Catheter ablation of ventricular tachycardia (VT) currently has an important role in the treatment of incessant ventricular tachycardia and reduction of the number of episodes of recurrent ventricular tachycardia.
Conventional mapping techniques require ongoing tachycardia and haemodynamic stability during the procedure. However, in many patients with scar-related ventricular tachycardia, non-inducibility of clinical tachycardia, poor induction reproducibility, haemodynamic instability, and multiple ventricular tachycardias with frequent spontaneous changes of morphology, preclude tachycardia mapping. To overcome these limitations, new strategies for mapping and ablation in sinus rhythm (SR) – substrate mapping strategies – have been developed and are currently used by many centres.
This review summarizes the progresses recently achieved in the ablative treatment of ventricular tachycardia using a substrate mapping approach in patients with structural heart disease.
La ablación de la taquicardia ventricular está adquiriendo gran importancia en el tratamiento de la taquicardia ventricular incesante así como en la reducción y prevención de episodios en pacientes con taquicardia ventricular monomorfa sostenida.
El abordaje convencional requiere la inducción de la taquicardia ventricular y la tolerancia de la misma durante el procedimento. Sin embargo, en muchos pacientes con taquicardia ventricular, en contexto de un infarto previo, no es factible la inducción de la taquicardia clínica, la inducción presenta baja reproducibilidad, la taquicardia se acompaña de inestabilidad hemodinámica o se presentan múltiples morfologías con variaciones espontáneas de una morfología a otra que dificultan el mapeo durante la taquicardia. Para superar a estas limitaciones, se han desarrollado las técnicas de mapeo y ablación de sustrato en ritmo sinusal, que actualmente se llevan a cabo en muchos centros.
Esta revisión se centra en los avances realizados en los últimos años en el campo de la ablación de sustrato de la taquicardia ventricular en el paciente con cardiopatía estructural.
Catheter ablation of ventricular tachycardia (VT) is an effective therapy in patients with recurrent episodes of sustained VT.1,2 Most of these patients have an arrhythmogenic substrate characterized by the presence of ventricular scar. Scar can be, not only the result of necrosis due to prior myocardial infarction (MI) in the setting of coronary artery disease (CAD), but can also result from fibrosis occurring in nonischemic cardiomyopathy (NICM) or related to cardiac surgery for congenital heart disease.3
Because most VT result from a reentrant mechanism that is dependent on channels of slow conduction occurring through bundles of viable cells within the scarred tissue, these “isthmuses” of slow conduction and their exit site are the main target of the catheter ablation procedure.3 Identification of this target ablation site can be performed during tachycardia by conventional strategies using activation and entrainment mapping techniques, or in sinus rhythm (SR) by substrate mapping strategies (Figs. 1 and 2).
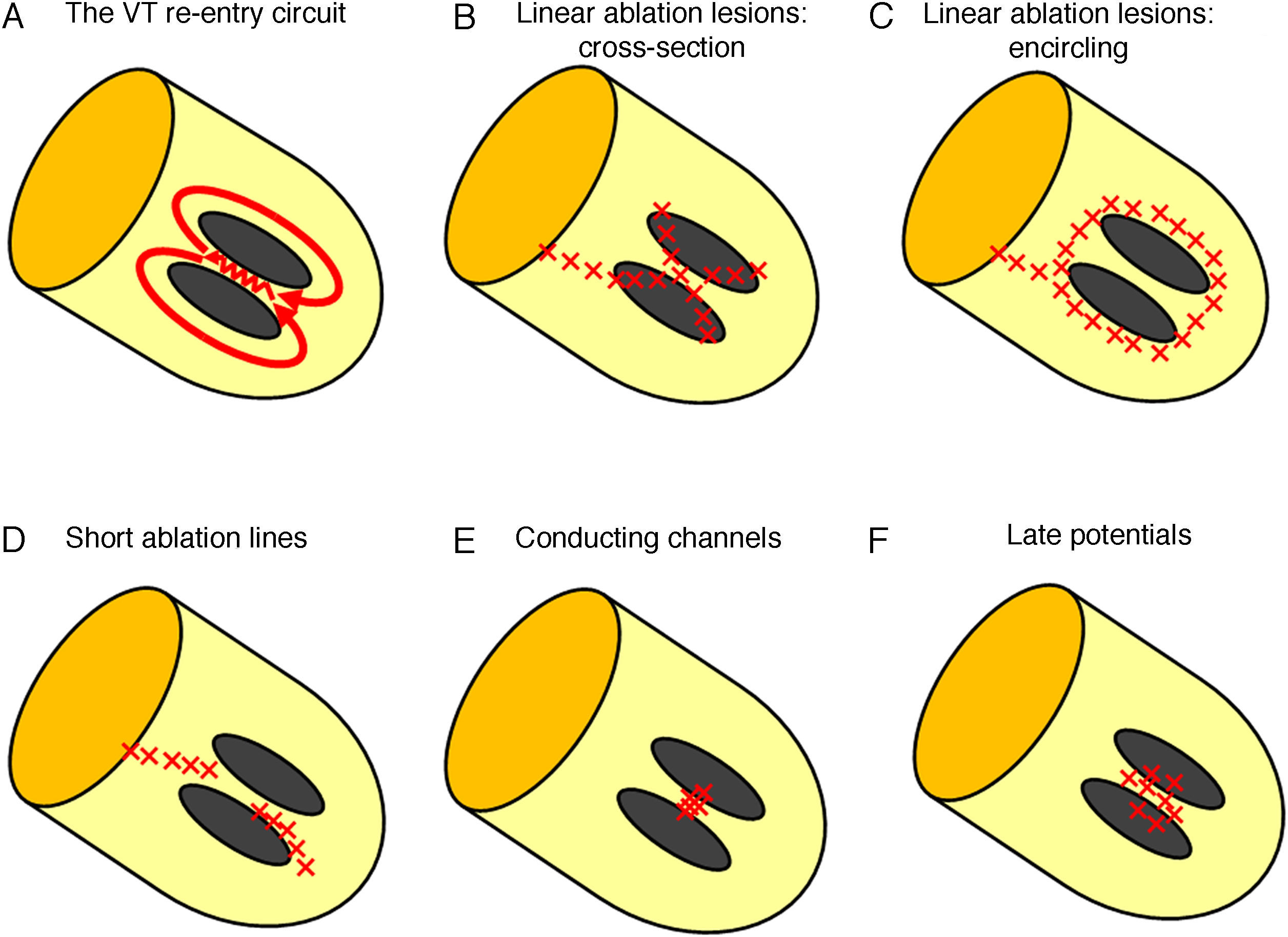
Techniques for substrate ventricular tachycardia ablation. (A) The re-entry circuit for ventricular tachycardia. Two scar areas are shown in grey; the zig-zag line depicts the area of isthmus of slow conduction inside the channel. (B) Linear ablation technique with cross-section of the scar. Linear lesions, identified by contiguous red “×”, extend from dense scar and cross border zone sites showing best pacemaps until the mitral annulus is reached. (C) Linear ablation technique with scar encircling. Linear lesions encircle the borders of the scar and extend until the valvular annulus is reached. (D) VT ablation with short lines. The initial ablation site is selected on the basis of isthmus identification by entrainment or by pacemapping. Additional radiofrequency lesions are applied during sinus rhythm, extending approximately parallel to the border zone of the infarct over 1–2cm until pacing at 10mA at 2ms stimulus strength failed to capture in the region. (E) Conducting channels ablation. Conducting channels are identified by sequential lowering of both upper and lower cut-offs for scar identification. Ablation is performed in the area of the conducting channel. (F) Complete late potentials abolition. Late potentials are identified by manually tagging the latest component of the local electrogram on the electroanatomical map. Ablation is aimed at the complete abolition of late potential activity.
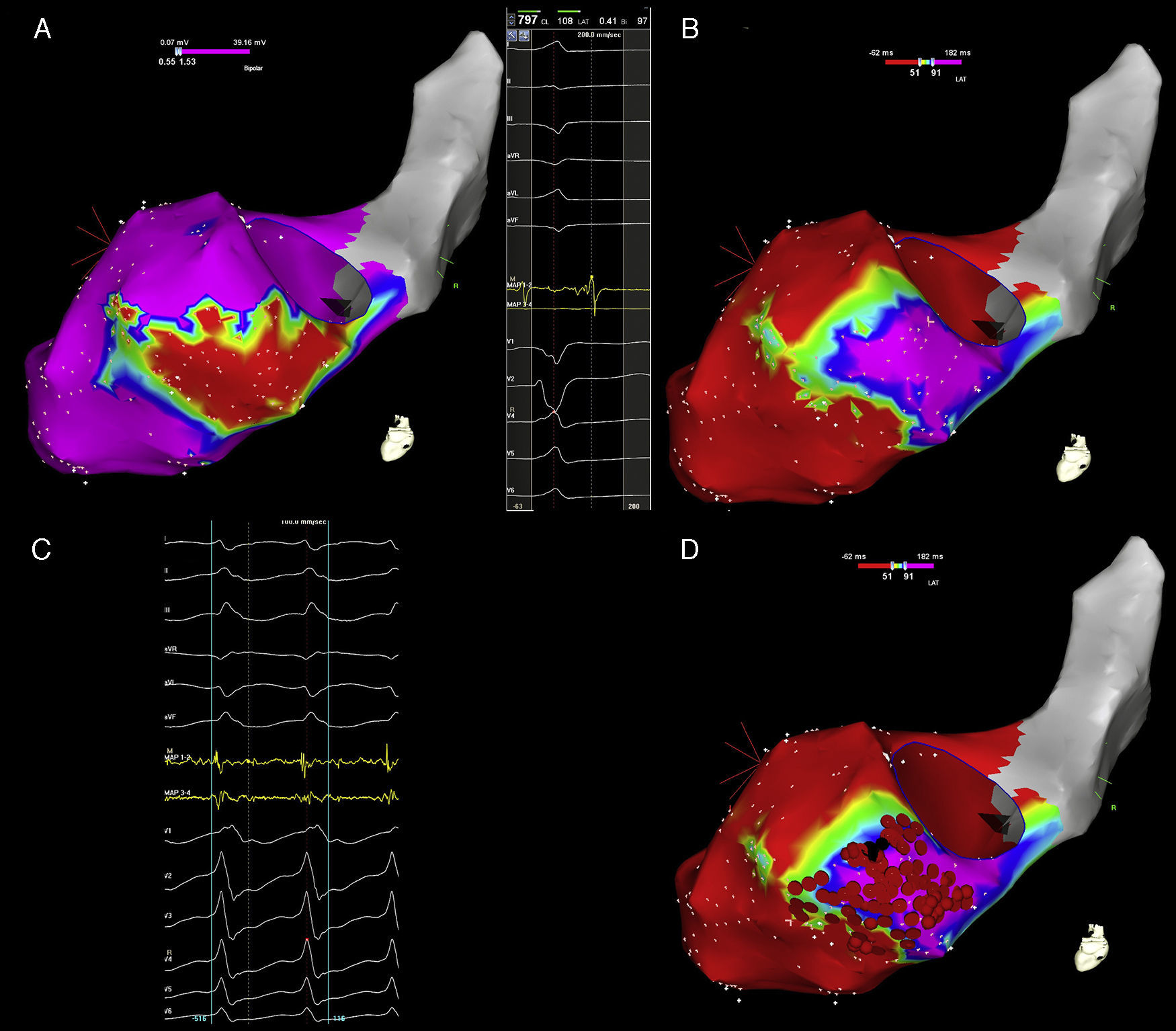
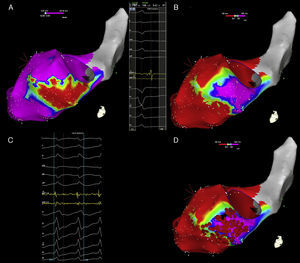
Electroanatomical mapping of the left ventricle with CARTO system and ablation of ventricular tachycardia with activation mapping and late potentials abolition, in a patient with a previous inferior myocardial infarction. (A) Bipolar electroanatomical mapping. The map shows the presence of a large scar area (colour coded in red) in the basal and mid segments of the inferior wall. Settings for scar and border zone identification were <0.5mV and 0.5–1.5mV, respectively. (B) Late potentials map. The left part of the panel shows a late potential recorded far after the end of the surface QRS. The right part of the panel shows the distribution of late potentials in sinus rhythm. The lower cut-off for the activation map was set at the end of the surface QRS complex (51ms); the upper cut-off was set at the timing of the latest late potential recorded (91ms). Areas without late potentials occurring after the end of surface QRS are colour coded in red; the area with late potentials is coded in a gradient of colours ranging from yellow (early after the end of surface QRS) to pink (latest potentials recorded). (C) Activation mapping. The clinical ventricular tachycardia was induced by programmed ventricular stimulation. The distal electrode of the ablation catheter recorded a continuous diastolic activity preceding the surface ventricular tachycardia QRS complex by >70ms. (D) Radiofrequency ablation. Black dots mark the site of successful ablation of the clinical VT, inside the area where late potentials were previously recorded in sinus rhythm. Red dots mark the site of ablation in sinus rhythm, aimed at the complete abolition of late potential activity.
Standard mapping techniques are based on the identification of the critical isthmus of the VT reentrant circuit during ongoing tachycardia, by activation mapping and entrainment mapping techniques.3 Activation mapping during tachycardia allows the acquisition of activation sequence maps by determining the electrogram timing in relation to the QRS onset. It is possible to build up a picture of the VT circuit, aiming at the identification of the zone of slow conduction, as well as the exit site. The former can be identified by diastolic or double potentials occurring during the slow conducting inner part of the circuit, whereas the latter can be identified by the earliest presystolic ventricular electrogram recorded from the mapping catheter electrode.3
Entrainment mapping with concealed fusion identifies the catheter to be in the isthmic zone of slow conduction,4 but it can only prove the location to be an integral part of the reentry circuit; the measurement of the post-pacing interval or the stimulus-QRS/electrogram-QRS interval relation may allow the distinction from bystander sites.4 Termination of VT by a pacing stimulus that fails to capture or by mechanical pressure can also be used to recognize the location on the isthmic zone, even if the sensibility of these criteria is limited.
Limitations of conventional techniquesSince conventional mapping techniques require mapping of the clinical VT during ongoing tachycardia, they have important limitations. If the clinical VT is not inducible with programmed ventricular stimulation (a not uncommon picture in the setting of general anaesthesia), mapping the VT circuit will not be possible, and non-inducibility of VT cannot be used as an end-point for the procedure. In patients with inducible VT at baseline, mechanical block of the VT by catheter manipulation can occur in up to 20% of patients during the procedure, precluding ablation during ongoing VT.5 Even if a sustained hemodynamically tolerated VT is induced, entrainment techniques rely upon the maintenance of VT and reproducibility of VT induction, which is usually highly variable.6
Not infrequently, the clinical VT is poorly tolerated or hemodynamically unstable requiring prompt cardioversion or defibrillation (DC) shock, thus rendering these VTs “unmappable” by conventional techniques. This is to be expected in patients with prior MI in the setting of CAD and poor ventricular function. In patients with very low ejection fraction, haemodynamic deterioration usually occurs with long-lasting VT, and repeated cardioversion or DC-shocks, delivered to terminate it, can be deleterious or not desirable. The same is true for repeated aggressive ventricular stimulation protocols. Intra-aortic balloon pump could partially improve haemodynamic tolerance, but it adds technical complexity to the procedure.7
Moreover, multiple VT morphologies, other than the clinical VT, can be induced in the same patient, rendering the identification of the target VT more difficult to achieve. These VT of undefined clinical significance may not occur spontaneously in the clinical setting, and whether they should be targets of ablation remain uncertain.3 In these patients multiple reentry circuits are often present, and an average of 3–4 different VT can often be induced.8 Localization of all VT circuits by extensive mapping can be difficult to achieve, especially in the presence of unstable VT.8 In this case, VT can be unsuitable for mapping due to haemodynamic instability, frequent changes of VT morphology, or inability to reproducibly induce theVT.8
In the setting of non-inducibility of VT, haemodynamic intolerance of induced VT, or inducibility of non-clinical arrhythmias, a substrate-based mapping strategy can be used.
Substrate mappingCharacterization of the abnormal myocardial substrate is based on the identification of areas of scar and electrogram characteristics that suggest abnormal myocardial conduction. Cassidy et al.9 reported that local bipolar electrograms from VT sites of origin, obtained during intraoperative electrogram mapping in SR, were of significantly lower amplitude and longer duration compared to other sites. They defined as normal electrograms those with an amplitude ≥3millivolts (mV) and ≤70milliseconds (ms) duration.9 Most abnormal electrograms were defined as “fractionated” or “late electrograms”.9 They also noticed that patients with a clinical history of sustained VT exhibited a greater percentage of abnormal endocardial electrograms and more evidence of slow endocardial conduction during mapping in SR than patients with no history of VT or history of non-sustained VT.10 Electrophysiological substrates were also different in patients with ischaemic and non-ischaemic disease.10
Marchlinski et al.11 hypothesized that in patients with unmappable VT, voltage mapping in SR could be used to define abnormal endocardium. Bipolar catheter mapping using the CARTO mapping system was performed during baseline rhythm to define endocardium with abnormal electrogram amplitude.11 In either ischaemic or nonischemic patients, they were able to identify areas of very abnormal signals with an amplitude<0.5mV (dense scar), surrounded by large “border zone” areas with amplitudes between 0.5 and 1.5mV located between the scar and the normal myocardium.11 Based on the study by Marchlinski et al.,11 “normal” ventricular myocardium is currently defined by a bipolar endocardial voltage ≥1.5mV and a bipolar epicardial voltage ≥1mV, “scar” as an area with a signal amplitude ≤0.5mV; areas with a bipolar voltage 0.5–1.5mV within the endocardium and 0.5–1.0mV in the epicardium are defined as “border zone”.11 Validation of these voltage criteria was recently obtained by myocardial viability studies using fluoro-deoxyglucose (FDG) Positron-Emission Tomography (PET).12 In this interesting study, FDG uptake pointed out scar tissue in areas with bipolar voltage<0.5mV, confirmed the presence of viable myocardium in areas >1.5mV, and showed predominantly viable myocardium in the so-called low-voltage border zone.12 A good correlation of electrogram voltage maps with contrast-enhanced Magnetic Resonance Imaging (CE-MRI) scars was also documented.13 Scar locations identified by CE-MRI were correlated with VT reentry circuit isthmus sites.13
Hsia et al.14 found that in patients with monomorphic VT and NICM the site of origin of VT, identified by entrainment with concealed fusion or early presystolic activity and/or pace mapping, corresponded to areas of electrogram abnormalities. Zeppenfeld et al.15 evaluated the significance of LV electrogram characteristics and they reported that the presence of several features, namely an amplitude <1mV, a duration ≥40ms, and the presence of more than four deflections, could predict a successful VT ablation site with 86% sensitivity and 94% specificity in patients with previous MI.15 Among electrogram morphologies that can be found in infarct areas, fractionated potentials are one of the most frequently encountered. Additionally, double potentials, suggesting local conduction block, and late potentials (LPs), suggesting slow conduction, are also frequently seen.
In the non-homogeneous scar tissue, areas of higher voltage, corresponding to surviving bundles of myocardial cells, can function as conducting channels that can be identified by corridors of continuous electrograms inside the scar.16 These viable bundles, although showing higher voltage than the non-conducting surrounding scar, are usually low voltage areas, and can be missed if a value of<0.5mV is simply used to define scar. Arenal et al.16 used different levels of voltage scar definition to identify conducting channels. By applying a step wise reduction on the definition of scar, from 0.5 to 0.1mV, carefully decreasing it in 0.1mV steps, the majority of conducting channels were identified with voltage scar definitions ≤0.2mV.16 The relationship between conducting channels and portions of the reentry circuits was analysed by Hsia et al.17 Entrance sites, isthmus, exit sites and outer loops were identified by entrainment mapping.17 They found that most entrance and isthmus sites were located in dense scar whereas exits were located in the border zone.17 The voltage threshold in the conducting channels ranged from 0.1 to 0.7mV and identification of conducting channels was achieved by careful adjustment of the voltage threshold.17
Techniques for substrate ablation of ventricular tachycardiaVarious techniques have been proposed for substrate mapping VT ablation (Fig. 1, Table 1). Marchlinski et al.11 demonstrated that the application of an extensive set of ablation lesions targeting the border zone during SR could control unmappable VT. They proposed a voltage mapping strategy in SR to define abnormal endocardium, followed by application of linear radiofrequency lesions extending from the dense scar to the normal myocardium or anatomic barriers, or at sites with a good pace mapping.11 Linear ablation lesions along the infarct border zone see med effective in controlling unmappable VT.11
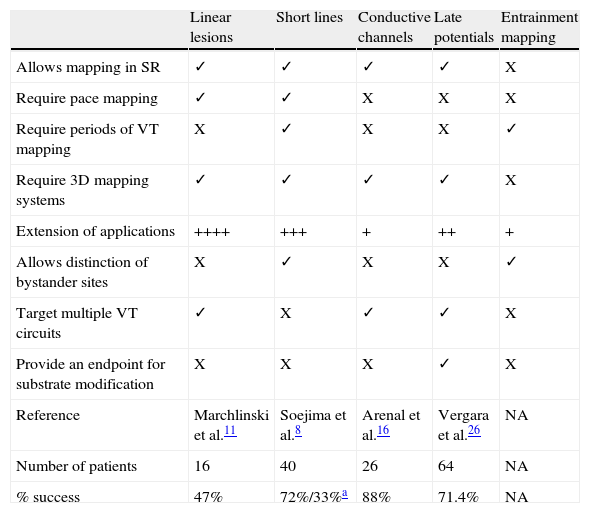
Differences between the techniques for substrate ventricular tachycardia ablation.
| Linear lesions | Short lines | Conductive channels | Late potentials | Entrainment mapping | |
| Allows mapping in SR | ✓ | ✓ | ✓ | ✓ | X |
| Require pace mapping | ✓ | ✓ | X | X | X |
| Require periods of VT mapping | X | ✓ | X | X | ✓ |
| Require 3D mapping systems | ✓ | ✓ | ✓ | ✓ | X |
| Extension of applications | ++++ | +++ | + | ++ | + |
| Allows distinction of bystander sites | X | ✓ | X | X | ✓ |
| Target multiple VT circuits | ✓ | X | ✓ | ✓ | X |
| Provide an endpoint for substrate modification | X | X | X | ✓ | X |
| Reference | Marchlinski et al.11 | Soejima et al.8 | Arenal et al.16 | Vergara et al.26 | NA |
| Number of patients | 16 | 40 | 26 | 64 | NA |
| % success | 47% | 72%/33%a | 88% | 71.4% | NA |
SR: sinus rhythm; VT: ventricular tachycardia; 3D: tridimensional; %: percentage; NA: not applicable.
Soejima et al.8 suggested that, even when multiple and unstable VT are present, less extensive ablation lesions could be effective, if mapping during VT could identify a reentry isthmus. They proposed that the application of short ablation lines guided by the identification of reentry isthmuses by limited periods of entrainment mapping and by target sites identified by a good pace mapping match.8 After the initial ablation site was selected, additional lesions were applied during SR, extending parallel to the border zone of the infarct, until pacing failed to capture or the mitral annulus was reached.8 Inducibility of VT was significantly lower and shorter ablation lines were needed in the sub-group of patients in whom an isthmus was identified, as opposed to the sub-group in which ablation was guided by pacemapping.8
The recognition of conducting channels on voltage maps, along with the identification of electrograms with isolated delayed components in the scar tissue was proposed by Arenal at al.16 as another method for VT substrate modification. Ablation aimed at transection of these channels by radiofrequency pulses suppressed VT inducibility in 88% of patients.16 Additionally, identification of the narrowest part of the reentry channel may reduce the extension of radiofrequency applications.16
Late potentials“Late potentials” (LPs), characterized by fractionated, low amplitude, multiple component electrograms occurring after the end of the surface QRS complex, are usually found in areas of slow conduction and can represent conduction on the reentry circuit isthmus.18–20 The signal originates from conduction in surviving isolated bundles of myocardial fibres within fibrotic scar tissue.18 These fractionated electrical electrograms extending after inscription of the QRS during SR can be the result of delayed conduction velocity and non-uniform anisotropy.18
As previously discussed, LPs were first noticed in the era of surgical therapy of VT by Cassidy et al.9 In patients undergoing radiofrequency ablation for VT after MI, LPs were found in 33% of mapped sites by Harada et al.18 LPs were shown at 71% of sites identified by entrainment as being central or proximal in the reentry circuit, but were also frequently encountered at bystander sites (33%).18 During pace-mapping, the stimulus-QRS complex was longer at LPs sites, suggesting slow conduction.18 A higher incidence of LPs was found near isthmus compared to entrance or exit sites by Hsia et al.19; they also found significantly longer QRS-LP intervals near entrance and isthmus compared to exits.19 Regions with LPs are more likely to be a part of reentry circuits, as radiofrequency current application at those sites more frequently terminated VT than at sites without LPs.18 The amplitude of the LPs present at sites where ablation terminated VT was significantly decreased after radiofrequency delivery.18
Looking for electrogram features that could be helpful in identifying VT isthmus during SR, Bogun et al.20 found that sites with LPs more frequently showed a perfect or good pace mapping, and ablation at these sites resulted in 84% freedom from recurrent VT.20
LPs were used by Arenal et al.21 as a target for catheter ablation for unmappable VT. Electrograms showing isolated and delayed components were identified during mapping in SR and right ventricular apex pacing.21 Ablation targeting these potentials was able to suppress most clinical VT in that cohort of 24 patients.21 Curiously, they realized that, in some patients, mapping during right ventricular pacing was more sensitive than SR mapping in the identification of these abnormal eletrograms,21 possibly related with a change in the direction of the activation front.21 In fact, other studies have already noticed that changing the direction of depolarization can influence the ability to identify target electrograms.22 When mapping was performed during atrial and ventricular pacing, sites showing multipotential electrograms during both atrial and ventricular pacing were more frequently related to a circuit isthmus than those in which they were recorded during one activation sequence only.22
The presence of LPs itself may characterize a subgroup of patients with a more favourable prognosis after ablation.23 In the experience of Nakahara et al.,23 a LP-targeted ablation strategy was more effective in ischaemic cardiomyopathy than NICM patients, while Kuhne et al.24 demonstrated that the presence of LPs was able to predict a better out come after the ablation also in patients with NICM.
In light of what was previously said, it seemed that these LPs “showed us the way” to crucial VT ablation sites. In fact, prior studies in the era of surgical therapy of VT had already shown that subendocardial resection, eliminating endocardial LPs, could also suppress VT inducibility at programmed stimulation.25 If surgical removal of this arrhythmia substrate was able to treat VT,25 could abolition of these LPs be used as an end point of successful VT ablation?
Recently, our group described a strategy for substrate modification in patients with VT, aimed at the electro anatomic definition of all areas of LPs and their complete elimination.26 Activation delay of LPs recorded after the end of the surface QRS was manually tagged on a previously acquired anatomical template of the left ventricle (Fig. 2). The colour-coded maps of LPs obtained were used to define the location and extension of LPs areas, which were then compared to the ones obtained after ablation. Complete LP activity abolition was used in our Laboratory as the primary end point for VT ablation in 64 patients with CAD or idiopathic dilated cardiomyopathy.26 After the substrate map was obtained, programmed ventricular stimulation from the right ventricular apex and multiple left ventricular sites was performed. If hemodynamically untolerated VT were induced, they were terminated by over drive pacing or DC-shock, and ablation proceeded in SR, targeting areas with LPs. If a hemodynamically tolerated VT was induced, radiofrequency ablation was performed during tachycardia, guided by activation mapping. In this case, even after termination of VT during radiofrequency delivery, ablation was continued during SR aiming at the complete abolition of all LPs. In patients without inducible VT but in which an electrocardiographic documentation of the clinical VT was available, pacemapping from the catheter placed in the areas of LPs was compared to the clinical VT morphology. In patients without inducible VT in which only implantable cardiovertor–defibrillator stored electrograms of the clinical arrhythmia were available, no pacing manoeuvres were performed and ablation was performed in SR, aiming at the complete abolition of all LPs.26
At the end of the procedure, prevention of VT inducibility was achieved in 71.4% of patients with previously inducible VT. VT was still inducible in 62% of patients with incomplete LPs abolition whereas in only 16% of those with complete LPs abolition (P<0.01). Complete abolition of LPs was a valuable predictor of the absence of VT recurrences. After a 13 months follow-up period, VT recurrence occurred in 9.5% of patients with LPs abolition and in 75% of those with incomplete abolition (P<0.0001). VT recurrence occurred in 12.5% of patients without VT inducibility after the ablation and in 50% of those with inducible VT (P=0.008). The sensitivity and the positive predictive value of complete LPs abolition (60% and 75%, respectively) were higher than those provided by programmed stimulation immediately after the procedure (50% and 50%, respectively).26
We thus showed that LPs abolition can be an effective endpoint of VT ablation, and that its prognostic value compares favourably with that achieved by post-ablation inducibility.26 Like other methods of substrate mapping, this technique allow to overcome the limitation of non-inducibility or poor haemodynamic tolerance, and it also has the advantage of overcoming the limitations of poor reproducibility of programmed ventricular stimulation. Unlike VT induction by programmed stimulation, LPs elimination is not affected by reproducibility issues, nor is it dependent on stimulation protocols, which can explain the better predictive value of LPs abolition in our study.26 Moreover, this technique can be very useful in that subset of patients with very compromised haemodynamic balance in whom it is advisable to minimize the time spent on VT and to reduce multiple VT inducibility tests. Another advantage of this technique over other methods of substrate mapping is that it provides an objective measure of substrate modification by the possibility to compare pre and post ablation LPs maps. Moreover, in patients with an extensive scar area, this strategy provides a more selective intervention, as opposed to the complete encircling of the scar, because it limits the ablation to a more selective area of LPs activity. As with other substrate mapping strategies, because LPs are also present in bystander sites, it is not possible to differentiate their location on the critical pathway or a bystander for a given VT circuit without using entrainment manoeuvres. Thus, LPs abolition can target not only the critical pathway, but also possible bystander circuits. In scar related VT, shared isthmuses are a common feature19 and multiple VT morphologies are frequently observed, often arising from several different circuits within the same area of the scar.19 In our understanding, in patients with multiple VT morphologies, LPs corresponding to a bystander site for one tachycardia circuit may participate in the circuit of a different tachycardia and, therefore, abolition of all LPs might reduce the arrhythmia recurrence in the long term. From an electrophysiological point of view, this approach changes the endpoint of the ablation from the elimination of a single arrhythmia to the complete modification of the arrhythmia substrate. We suggest that LPs elimination can be used as an additional endpoint to the VT ablation procedure.
ConclusionsSuccessful catheter ablation of VT relies on the identification of the VT circuit. Entrainment mapping during ongoing tachycardia can identify the critical components of the reentry circuit, but requires reproducible inducibility of the arrhythmia, stable VT morphology and haemodynamic tolerance of the clinical VT.
It has been shown that substrate mapping during SR is a successful technique for VT ablation. LPs, identified during mapping in SR, suggest slow conduction and are useful to identify the reentry circuit. The strategy of LPs mapping and their complete elimination reduces VT recurrences and provide an objective measure of substrate modification. We suggest that LPs abolition could be taken into account in substrate-based ablation strategies as an additional technique for reduction of VT recurrence.
Since no data are currently available on the best method for substrate mapping, randomized studies should be performed in order to evaluate the relative efficacy of extensive linear ablation, short lines, conductive channels targeting, and LPs activity elimination approach.
FinancingThe authors did not receive any kind of financing to write this article.
Conflicts of interestDr. Paolo Della Bella (PDB) is a consultant for St. Jude Medical and has received honoraria for lectures from Biosense Webster, St. Jude Medical and Biotronik. No conflicts of interest for other authors exist.