Up to April 20, 2020, COVID-19 (English acronym for coronavirus disease 2019) has affected 204,178 people in Spain and caused the dismal number of 21,282 deaths1, including our colleague from Valencia, Dr. Vicente Sánchez. From here we extend our heartfelt sympathy and condolences to his family.
In the past 10 days the tide of the pandemic seems to be turning as shown by indicators in our country, with regularly decreasing numbers of contagions and daily deaths1. For this reason, health authorities are beginning to plan a possible transitional phase, known as “descaling”, (an English-language term that is not approved by the Royal Academy of the Spanish Language) for existing healthcare conditions.
However, it is well-known that accessibility to SARS-CoV-2 tests (severe acute respiratory syndrome coronavirus 2), the virus that has caused COVID-19, has been and continues to be limited for health professionals.
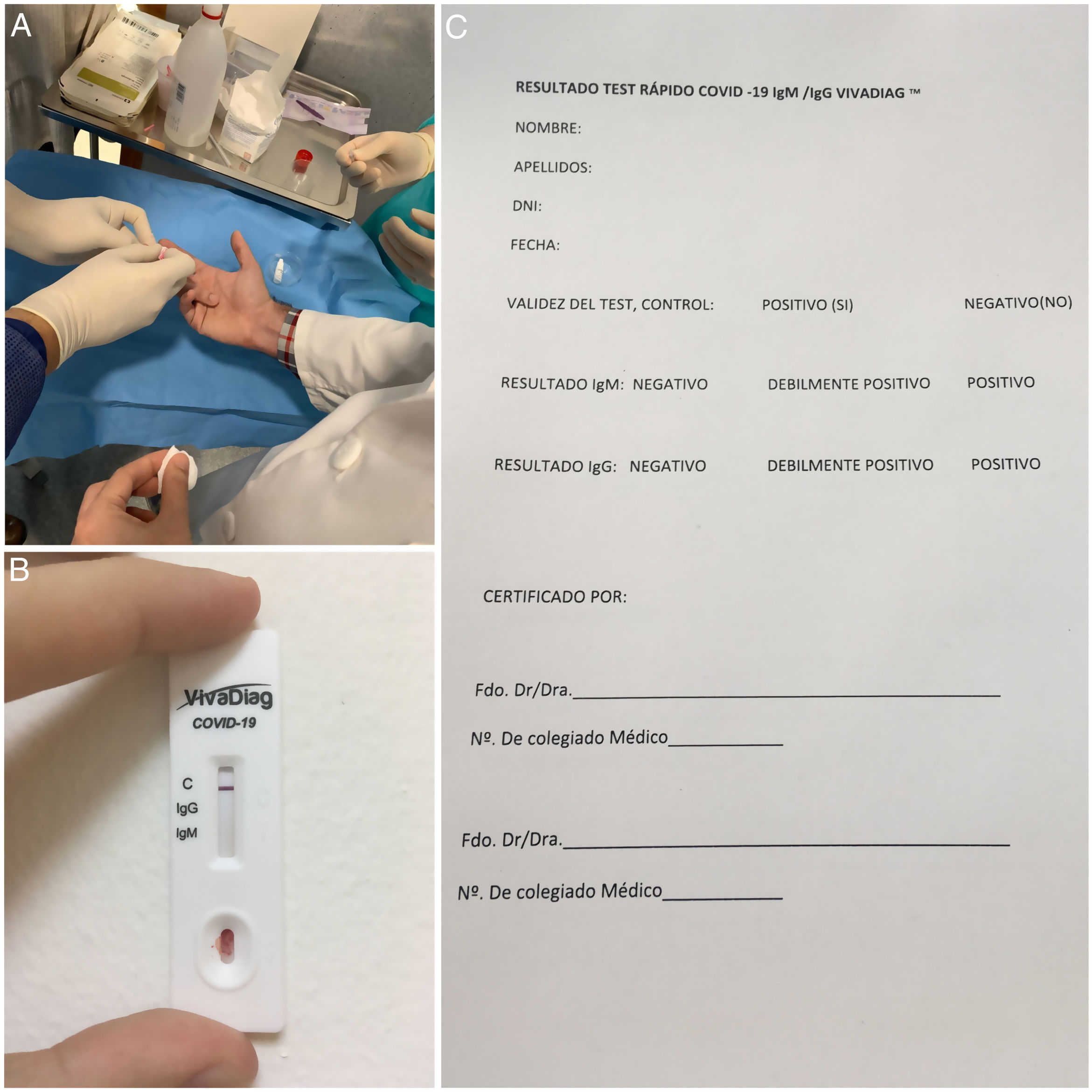
In these circumstances, in our department we have personally purchased 40 VivaDiagTM quick tests for COVID-19 IgM/IgG (VivaChek™ Biotech Co., Ltd., Hangzhou, China). This test makes a qualitative assessment of IgM and IgG antibodies against SARS-CoV-2. The cost of this test is low and it is very simple to make, as can be seen in Figure 1A . No equipment is necessary to assess the results of the test, which can be read in 15min (Fig. 1B). In what concerns diagnostic reliability, the manufacturers report a specificity of 100% and sensitivity of 81.25% between day 4 and day 10 of the infection, and 97.1% between between days 11 and 242. However, as indicated by the website of the Health Ministry, the National Microbiology Center has assessed the reliability of this test with samples of patients from hospitals in different regions of Spain, and confirm 100% specificity as reported by the lab but lower sensitivity (64%) when applied to patients without taking into account evolution time, and of approximately 80% in patients with over 7 days evolution of the disease3. The main drawback of these quick tests is that they are not useful during the first days of infection because antibodies against virus start being produced as from day 6 since the onset of symptoms3. Therefore, it would be convenient to combine this test with the polymerase chain reaction (PCR) which continues to be the reference diagnostic test.
The study initiated by our department had 2 main objectives: to determine whether an ophthalmologist in the department, none of whom had symptoms, was an asymptomatic case, and also to determine whether anyone had endured the infection asymptomatically and was immune to the virus. The idea was to plan clinic and surgical care strategies for the future. None of the 40 tested ophthalmologists was positive for IgM and/or IgG. This result was verified by 2 ophthalmologists of our department, obviously not those who were tested, utilizing a certification record specifically designed for the purpose (Fig. 1C).
In the light of the results of this study, it is clear that we should continue maintaining safety measures in our healthcare practice, both in consultations and surgery rooms, at least until an effective vaccine becomes available. Consequently, we call on ophthalmologists who have not yet tested their immunity against the virus to do so at the earliest possible time, particularly if they work in the regions that are most affected by the pandemic. Let us remain steadfast, we shall prevail.
Please cite this article as: Garrido-Hermosilla AM, Caro-Magdaleno M, Moreno-Galdo JF, Rodríguez-de-la-Rúa-Franch E. Auditoría inmunitaria de COVID-19 en el servicio de oftalmología de un hospital de tercer nivel antes del desconfinamiento. Arch Soc Esp Oftalmol. 2020;95:311–312.