 0, and after a two week washout period in the case of subjects already receiving monotherapy, all patients received oral FMS 60mg once a day (q.d.) during the first 8 weeks of the planned treatment period. Patients with a DBP <90mmHg at treatment week 8 continued receiving the same dose for up to 24 weeks; those with week 8 DBP ≥90mmHg were then randomized to receive either FMS 120mg q.d. or a fixed-dose combination tablet of fimasartan+hydrochlorothiazide (FMS/HCTZ) 60mg/12.5mg q.d. At week 12, patients randomized at week 8 maintaining a DBP≥90mmHg were escalated to open treatment with the fixed dose combination tablet of FMS 120mg/HCTZ 12.5mg q.d. for the remaining 12 weeks of the study (Fig. 1). At all visits, subjects were instructed to take one tablet of their assigned medication in the morning, with or without food.
Included subjects underwent a medical evaluation including physical exam, vital signs, safety laboratory analyses, assessments of treatment adherence (by means of tablet counting) and assessments of occurrence of adverse events at baseline and at treatment weeks 4, 8, 12, 16, 20 and 24.
Randomization Method. At the end of treatment week 8, subjects not achieving the target (DBP <90mmHg) were randomized by means of a computer-generated randomization list (simple randomization with a 1:1 allocation ratio), to receive either FMS 120mg q.d. or FMS/HCTZ 60mg/12.5mg q.d. The randomization sequence was administered by an independent third party, so that sponsor's staff, investigators and enrolled subjects were blinded to the randomization list.
Blood pressure measurement was performed on the same arm at each clinic visit by either the principal investigator or designated medical personnel experienced and trained in the use of identical calibrated sphygmomanometers (Tycos 767, Welch Allyn) provided to each participating site with appropriate cuffs to accommodate the subject's arm circumference. Subjects had to refrain from performing exercise and/or consume tobacco or coffee for at least 30min prior to blood pressure measurements. Following 5min of rest in the sitting position, the average of two consecutive blood pressure measurements (2min apart from each other) was obtained with the individual and average results recorded on the subject's charts.
Efficacy endpointsThe primary study endpoint was the change from baseline DBP at treatment week 8 (FMS 60mg q.d). Secondary efficacy endpoints included week 8 SBP change from baseline, treatment-week 12 blood pressure changes from treatment week 8 (subjects randomized to FMS 120mg q.d. or FMS+HCTZ 60mg/12.5mg q.d.), treatment week 24 blood pressure changes from treatment week 12 (non-responding randomized subjects escalated to daily FMS+HCTZ 120/12.5mg doses at treatment week 12), blood pressure changes from baseline in subjects receiving 60mg FMS q.d. during the 24 week treatment period and between randomized groups differences on treatment week blood pressure changes from treatment week 8.
Safety endpointsSafety endpoints consisted on the incidence of adverse events, including clinically significant treatment emergent changes on vital signs, ECG parameters, and clinical laboratory measurements (complete blood counts, blood chemistry and urine examinations).
Statistical analysisA sample size of 248 subjects was estimated as sufficient to detect a 1.4mmHg difference in the change from baseline DBP between FMS and an assumed weighted mean change from baseline DBP of −8.375mmHg for telmisartan as estimated from previously published results,10–16 assuming a DBP standard deviation of 6mmHg; with 95% power and a two-tailed 5% significance level, using the sampsi command on Stata-12 (one sample comparison of mean to hypothesized value)17; a final sample size of 270 subjects was determined to account for an estimated 8% drop-out rate assumed based on the dropout rate observed in similar studies.10,11,16 Blood pressure changes from baseline and response rates were analyzed on an intention to treat (ITT) population consisting on all subjects taking at least one dose of study medication and one post-baseline blood pressure measurement using the multivariate normal procedure on NCSS10 (NCSS Statistical Software)18 to impute missing data. Safety analysis was performed on all subjects who received at least one dose of the study medications. To explore the robustness of the primary efficacy analysis, efficacy assessments were also conducted on a per-protocol population consisting on all subjects who completed the planned treatment period with no major protocol deviations (protocol deviations: DBP <90mmHg at screening: 8 cases, included subjects with grade 3 [SBP >180mmHg]: 3 cases, subjects assigned to an erroneous treatment at week 8: 6 cases, 1 subject erroneously assigned to treatment with FMS 60mg+HCTZ 12.5mg at week 4, 1 subject erroneously assigned to FMS 120mg+HCTZ 12.5mg at week 16 and 28 additionally withdrawn subjects). Within-group changes from baseline/reference point in time (i.e., week 8 for randomized subjects and week 12 for subjects escalated to the fixed dose combination of FMS 120mg+HCTZ 12.5mg q.d.) were analyzed using paired t tests, whereas differences between randomized treatment groups were analyzed using Student's t tests in the case of independent means and relative risks in the case of proportions of subjects achieving therapeutic target in both groups. Blood pressure changes from baseline observed in the subset of patients treated with 60mg FMS once daily over the 24 week treatment period were analyzed by means of repeated measures analysis of variance. We used two tailed tests performed at a 5% significance level.
Ethical considerations. This study was conducted in compliance with the principles established in the Declaration of Helsinki. The protocol was approved by the Commission for Sanitary Approval (CAS) of the Mexican Comisión Federal Para la Protección Contra Riesgos Sanitarios (COFEPRIS). Consent was granted by signing a written patient information and informed consent form, previously reviewed and approved by both relevant local ethics committees and the CAS.
ResultsThis study was conducted from April 2013 to August 2014.
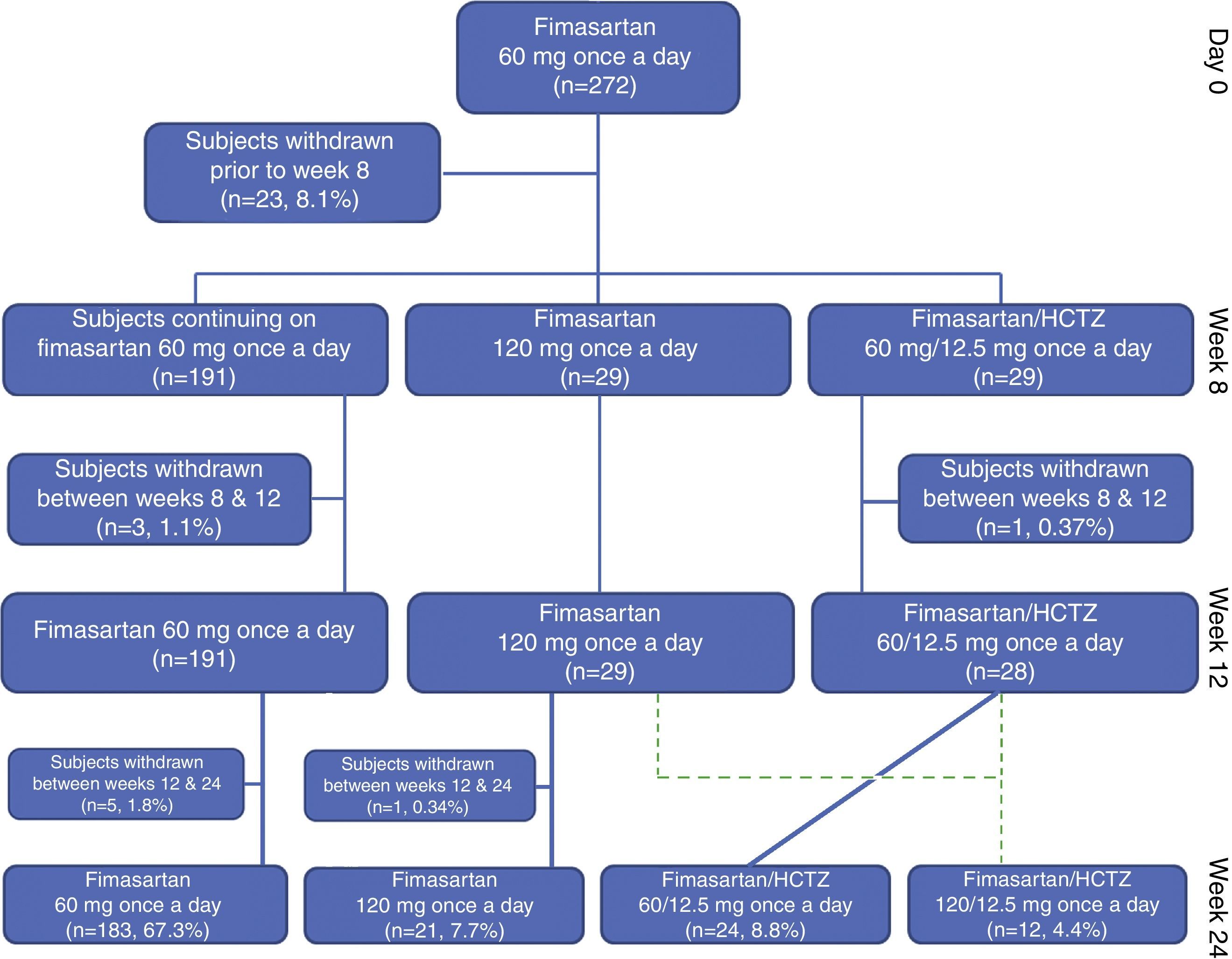
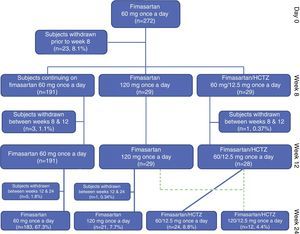
Study populationTwo hundred and seventy two (104 [38.2%] treatment naïve and 168 [61.8%] treatment experienced patients completing the planned washout period), eligible Mexican subjects, started the initial 8 weeks of planned treatment with FMS 60mg q.d. with 33 subjects being withdrawn before completing the planned 24 week treatment period of the study (lost to follow-up: 17 [6.25%], consent withdrawal: 5 [1.84%], non-serious adverse event: 4 [1.47%], treatment non-compliance: 3 [1.10%], investigator's decision: 1 [0.37%], conflict of interest: 1 [0.37%], serious adverse event: 1 [0.37%] and DBP <90mmHg: 1 [0.37]); therefore 239 (87.9%) enrolled subjects completed the trial according to the study protocol (Fig. 2). Only five subjects where withdrawn because of the occurrence of non-serious and serious adverse events.
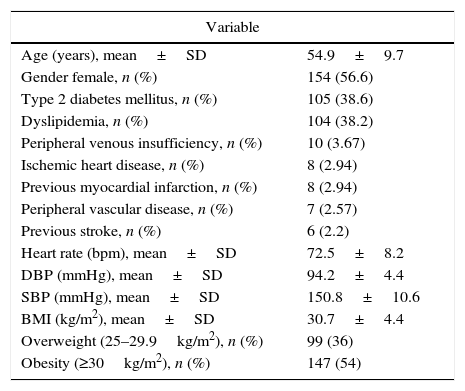
The study population comprised a broad range of patients with essential hypertension, including a significant proportion of subjects with co-morbidities such as obesity (with mean waist–hip ratios of 0.90±0.07 and 0.97±0.05 for female and male subjects, respectively), diabetes, dyslipidemia and coronary heart disease (Table 1). Estimated global assigned treatment adherence rate was 98.6%.
Total study population baseline clinical characteristics.
| Variable | |
|---|---|
| Age (years), mean±SD | 54.9±9.7 |
| Gender female, n (%) | 154 (56.6) |
| Type 2 diabetes mellitus, n (%) | 105 (38.6) |
| Dyslipidemia, n (%) | 104 (38.2) |
| Peripheral venous insufficiency, n (%) | 10 (3.67) |
| Ischemic heart disease, n (%) | 8 (2.94) |
| Previous myocardial infarction, n (%) | 8 (2.94) |
| Peripheral vascular disease, n (%) | 7 (2.57) |
| Previous stroke, n (%) | 6 (2.2) |
| Heart rate (bpm), mean±SD | 72.5±8.2 |
| DBP (mmHg), mean±SD | 94.2±4.4 |
| SBP (mmHg), mean±SD | 150.8±10.6 |
| BMI (kg/m2), mean±SD | 30.7±4.4 |
| Overweight (25–29.9kg/m2), n (%) | 99 (36) |
| Obesity (≥30kg/m2), n (%) | 147 (54) |
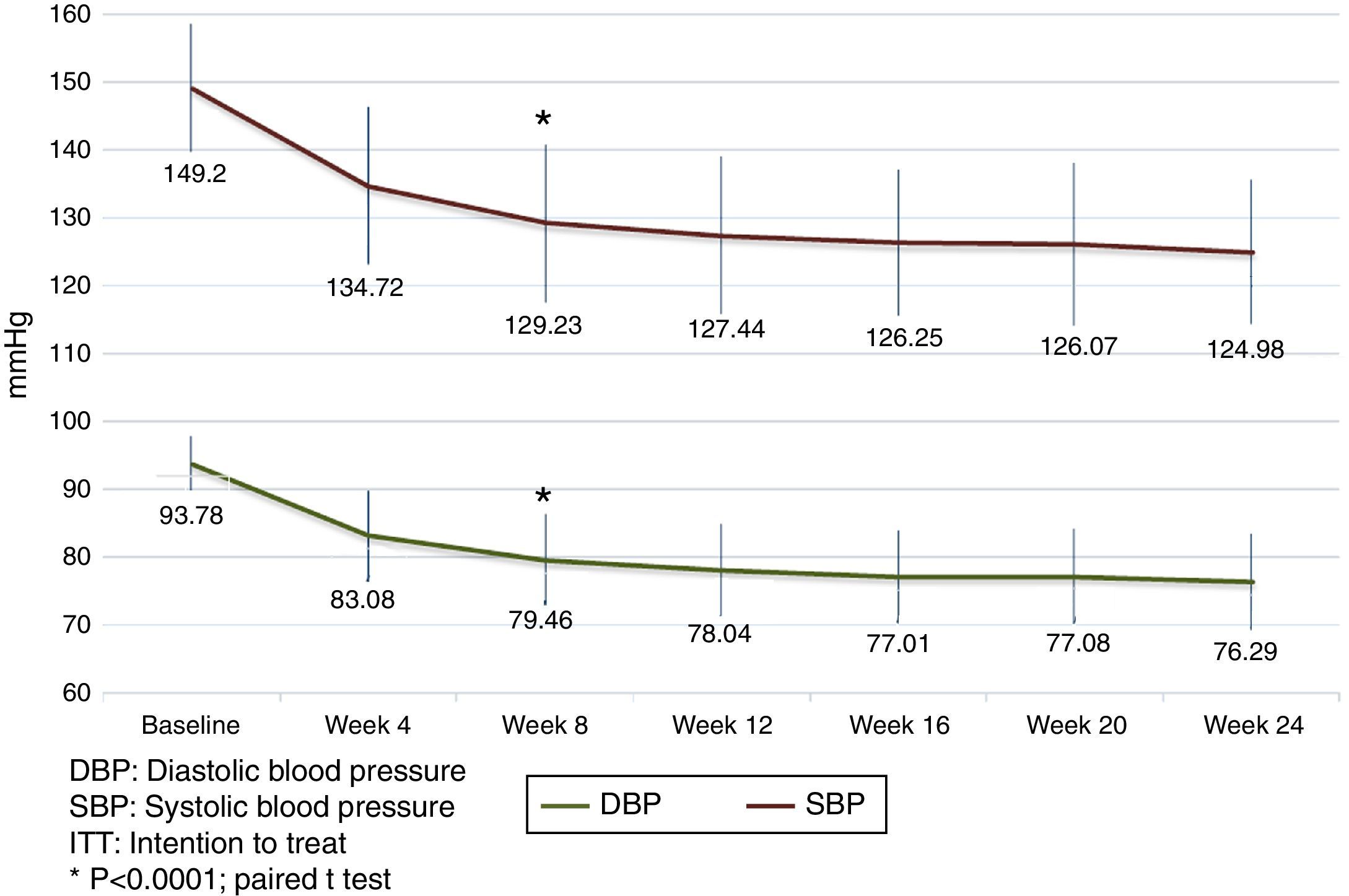
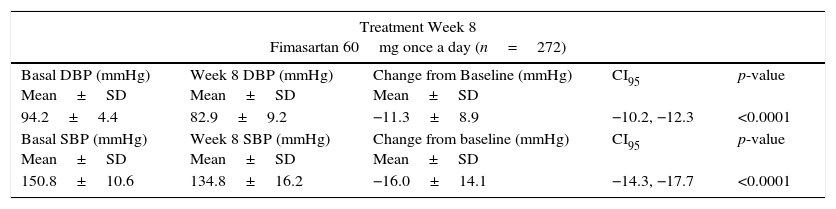
By treatment week 8, FMS 60mg q.d. reduced both DBP and SBP by 11.3±8.9mmHg (p<0.00001) and 16.0±14.1mmHg (p<0.00001), respectively; with 75.4% (205/272) of the subjects achieving their treatment target (defined as a SiDBP <90mmHg). By treatment week 12, 29 and 28 patients randomized to FMS 120mg q.d. or to the fixed dose combination of FMS+HCTZ 60/12.5mg q.d. showed significant blood pressure decreases from their week 8 values, with 58.6% (17/29) and 78.6% (22/28) subjects achieving treatment target. At week 12, twelve non-responding randomized subjects (8 from the FMS 120mg q.d. and 4 from the FMS+HCTZ 60/12.5mg q.d. groups), received FMS 120mg/HCTZ 12.5mg q.d. until the end of the planned treatment period and showed significant DBP and SBP reductions from their week 12 values, with 75% of them achieving treatment target by week 24 (Table 2).
Blood pressure (BP) changes from reference values.
| Treatment Week 8 Fimasartan 60mg once a day (n=272) | ||||
|---|---|---|---|---|
| Basal DBP (mmHg) Mean±SD | Week 8 DBP (mmHg) Mean±SD | Change from Baseline (mmHg) Mean±SD | CI95 | p-value |
| 94.2±4.4 | 82.9±9.2 | −11.3±8.9 | −10.2, −12.3 | <0.0001 |
| Basal SBP (mmHg) Mean±SD | Week 8 SBP (mmHg) Mean±SD | Change from baseline (mmHg) Mean±SD | CI95 | p-value |
| 150.8±10.6 | 134.8±16.2 | −16.0±14.1 | −14.3, −17.7 | <0.0001 |
| Treatment Week 12 Fimasartan 120mg once a day (n=29) | ||||
|---|---|---|---|---|
| Week 8 DBP (mmHg) Mean±SD | Week 12 DBP (mmHg) Mean±SD | Change from Week 8 (mmHg) Mean±SD | CI95 | p-value |
| 96.1±5.2 | 87.9±8.0 | −8.3±8.1 | −5.2, −11.4 | <0.0001 |
| Week 8 SBP (mmHg) Mean±SD | Week 12 SBP (mmHg) Mean±SD | Change from Week 8 (mmHg) Mean±SD | CI95 | p-value |
| 155.6±14.0 | 143.3±12.4 | −12.3±12.5 | −7.5, −17.0 | <0.0001 |
| Treatment Week 12 Fimasartan/Hydrochlorothiazide 60/12.5mg once a day (n=28) | ||||
|---|---|---|---|---|
| Week 8 DBP (mmHg) Mean±SD | Week 12 DBP (mmHg) Mean±SD | Change from Week 8 (mmHg) Mean±SD | CI95 | p-value |
| 95.5±3.9 | 84.7±7.9 | −10.8±7.4 | −7.9, −13.6 | <0.0001 |
| Week 8 SBP (mmHg) Mean±SD | Week 12 SBP (mmHg) Mean±SD | Change from Week 8 (mmHg) Mean±SD | CI95 | p-value |
| 156.2±13.7 | 139.4±16.4 | −16.8±13.2 | −11.6, −21.9 | <0.0001 |
| Treatment Week 24 Fimasartan/Hydrochlorothiazide 120/12.5mg once a day (n=12) | ||||
|---|---|---|---|---|
| Week 12 DBP (mmHg) Mean±SD | Week 24 DBP (mmHg) Mean±SD | Change from Week 12 (mmHg) Mean±SD | CI95 | p-value |
| 95.4±7.2 | 84.5±8.9 | −10.8±7.2 | −5.9, −15.7 | 0.0006 |
| Week 12 SBP (mmHg) Mean±SD | Week 24 SBP (mmHg) Mean±SD | Change from Week 12 (mmHg) Mean±SD | CI95 | p-value |
| 153.9±12.8 | 139.4±13.8 | −14.5±10.6 | −7.4, −21.7 | 0.001 |
SD, standard deviation.
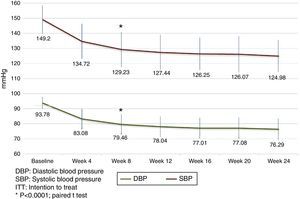
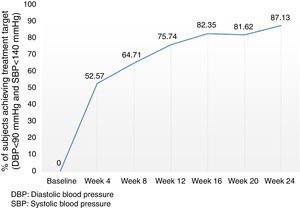
Analysis of the time course of the blood pressure lowering effect in the subgroup of subjects assigned to treatment with 60mg fimasartan once daily throughout the study showed sustained systolic and diastolic pressure decreases which were statistically significant at all post-baseline assessments (i.e., p<0.0001 for all pairwise comparisons between weeks 4, 8, 12, 16, 20 and 24 blood pressure decreases and baseline), with a trend to further blood pressure decreases throughout the study (Fig. 3). Of note, the treatment-to-target escalation strategy explored in this study resulted in a high proportion of participating subjects achieving both a DBP <90mmHg and a SBP <140mmHg (87.1%) by the end of the planned 24-week treatment period (Fig. 4).
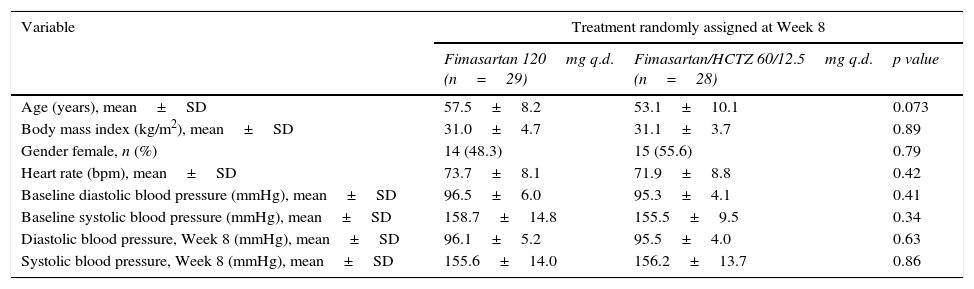
Baseline comparison of subjects randomized at week 8 showed modest, non-significant differences between resulting groups with subjects assigned to FMS/HCTZ 60/12.5mg being younger (53.1±10.1mmHg vs. 57.5±8.2mmHg, p=0.073) and with a slightly lower baseline SBP (155.5±9.5mmHg vs. 158.7±14.8mmHg, p=0.34) in comparison with subjects randomized to FMS 120mg q.d. (Table 3). Between-treatment group comparisons showed that patients randomized to FMS/HCTZ 60/12.5mg q.d. had greater reductions on both SBP and DBP which were not statistically significant (mean difference±SD [95% confidence interval] of 2.51±7.77 [−1.62, 6.63] and −4.49±12.86 [−2.33, 11.32] for the DBP and SBP, respectively. In comparison with subjects assigned to FMS 120mg q.d., the proportion of subjects assigned to FMS+HCTZ 60/12.5mg q.d. who achieved treatment target was higher (12/29, 58.6% vs. 22/28, 78.6%); this difference was, however, not statistically significant (RR=1.34 [95% confidence interval: 0.94, 2.01], p=0.11).
Subjects randomized at Week 8: baseline clinical characteristics (n=57).
| Variable | Treatment randomly assigned at Week 8 | ||
|---|---|---|---|
| Fimasartan 120mg q.d. (n=29) | Fimasartan/HCTZ 60/12.5mg q.d. (n=28) | p value | |
| Age (years), mean±SD | 57.5±8.2 | 53.1±10.1 | 0.073 |
| Body mass index (kg/m2), mean±SD | 31.0±4.7 | 31.1±3.7 | 0.89 |
| Gender female, n (%) | 14 (48.3) | 15 (55.6) | 0.79 |
| Heart rate (bpm), mean±SD | 73.7±8.1 | 71.9±8.8 | 0.42 |
| Baseline diastolic blood pressure (mmHg), mean±SD | 96.5±6.0 | 95.3±4.1 | 0.41 |
| Baseline systolic blood pressure (mmHg), mean±SD | 158.7±14.8 | 155.5±9.5 | 0.34 |
| Diastolic blood pressure, Week 8 (mmHg), mean±SD | 96.1±5.2 | 95.5±4.0 | 0.63 |
| Systolic blood pressure, Week 8 (mmHg), mean±SD | 155.6±14.0 | 156.2±13.7 | 0.86 |
SD, standard deviation.
The exploratory analysis of changes from reference blood pressure values and response rates performed in the per protocol population (n=225) was consistent with results obtained with the ITT population in terms of the magnitude of effect and statistical significance, thus supporting the robustness of the primary efficacy analyses (Supplementary Data).
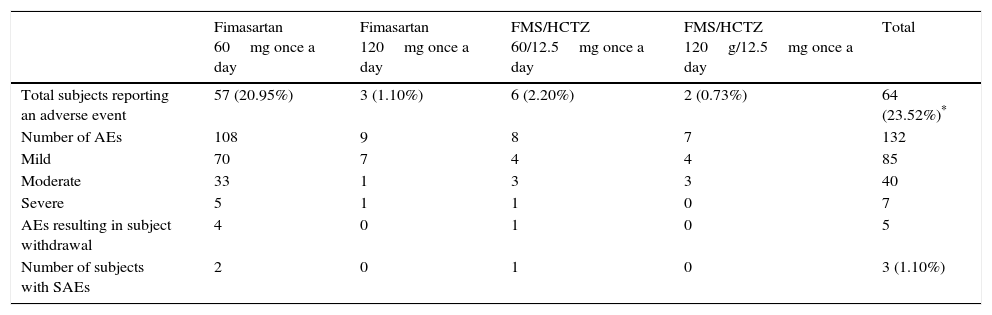
SafetyDuring the whole study period, 64 of 272 subjects (23.5%) experienced 132 adverse events (AEs); most of which were mild to moderate in intensity (125/132 events, 94.7%) (Table 4). Adverse events considered as causally related with the study drugs by the participating investigators were identified in 25/272 (9.19%) subjects. The most frequent related AEs were headache (3.7%), dry mouth (1.1%), non-clinically significant, transient liver enzymes increase (1.1%) and dizziness (0.7%). Two non-serious events (0.7%) of hypotension attributed to FMS were reported, one event was solved with treatment discontinuation and the other resolved spontaneously. Three serious adverse events (one event of narrow angle glaucoma exacerbation and two events of traumatic bone fracture) were observed, all of which resolved with no sequels. None of the serious adverse events were considered by the investigators as related to the study drug. These observations are in accordance with previously reported results.3,4
Treatment emergent adverse events.
| Fimasartan 60mg once a day | Fimasartan 120mg once a day | FMS/HCTZ 60/12.5mg once a day | FMS/HCTZ 120g/12.5mg once a day | Total | |
|---|---|---|---|---|---|
| Total subjects reporting an adverse event | 57 (20.95%) | 3 (1.10%) | 6 (2.20%) | 2 (0.73%) | 64 (23.52%)* |
| Number of AEs | 108 | 9 | 8 | 7 | 132 |
| Mild | 70 | 7 | 4 | 4 | 85 |
| Moderate | 33 | 1 | 3 | 3 | 40 |
| Severe | 5 | 1 | 1 | 0 | 7 |
| AEs resulting in subject withdrawal | 4 | 0 | 1 | 0 | 5 |
| Number of subjects with SAEs | 2 | 0 | 1 | 0 | 3 (1.10%) |
Non-clinically relevant changes of serum creatinine and potassium (K+) were observed. Serum creatinine change from baseline at week 24 was 0.02±0.1mg/dL (from a baseline value of 0.83mg/dL) while serum K+ change from baseline at week 24 was 0.06±0.40 mEq/L (from a baseline value of 4.11mEq/L). No adverse events related with clinically significant changes of serum Cr and/or K+ values were observed.
DiscussionA series of observational studies and clinical trials indicate that commonly used antihypertensive agents may produce variable blood pressure lowering responses and potentially differing long-term outcomes in different ethnic populations when used as monotherapy.8,19–21 Still, specific information on the safety and efficacy of available anti-hypertensive medications – both in terms of blood pressure lowering effects and clinically relevant outcomes, in diverse ethnic groups is lacking.22 Data on the efficacy and safety of antihypertensive agents, specially ARBs, among Mexicans remains limited due to the under-representation of this population in clinical trials; with most of the data primarily restricted to retrospective subgroup analyses.23,24 The results of our study represent a contribution toward filling this gap.
In this Mexican experience with grade 1–2 essential hypertension subjects, FMS monotherapy at a dose of 60mg q.d. resulted in sustained, clinically and statistically significant blood pressure reductions from baseline with 75.4% of subjects achieving the DBP treatment target (DBP <90mmHg). Interestingly, rather than showing an additive blood pressure lowering effect of subsequent treatment escalation, our treat to goal escalation strategy allowed the identification of subsets of non-responders at weeks 8 and 12 with blood pressure levels very similar to their baseline values and who eventually showed clinically relevant blood pressure value decreases with subsequent treatment escalation, resulting in a high proportion of subjects (87.1%) achieving both a DBP <90mmHg and a SBP <140mmHg. We think that this response pattern merits further analysis to determine whether other factors such as renin-angiotensin system-related polymorphisms may influence treatment response in this population.
In conducting this experience, we sought to obtain a reliable estimate of FMSs blood pressure lowering effect in a sample of Mexican subjects by systematically studying a patient cohort based on the same general selection criteria used during the phase III assessment of the efficacy and safety of the drug in the Korean population.3,4,25 The resulting estimates for both DBP and SBP 8 week change from baseline with FMS 60mg q.d. in our experience were consistent with those published by Lee et al.3 (−11.3±8.9 vs −11.0±7.6 and −16.0±14.1 vs −17.8±12.5, respectively) and similar to those reported by Lee H. and Kim K.S. et al.4 (−11.3±8.9 vs. −14.0±7.2 and −16.0±14.1 vs 18.9±9.0, respectively), thus supporting the notion of a similar efficacy of FMS in both the Korean and Mexican population.
The above results are consistent with our initial assumption of minimal to no effect of intrinsic ethnic factors on the tolerability and efficacy of FMS based on its linear pharmacokinetics, flat efficacy/concentration curve, wide therapeutic dose range (which suggest a low potential for differences in tolerability among differing ethnic groups), its minimal hepatic metabolism and low potential for drug-drug interactions as described on the ICH guidance document E5R1 regarding the acceptance of foreign data,26 and supports the usefulness of this document as a guidance for the regulatory acceptance of foreign clinical data.
Observed differences in terms of the blood pressure lowering effect between subjects randomized to 120mg FMS q.d. or to 60/12.5mg FMS/HCTZ q.d., are consistent with existing evidence of a superior effect of combined treatment over monotherapy up-titration,27 but lacked the power needed to reach statistical significance; considered in the context of existing evidence, this result suggests that, in the absence of contraindications to the use of HCTZ, the FMS/HCTZ combination should be the preferred treatment escalation strategy for patients not responding to FMS monotherapy.
The safety and tolerability findings among this cohort of Mexican subjects exposed to FMS alone or in combination with HCTZ were similar to those observed during the initial clinical development of the compound in Korea.
Limitations of this study include its open and non-controlled nature and the fact that sample size estimation was not conducted to provide sufficient power for randomized treatment-group comparisons; the fact that the study was prospectively conducted in a cohort of subjects with a pattern of co-morbidities typically observed among Mexican essential hypertension patients, with a clearly defined “zero time” to determine eligibility and baseline characteristics, and selection criteria and statistical methods (e.g., ITT analysis) similar to those used in controlled clinical trials previously conducted with fimasartan allowed us to obtain estimates of the safety and efficacy of the drug consistent with those observed in Korean subjects.25
ConclusionFimasartan 60 or 120mg, alone or in combination with 12.5mg HCTZ once a day is safe and effective in Mexican patients with grade 1–2 essential hypertension.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThus study was funded by Específicos Stendhal S.A. de C.V. with additional financial support from the Mexican Consejo Nacional de Ciencia y Tecnología (Project number: PEI-467/2013-199007).
Author contributionsErnesto Germán Cardona-Muñoz M.D., Maricela Vidrio-Velázquez M.D., Arturo Guerra-López M.D., Efrain Villeda-Espinosa M.D., Armando García-Castillo M.D., Guillermo González-Gálvez M.D., Sara Pascoe-González M.D., Jose Luis Leiva-Pons M.D., Ramiro Banda-Elizondo M.D., Agustin López-Alvarado M.D., Raul Gerardo Velasco-Sanchez M.D. and Ramón M. Esturau-Santalo M.D. received study drug, blood pressure measurement devices and financial support from Específicos Stendhal S.A. de C.V. for their participation in the study.
Conflicts of interestIgnacio Conde-Carmona M.D. and Gerardo Sánchez-Mejorada M.D. are employees of Específicos Stendhal S.A. de C.V.
We would like to thank Laura García PhD and team at Global Clintrial S.A. de C.V. for their logistic, regulatory, monitoring and data management support during the conduction of the study.