Introduction
The mitral valve is a complex morphologic and functional apparatus formed by the annulus, leaflet valves, tendi-neal chords, papillary muscles and left ventricular wall.1 Pathologic lesions that affect one or more of these components result in a valvular stenosis and/or insufficiency.2
Appropriate diagnosis of the valvular malformation and the assessment of its severity by transthoracic echo-cardiography have been complemented by transesophageal echocardiography which improve knowledge of production mechanisms of the mitral lesions. This, in fact, has contributed to the development of the current interest in surgical repair techniques as an alternative to mitral valve prosthetic replacement.3
The advantages of mitral valve surgical repair techniques over mitral valve prosthetic replacement in adults has been widely accepted.4 However, the development of these techniques in pediatric patients has been slow because of the great variety in the presentation of congenital mitral valve malformations and the still unknown effect of growth over the complex mitral valve apparatus.5-16 Additionally, experience in surgery of mitral valve malformations in Mexico is poor and limited to stenotic lesions.17 Congenital insufficiency, which is the most frequent mitral valve lesion, is not well described in our country.
The objective of this study is to review, the early and mid-term results in the surgical repair of congenital mitral valve malformations at our institution.
Methods
Group of study. Between January 2002 and May 2007, we retrospectively studied all pediatric patients in whom surgical valve repair was performed because of mitral congenital malformation in the National Institute of Cardiology Ignacio Chavez.
From a total of 27 patients with surgical mitral valve repair, we excluded those with acquired mitral valve disease, cyanotic congenital heart disease, atrioventricular septal defect, L-transposition of great arteries, single ventricle, Marfan syndrome, and anomalous left coronary origin from pulmonary artery (ALCAPA). We included in our study 14 patients with congenital mitral valve malformations.
Preoperatory echocardiography diagnosis of the mitral valve malformations was performed in all cases in order to establish the type of valvular lesions according to the pathophysiological Carpentier's classification.8 In those patients with complex mitral valve lesions, the predominant lesion was used for their classification.
Surgical technique: Surgical approach for all patients was through a median sternotomy. Cardiopulmonary bypass was established by aortic and bicaval cannulation. After aortic cross clamp, we used moderate hypothermia and antegrade blood cardioplegia for myocardical protection. Only in one case we used cristaloid antegrade and retrograde cardioplegia. The mitral valve was approached by left atriotomy in all cases, and several mitral surgical repair techniques were used for every patient.
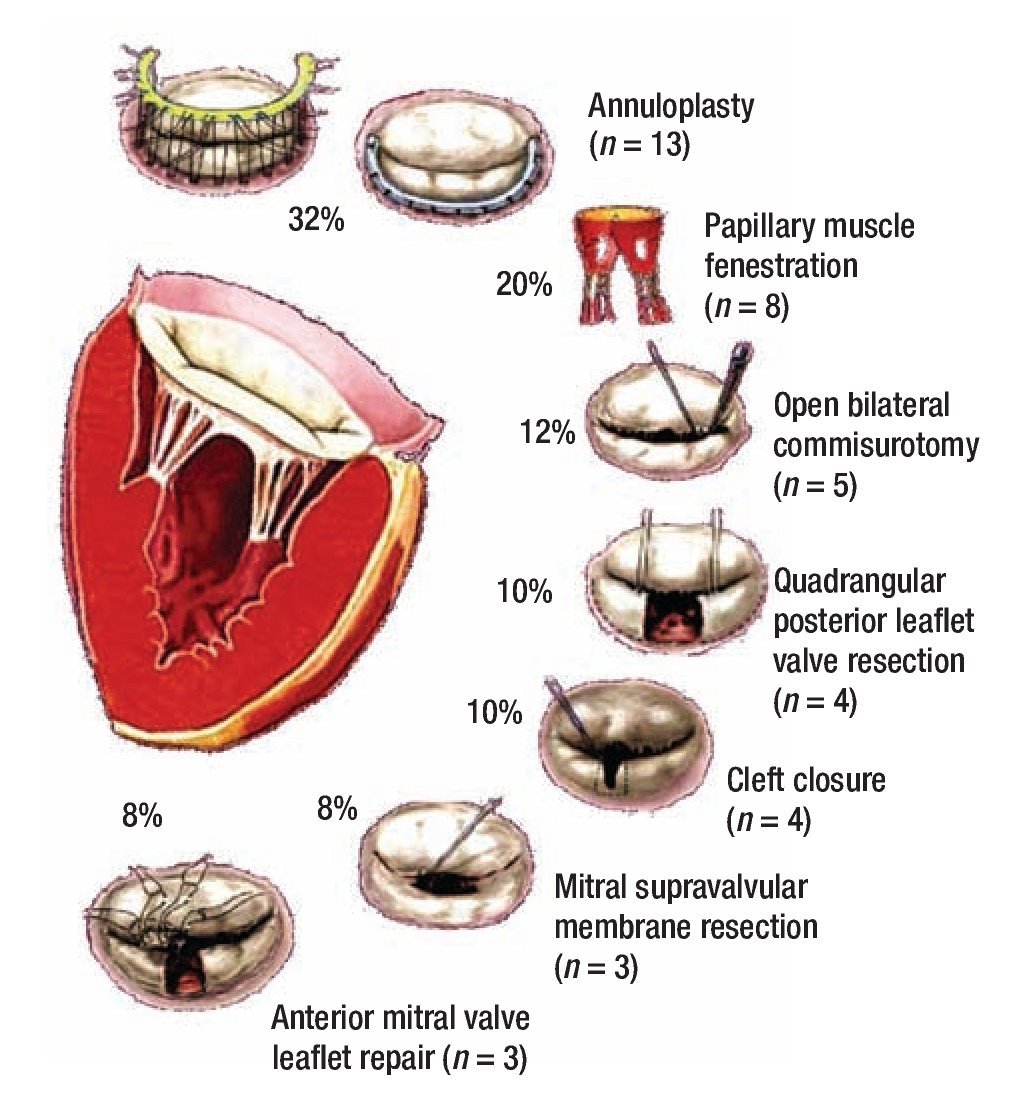
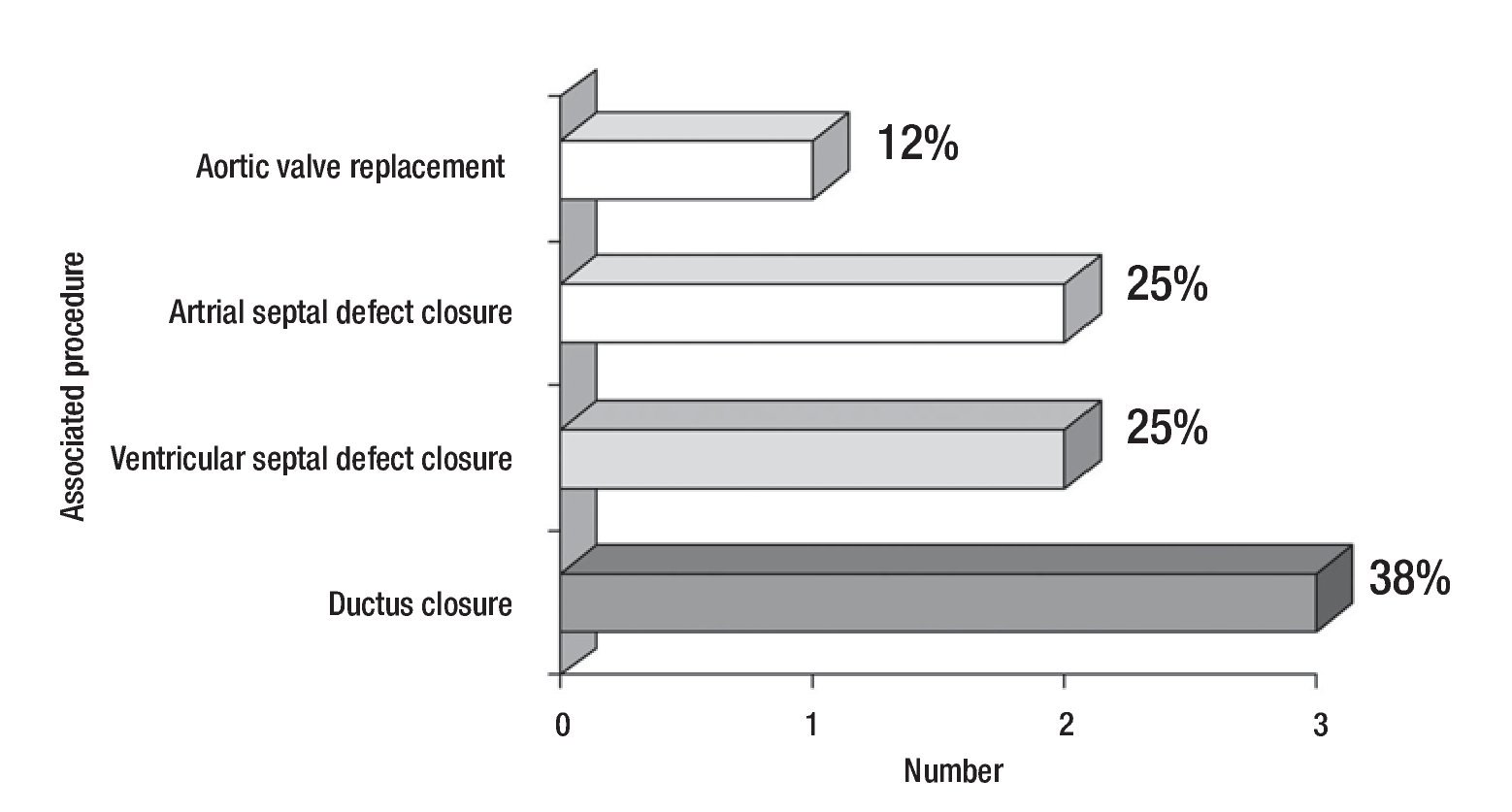
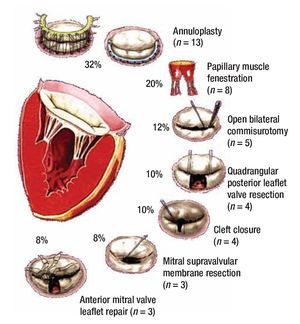
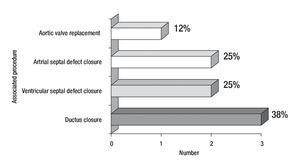
Figure 1 describes the surgical techniques used for mitral valve repair, and Figure 2 the associated procedures. After weaning of cardiopulmonary bypass was completed, and before the decanulation time, we rutinarily performed an intraoperatory transesophageal echocardiography to evaluate mitral competence after valve repair.
Figure 1. Schematic diagrams of surgical mitral valve repair techniques and number of cases.
Figure 2. Number and percentage of associated surgical procedures.
Follow-up strategy: We carried out a clinical and echocardiographic early and mid-term follow-up. All survivors had a clinical follow-up evaluation of their functional class stratification using the New York Heart Association classification (NYHA) for children and young patients,18 and the modified Ross classification for neonates and infants.19 Echocardiographic follow-up was obtained in all of the symptomatic patients and in 50% of the asymptomatic ones. The degree of mitral echocardiographic insufficiency was estimated by a semiquantitative method based in the maximum lenght and width of the regurgitant jet in relation to the left atrium as follows: 0 = none, without regurgitation; 1 = mild, regurgitant jet less than 1/3 the lenght and width of the left atrium; 2 = moderate, regurgitant jet between 1/3 and 1/2 of this lenght and width; 3 = moderate to severe, regurgitant jet between 1/2 and 2/3; and 4 = severe, regurgitant jet larger than 2/3 of the lenght and width of the left atrium. We also evaluated the regurgitant jet direction. Degree of stenosis was minimum, moderate or severe depending on the transvalvular mitral valve gradient and the area of the orifice of this valve in relation to the patient´s age and weight.20
Statistical analysis: Information was obtained from our institutional clinical database, stored in an electronic Excell page and processed with a SPSS statistical software. Numeric results are presented as a mean, variability ranges, and percentages in relation to the risk population. Overall survival rate and freedom from reoperation for mitral valve prosthetic replacement survival rate were calculated using the Kaplan - Meier method.
Results
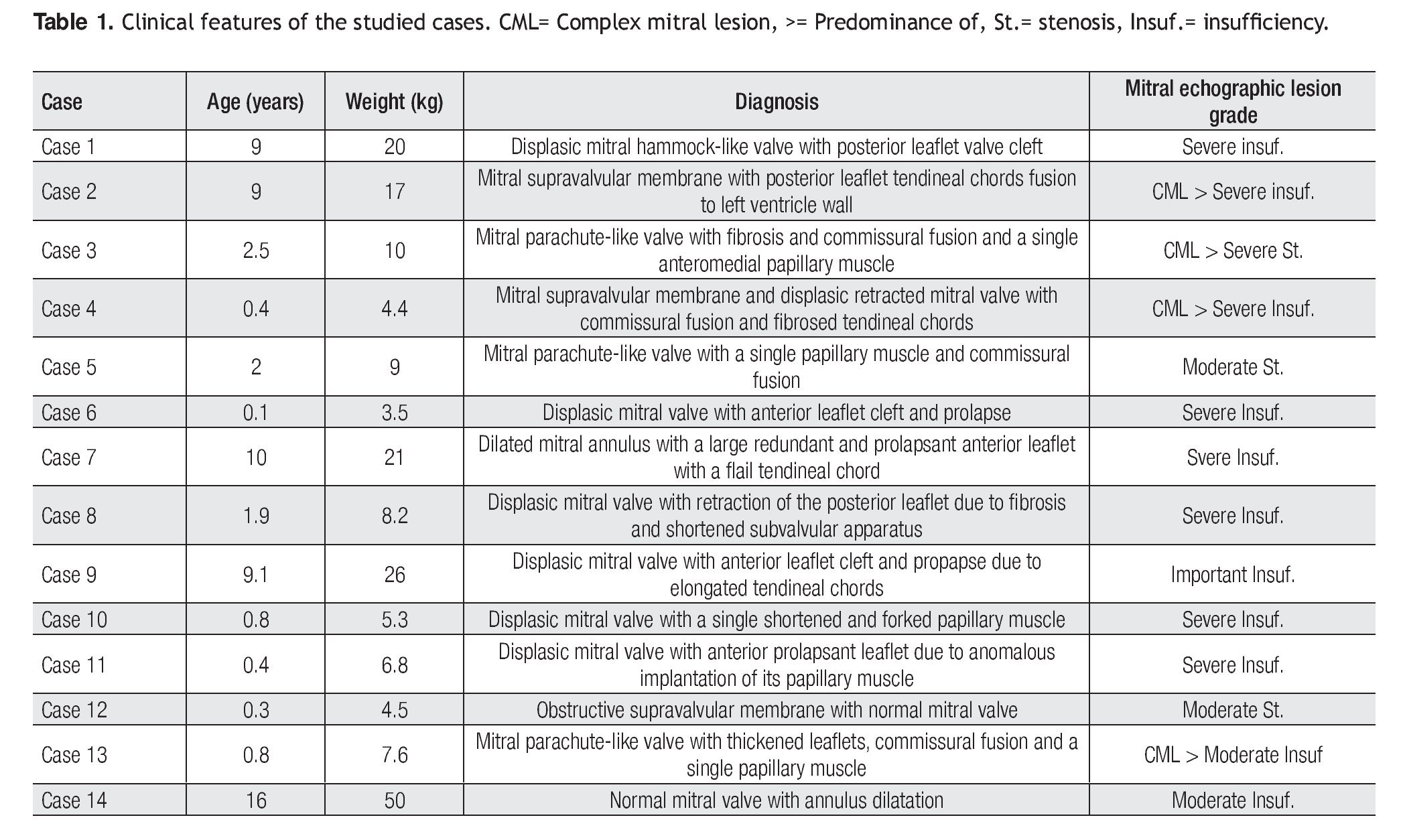
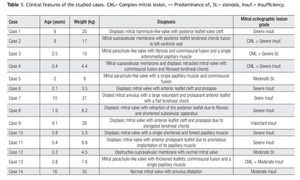
Clinical features of the 14 patients (eight males and six females) of our study are summarized in Table 1. Age ranged between one month and 16 years (mean 4.4 years), with a mean weight of 13.8 kg (range, 3.5 to 50 kg), mean length of 92 cm (range, 50 to 155 cm), and body surface area of 0.6 m2 (range, 0.22 to 1.46 m2).
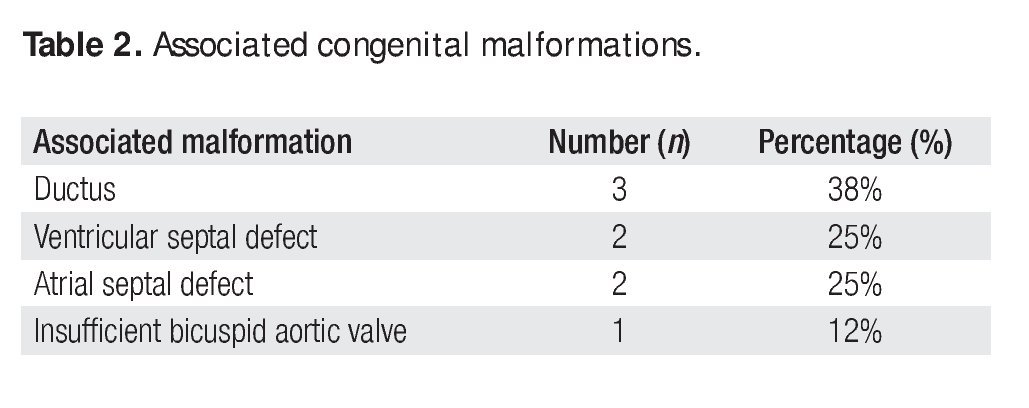
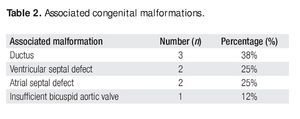
Twelve (86%) of these patients did not have previous cardiovascular surgical or interventional procedures. Two cases had an associated aortic coartation that was treated by surgical coartectomy in one of them (7%). In the other case (7%) an interventional balloon dilatation of the coartated segment was performed. Associated congenital malformations occurred in eight patients (57%), as shown in Table 2.
Sinus rhythm was present in all patients at the time of admission to the hospital, and in two of them (14%) the electrocardiogram (ECG) showed a right bundle branch block. Half of the patients were in heart failure preoperatively, but only one (7%) required inotropic support before surgery. None of the patients needed mechanic ventilation, and all of them received an appropriate preoperatory medical treatment in order to arrive to surgery in the best hemodynamic conditions as possible.
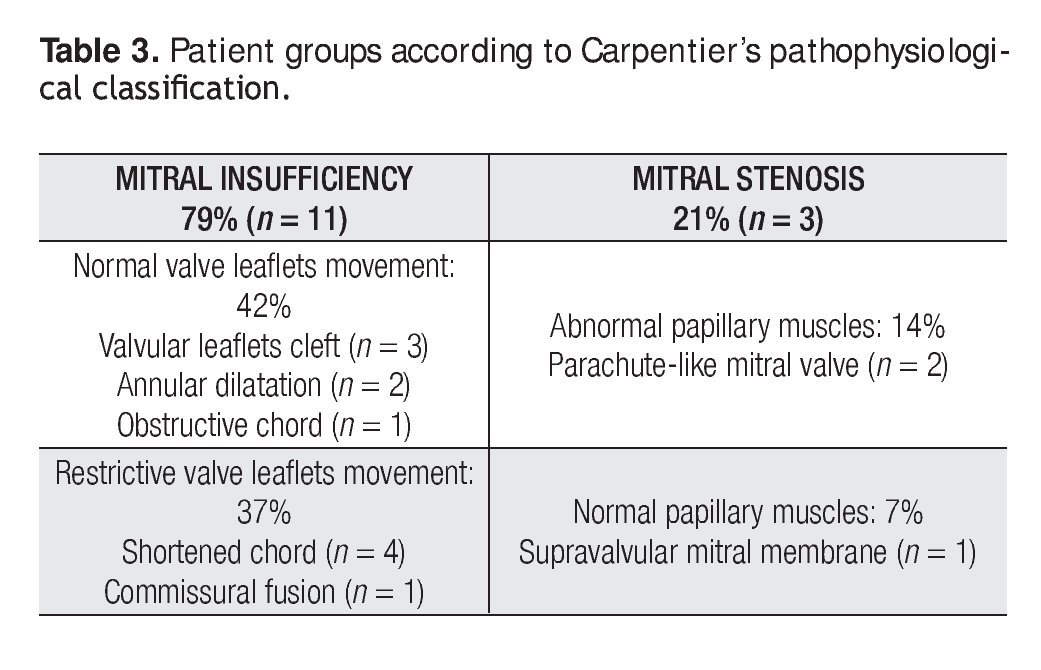
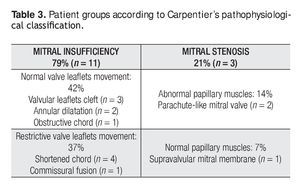
Mitral valve repair was possible in all of the cases, with a mean cardiopulmonary bypass time of 96min (range, 44 to 174min), aortic cross-clamp of 61 min (range, 18 to 134min) and 29°C hypothermia (range, 25 to 32°C). Mitral predominant lesion was incompetence in 11 patients (79%) and stenosis in three (21%). Table 3 shows the groups of patients according to Carpentier's pathophysiological classification.8
The surgical techniques used were mitral annuloplasty, papillary muscles fenestration, open bilateral commisurotomy, valve cleft closure and supravalvular mitral membrane resection. Less frequently used techniques were anterior valve leaflet repair (with bovine pericar-dial patch enlargement, tendineal chords resuspension or reduction) and quadrangular posterior leaflet valve resection. We used bovine pericardium for all mitral annuloplasties, except in one case that received a rigid Carpen-tier hemiannulus. Concurrent repair of associated lesions included ductus closure in three cases, ventricular septal defect closure in two cases, and atrial septal defect closure in two cases. Additionally, we performed an aortic valve replacement in the oldest patient of our series (16 years) who had a severe regurgitant congenitally bicuspid aortic valve in association with a moderate mitral valve insufficiency (Figure 2).
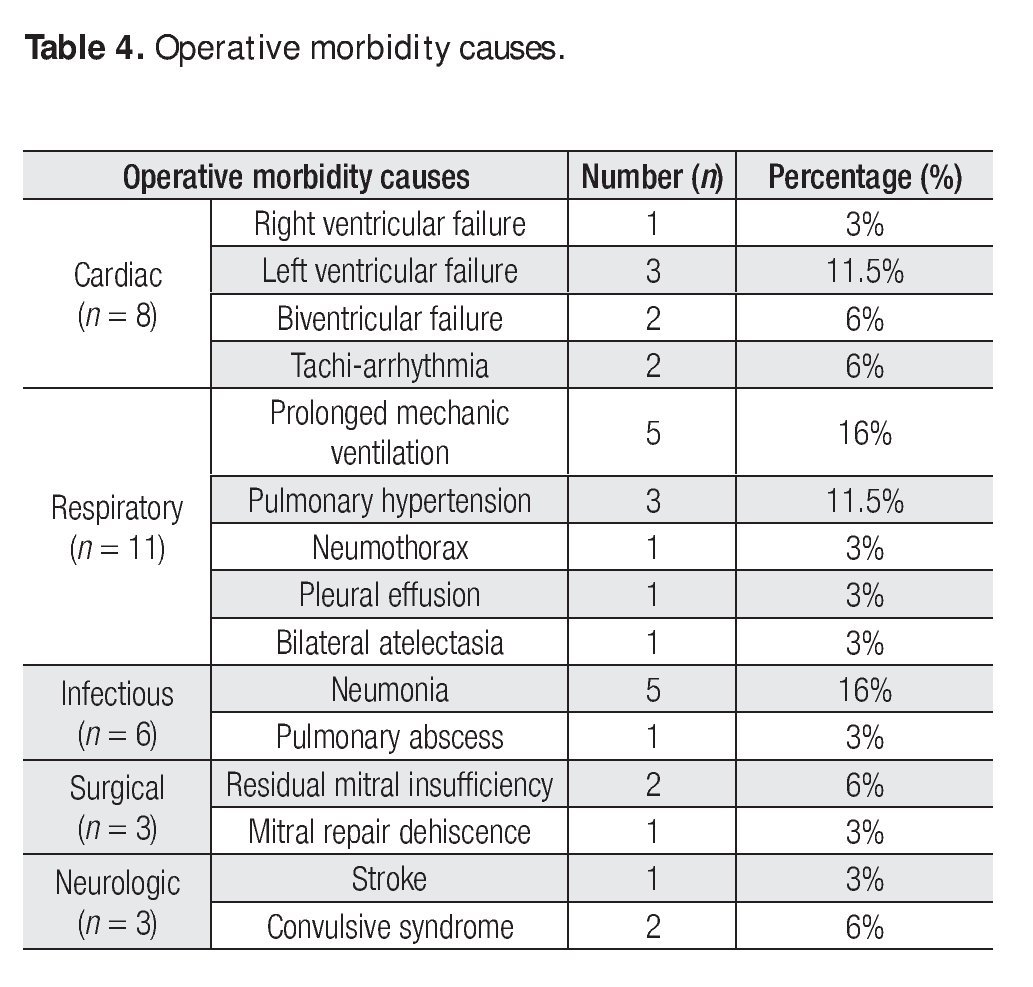
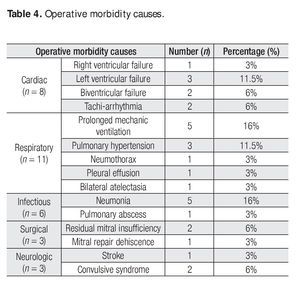
There were no operative complications and operative morbidity rate was 77%. Morbidity events are described in Table 4. There was only one early death (operative mortality of 7%), from hypovolemic shock from a subclavian artery lesion due to a re-introduction of a central venous catheter.
The 13 survivors had a mean postoperative hospitalization stay of 15.5 days (range, 4 to 56 days), and 3.7 days (range, 1 to 11 days) in the Intensive Care Unit, with a mean mechanic ventilation time of 4 days (range, 2 hours to 20 days). There were four patients who needed reoperation during the early postoperative period (30.7%), with a mean of 1.2 (range, 1 to 2) reoperations per patient with mean time of 11.5 days (range, 1 to 28 days) after the first surgery. In two cases, reoperations were needed for extracardiac reasons (tracheostomy with gastrostomy, and left superior lung abscess drainage). In another case, reoperation was to perform a pericardial window because of pericardial effusion. Finally, two of the reoperations were for mitral valve prosthetic replacement because of early failure of the mitral valve repair. The first patient was a six month male infant with a mitral supravalvular membrane, a dysplastic stenotic mitral valve due to tendineal chord fibrosis, commissural fusion and retraction of the anterior mitral valve leaflet. In the operation we resected the mitral supravalvular membrane, performed a commisurotomy and a bovine pericar-dial patch ampliation of the anterior mitral valve leaflet. Although transesophageal echocardiography reported a moderate residual mitral valve regurgitation, weaning of cardiopulmonary by-pass at the first time was successful without early hemodynamic deterioration. In the early postoperative period he developed clinical and radiologic signs of acute pulmonary edema, and six hours later was reoperated for mitral valve replacement with an aortic St. Jude 17 mechanical prosthesis placed invertedly in a supra annular mitral position, with a successful postoperative course. The second patient was a eight months female infant who had a dysplastic mitral valve with a single shortened and forked papillary muscle. We performed a bilateral commisurotomy, papillary muscle fenestration and an annuloplasty with a bovine pericardial hemiannulus. Additionally she had a ventricular septal defect that was repaired with a bovine pericardial patch and a patent ductus arteriosus that was closed at the same time. Moderate stenosis and regurgitation without residual septal defects was reported by the transesophageal intraoperative echocardiography. Postoperatively she had prolonged mechanic ventilation due to heart failure, and nine days later we decided to reoperate this patient for mitral valve replacement with a mechanical Carbomedics 17 mitral valve in annular position, with good postoperative results. Up to date, both patients are asymptomatic and with appropriate prosthetic valve performance at the echocardiographic control.
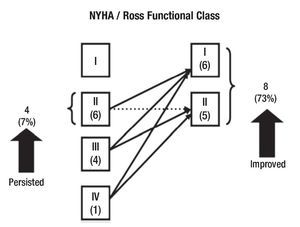
Clinical follow-up was made at a mean time of 29 months (range, 15 days to 57 months) in all of the survivors. Figure 3 shows functional class stratification according to the NYHA and the modified Ross classification evolution between preoperative and mid-term follow-up in patients with mitral insufficiency. All of the patients with mitral stenosis which had III NYHA/Ross functional class improved to class I. Morbidity rate at mid time follow-up was of 15.3% (two patients). One of these patients developed an embolic stroke with left hemiplegia and gastroesophageal reflux. The other one required a mitral prosthetic valve replacement two years after the first operation, because of mitral valve repair failure. Additionally, a patient in our series developed mitral valve repair failure at the mid time (22 months), of no important clinical significance. In this case, optimum medical treatment has allowed us to delay the time of reoperation until he reaches the adequate age to perform a definitive prosthetic valve replacement.
Figure 3. Change in NYHA/Ross functional class after mitral valve repair in patients with mitral insufficiency.
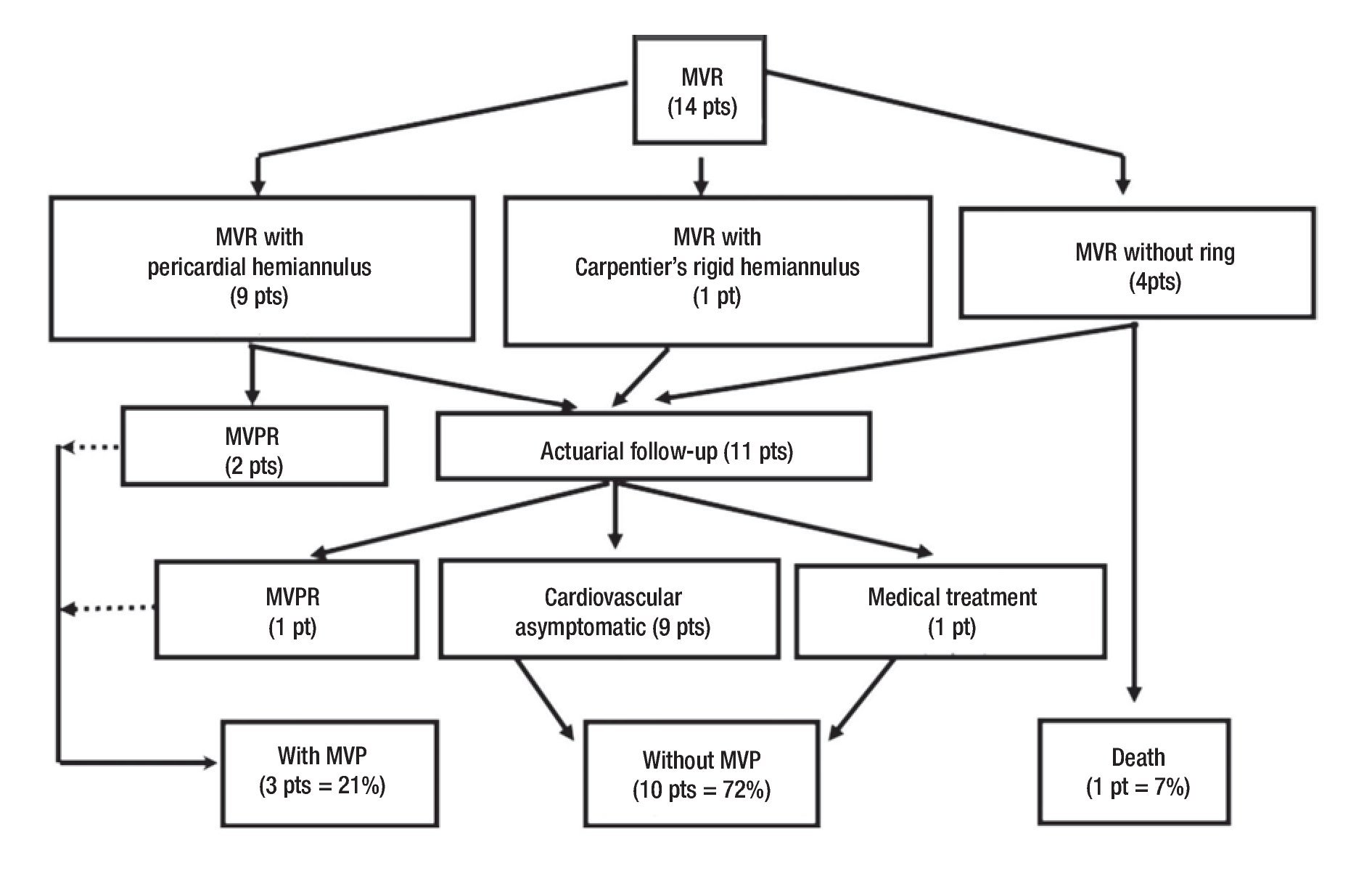
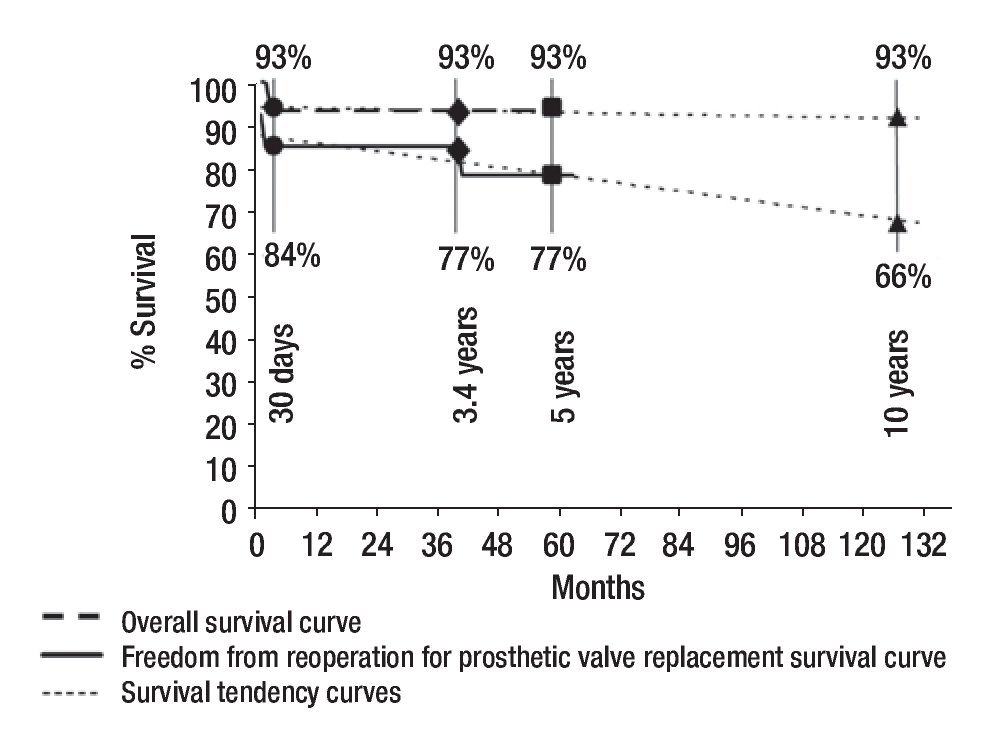
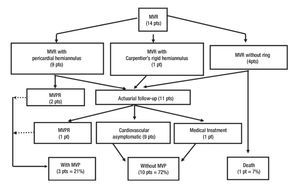
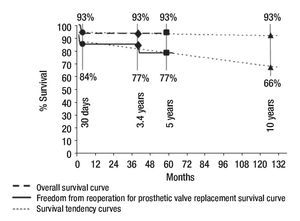
We did an echocardiographic follow-up in eight of the 13 survivors (65%) at a mean time of 1.8 years (range, one month to 2.6 years). All the remaining patients who did not have echocardiographic follow-up were asymptomatic. Only three of the patients who had echocardiographic follow-up were symptomatic. Therefore, we made an echo-cardiografic follow-up in all the symptomatic patients and in 50% of the asymptomatic ones at a mean time of 21 months (range, 10 to 33 months). In Figure 4 we resume the clinical outcome of our patients. There were no late deaths. Figure 5 shows that the overall survival rate after surgical mitral valve repair is of 93% at 30 days and does not vary up to 3.5 year follow-up. It also shows freedom from reoperation for mitral valve prosthetic replacement survival curve in the early and mid time follow-up. We can say that the successful rate observed in our series for surgical mitral valve repair was 84% at 30 days and 77% at 3.5 years. The estimated freedom from reoperation for mitral valve prosthetic replacement survival curve al 5 years was 77% and 66% at 10 years follow-up.
Figure 4. Clinical follow-up outcome of surgical treatment in congenital mitral valve malformations. MVR= Mitral valve repair, MVPR= Mitral valve prosthetic replacement, MVP= Mitral valve prosthesis, pt(s)= patient(s).
Figure 5. Early and mid-term curves and tendencies of overall survival and freedom from reoperation for mitral valve prosthetic replacement survival after surgical mitral valve repair.
Discussion
The spectrum of congenital mitral valve malformations ranges from the readily reparable anterior mitral leaflet cleft to the restrictive and challenging lesions of mitral stenosis. Whatever the abnormality, the incidence of associated heart defects is high6,9,11 and can have a significant impact on the eventual clinical outcome.
Because of the significant short- and long-term problems with mechanical and bioprosthetic mitral valves in children21,22, considerable attention has been paid to mitral remodeling techniques9,11,15 for both incompetent and stenotic valves. This trend has lowered the perioperative mortality from as high as 21% to 43% for mitral valve replacement5,23,24 to as low as 0% to 5% for mitral valve repair.9,10,15,16,23 Our overall operative mortality is along with these trends.
One of the most common causes of mitral insufficiency in our series was anterior leaflet valve cleft, as well as shortening of tendineal chords. The simple cleft closure with or without papillary muscles fenestration was the only procedure used for a correct mitral valve competence. More challenging is the infant and young child with annular dilatation and mitral insufficiency who will require native annular remodeling with a posterior hemiannulus. Although we used a rigid Carpentier hemiannulus in a young child, in infants and children in growing ages there are more problems in choosing a correct prosthesis size. Chavaud and associates9 have approached this problem by performing the posterior annular reduction without the use of a rigid prosthesis. Based on Rowlatt's,25 measurements they used Goretex or pericardial hemiannulus for the reduction of the posterior native mitral annulus, because the anterior aspect does not enlarge as much due to its fibrous constitution. In our series we only used one Carpentier rigid hemiannulus in a young child. All of the other children received a bovine pericardial hemiannulus. This biologic tissue is similar to the autologus pericardium, but has the advantage in that it is easier to manipulate. The manufacturing of this material in our institution and its availability in different sizes and thickness without an additional economic cost, makes bovine pericardium the material of choice to perform the mitral hemiannular posterior reduction in infants and children at growing ages in our series.
The most common cause of mitral stenosis in our series was the parachute-like mitral valve, which was treated with fenestration of papillary muscles in most of the cases. Another cause was supravalvular mitral membrane, usually associated with other stenotic lesions such as redundant valvular tissue, and commisure and/or papillary muscle fusion. For this reason, simple resection of the supravalvular membrane was not able to achieve an appropriate relief of the mitral stenosis, so we associated another procedure such as open mitral commisurotomy, papillary muscle fenestration, or resection of redundant valvular tissue.
There were three patients in our series who needed reoperation because of failure of the original mitral valve surgical repair. Transesophageal intraoperatory echo-cardiography was very useful to evaluate the immediate results of surgical mitral valve repair, and identify early technical failure. We only had a patient who developed hemodynamic instability and needed mitral valve pros-thesis replacement before chest closure. Hemodynamic status was acceptable in the other two cases after weaning of cardiopulmonary bypass. This fact allowed the surgeon for chest closure, but they developed cardiac failure in the Intensive Care Unit that requiring reoperation for mitral valve prosthetic replacement. One of them was reoperated before 30 days and the other after two years of the first mitral valve repair. The early reoperation rate compares favorably to other reports 5,6,13,15 of mitral valve repair in children. The immediate reoperation rate before chest closure on the basis of hemodynamic findings and intraoperative transesophageal echocardiography is not widely reported. In our series the outcome of the 3 reoperated patients (23%) was favorable after the mitral valve prosthetic replacement. Most reports dealing with congenital anomalies5,9,10 recognize the importance of intraoperative transesophageal echocardiography for functional evaluation giving the surgeon the option for reintervention as deemed necessary.
Although we believe that intraoperative transesophageal echocardiography is an important method that allows the surgeon to decide which is the best surgical mitral repair technique to use and to evaluate the early results, our final decision depends on the type of patient we deal with. The low socioeconomic level of the majority of our patients and their rural environment difficults an appropiate oral anticoagulation treatment, because of the lack of INR laboratory resources close to home. This is the reason why we try to preserve their native mitral valve the longer time as possible, even if we know by echocardio-graphic evaluation that the result is not always entirely satisfactory.
Our overall survival early and mid-term rate, as well as the freedom from reoperation for mitral valve prosthetic replacement survival rate were favorable and similar to the experience of other centers.5,9,10,15,26 The low incidence of thromboembolic episodes observed in our patients is also consistent with the findings of others5,9,10,15,26 and contributes to the low postoperative morbidity. We believe that anticoagulation therapy is not needed for patients with surgical mitral valve repair except for the first three months postoperatively in our only case where Carpentier´s rigid hemiannulus prosthesis was used. This is the time a rigid hemiannulus prosthesis requires to become endothelized.
Conclusion
Malformations of the mitral valve are not a common pathology, and our experience with this study has the proper limitations of a retrospective one. Although this series cannot lead us to drive definite conclusions, we can say that mitral valve repair in pediatric patients is probably the best technique option in the treatment of congenital malformations of the mitral valve, because of the satisfactory early and mid-term outcomes, low global mortality and a high freedom from reoperation for mitral valve prosthetic replacement survival rate. In addition, mitral valve repair allows preservation of the native valve without interfering with its growth, avoiding anticoagulation at an early age. On the other hand, the routine use of transesophageal intraoperative echocardiography must be highly recommended for evaluation of results in a surgical mitral valve repair procedure.
Corresponding author: Pedro José Curi Curi.
Juan Badiano Nº 1, Colonia Sección XVI, Tlalpan. Mexico City.
Telephone: 55545828. Fax: 52 (55) 55730994.
E-mail:pcuricuri@gmail.com
Received in July 9, 2009;
accepted in October 9, 2009.