To determine whether the β-lactam allergy delabeling was safe and cost-saving in Primary Care (PC) patients.
DesignWe have conducted a retrospective chart review of PC patients with β-lactam allergy label evaluated in our Allergy Unit between 2017 and 2022.
SiteAllergy Department. Hospital Virgen del Rocio (Sevilla).
ParticipantsA total of 391 patients labeled for β-lactam allergy in PC were studied.
Main measurements(a) Outcome evaluation of a β-lactam allergy delabeling procedure. (b) A ratio between the total e-prescribed antibiotic cost and the number of treatment days (the experimental daily antibiotic cost or EDAC) before and after delabeling was analyzed in delabeled and truly allergic patients.
ResultsThe results of skin testing were positive in 9.2% of the reported cases (36 of 391 patients). The reactions to oral provocation challenge (OPC) occurred in 2.14% of the patients who underwent negative skin testing to offending β-lactam (in 15 of 699 OPC). A total of 307 patients (78.5%) were delabeled; 70 (17.9%) had a β-lactam selective response and 14 (3.59%) reacted to both penicillin and cephalosporin. The EDAC before and after the procedure in delabeled patients was significantly lower (0.88 € vs 0.62 €, p<10−3), than that observed in truly allergic group (0.87 € vs. 0.76 €, p=not significant).
ConclusionTo delabel β-lactam allergy in Primary Care patients is safe in most patients, cost-saving in antibioticotherapy, and allows identify the main clinical β-lactam allergy phenotypes that benefit from this procedure.
Evaluar la seguridad del desetiquetado de alergia a β-lactámicos y su impacto económico en la prescripción antibiótica en atención primaria (AP).
DiseñoEstudio observacional retrospectivo en situación de práctica clínica habitual.
EmplazamientoUnidad de Alergología, Hospital Virgen del Rocío, Sevilla.
Participantes391 pacientes etiquetados de alergia a β-lactámicos en AP.
Mediciones principalesa) Evaluación de un procedimiento de desetiquetado de alergia a β-lactámicos. b) Se analizó la relación entre el coste total del antibiótico e-prescrito y el número de días de tratamiento (el coste diario antibiótico experimental [the experimental daily antibiotic cost, EDAC]).
ResultadosLas pruebas cutáneas a β-lactámicos fueron positivas en el 9,2% de casos (36 de 391 pacientes). Las reacciones durante la provocación oral controlada ocurrieron en el 2,14% de casos con pruebas cutáneas negativas a β-lactámicos (en 15 de 699 provocaciones). Un total de 307 pacientes (78,5%) fueron desetiquetados; 70 (17,9%) tuvieron una respuesta selectiva a un β-lactámico y 14 (3,59%) reaccionaron tanto a penicilina como a cefalosporina. El EDAC antes y después del procedimiento en los pacientes desetiquetados fue significativamente menor (0,88€ versus 0,62€, p<10−3), al observado en el grupo de pacientes alérgicos (0,87€ versus 0,76€, p no significativo).
ConclusionesDesetiquetar la alergia a β-lactámicos en pacientes de AP es seguro en la mayoría de los pacientes, ahorra costes en antibioterapia y permite identificar los principales fenotipos clínicos de alergia a β-lactámicos que se benefician de este procedimiento.
β-Lactam antibiotics are the most commonly used antibiotics in clinical practice.1 Up to 5–10 percent of patients seen in a Primary Care (PC) may report a β-lactam allergy,2,3 although in more than 90% they could tolerate a β-lactam antibiotic during the allergological workup.4,5
A recent British study has explored the views of PC physicians about their understanding and impact of β-lactam allergy.6 In general, they assumed that the diagnosis of β-lactam allergy was often based on patients’ own verbal reports (self-reported allergy) and/or in usually incomplete medical records. Despite the fact that PC physicians often knew that the allergy label was wrong in most cases, they were reluctant to initiate any proposal for an allergological study to proceed with the delabeling of β-lactam allergy and they usually prescribed an alternative antibiotic which could be easy to identify in the electronic prescribing programs that they usually used.6 Labeling patients for β-lactam allergy in PC meant an increased risk of receiving ≥1 antibiotic prescription per year, more contacts with the PC practitioner, as well as increased likelihood of receiving more expensive and less effective second-line antibiotics.7
For all these reasons, the β-lactam allergy delabeling is a procedure that has a high impact on health care costs, which has been proven in hospitalized patients8–11 and in some specific patient groups.12–14 However, despite the fact that a β-lactam allergy label modifies the PC physician's clinical decision making,7,15 the evaluation of this impact on health care costs in PC patients offers more scattered and scarce data16,17 and further studies are needed to verify if delabeling β-lactam allergy in PC patients could be cost-saving.
The primary objective of this study was to determine the diagnostic accuracy and safety of a procedure for β-lactam allergy delabeling in those patients coming from PC with a positive clinical history of β-lactam allergy, but with negative skin tests, who received during the study a full dose of a β-lactam.
As secondary objectives, a systematic application of the β-lactam allergy delabeling would allow: (1) to identify the main clinical phenotypes associated with β-lactam allergy that would benefit of this procedure and (2) to measure the effect of β-lactam allergy delabeling on the costs of prescribed antibiotic in PC.
MethodsPatientsWe have included any patients who has been referred from PC to the Allergy Department from Hospital Virgen del Rocio of Sevilla (Spain) reporting a β-lactam allergy label since January 2017–February 2022 and who underwent skin tests and/or oral provocation challenges (OPC) to any β-lactam. The allergological workup included an exhaustive clinical history according to the European Network on Drug Allergy questionnaire.18 This study was conducted as an audit of the safety and quality of patient care, and it was approved by the Research Ethics Committee of Hospitales Virgen del Rocío/Virgen Macarena of Sevilla.
Skin test with β-lactam antibioticsRoutine management included skin prick testing (SPT) and intradermal testing (IDT) performed using penicillin G (at 104UI/mL), amoxicillin (at 20mg/mL), clavulanic acid (at 20mg/mL) and DAP Penicillin Test Kit (Diater; Madrid, Spain) which consisted of benzylpenicilloyl octa-l-lysine at 0.04mg/mL, equivalent to 8.64×10−5M concentration of the benzylpenicilloyl moiety and the minor determinant (sodium benzylpenilloate) at 0.5mg/mL, equivalent to a concentration of 1.5×10−3M of sodium benzylpenilloate. In patients labeled as allergic to cephalosporin, SPT/IDT to the above mentioned betalactam determinants and the labeled cephalosporin (if available in intravenous form) were also carried out (at 2mg/mL). SPT results were read after 15min and IDT results were read after 20min. For both tests, a wheal of diameter of >3mm (surrounded by erythema) larger than negative saline control was considered as positive.
Oral provocation challenge protocolWe underwent a graded OPC with at least one β-lactam antibiotic if skin testing was negative with the offending β-lactam (Table 1). Patient informed consent was obtained before the procedure. Complete equipment for cardiopulmonary resuscitation was immediately available.
β-Lactam antibiotics and doses used for oral provocation challenge.
Only patients who gave negative results in SPT/IDT and OPC were also included in a resensitization study made between 15 and 60 days after the first OPC. All these patients were retested by performing skin test and OPC with a full dose of β-lactam antibiotic (see Table 1). If patients did not react to this dose, β-lactam allergy label was removed from the patient's chart.
Drug data sources and other economic variablesData were extracted from DIRAYA, an electronic health record and e-prescribing system of the Andalusian public health service, which is part of the National Health Service of Spain.19 Variables included: sex, age, name of antibiotic, antibiotic use in doses per day and days of treatment.
For the economic analysis for each antibiotic prescription before and after of β-lactam delabeling procedure, we used the Prescription Nomenclator of the Health Ministry of Spain. This is a drug database designed to provide basic prescription information to health care providers (https://www.sanidad.gob.es/profesionales/nomenclator.do) which included for all authorized and marketed medicines in Spain, data relating to their identification, the dose and its units, the route of administration and their cost.
We have determined for each patient the total expected of e-prescribed antibiotic cost and the total treatment days before and after delabeling. We then calculated the experimental daily antibiotic cost (EDAC) before and after β-lactam delabeling. EDAC was the result from dividing the total cost of the e-prescribed antibiotic used by the number of treatment days. All patients were evaluated at least 3 years before and 1 year after the β-lactam allergy delabeling.
Statistical analysisThe minimum sample size required by the study (margin error 5%, confidence level 95%) was estimated in 384 patients. Data were analyzed using SPSS software (IBM SPSS Statistics for Windows, Version 23.0, Armonk, NY, USA, IBM Corp), and Excel 2019 (Version 1809, Redmond, Washington, USA, Microsoft). For all parameters considered in the study, the approximation to normal of the distribution of the population was tested by Kolmogorov–Smirnov test. As the results were asymmetrically distributed, nonparametric tests were used. Descriptive statistics were presented as frequency (percentage) and means (with ranges). Wilcoxon's signed rank test was used to compare days of antibiotic treatment, the total cost of prescribed antibiotic treatment and the daily cost of antibiotic treatment before and after of β-lactam allergy delabeling intervention. We used the Kruskal–Wallis test to examine the changes of EDAC for each year in which the Allergy workup was performed from 2018 to 2021.
General outline of the study: β-Lactam allergy delabeling flow chart of 391 patients from Primary Care.
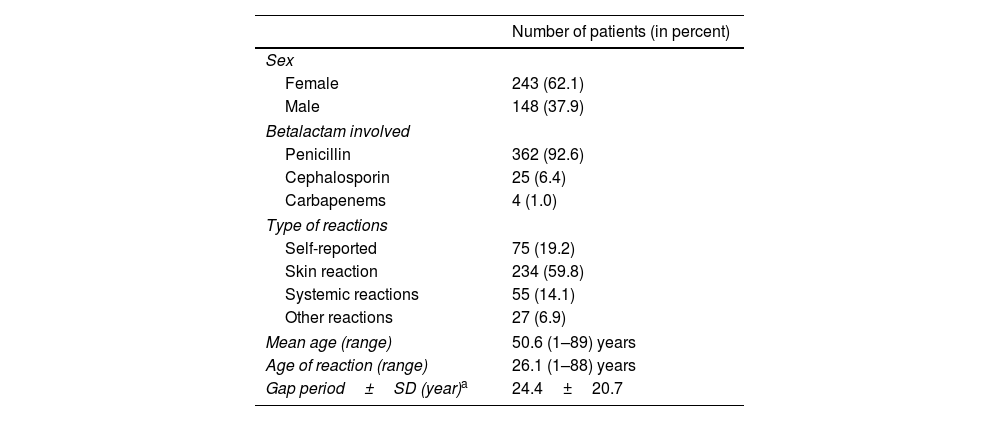
ResultsDemographic and clinical characteristics of the patientsWe have evaluated a group of 391 patients reporting a β-lactam allergy label referred by PC physician for an allergological workup. Table 2 summarizes the clinical characteristics of the patients included.
Baseline demographics and clinical characteristics of the 391 patients included.
| Number of patients (in percent) | |
|---|---|
| Sex | |
| Female | 243 (62.1) |
| Male | 148 (37.9) |
| Betalactam involved | |
| Penicillin | 362 (92.6) |
| Cephalosporin | 25 (6.4) |
| Carbapenems | 4 (1.0) |
| Type of reactions | |
| Self-reported | 75 (19.2) |
| Skin reaction | 234 (59.8) |
| Systemic reactions | 55 (14.1) |
| Other reactions | 27 (6.9) |
| Mean age (range) | 50.6 (1–89) years |
| Age of reaction (range) | 26.1 (1–88) years |
| Gap period±SD (year)a | 24.4±20.7 |
The results of skin testing were positive in 9.2% of the reported cases (36 of 391 patients). The reactions to OPC occurred in 2.14% of the patients who underwent negative skin testing to offending β-lactam (in 15 of 699 OPC). Thirteen cases showed urticaria and/or peripheral angioedema in-clinic observation, one patient had facial angioedema and abdominal pain and one had an anaphylactic episode). Eight patients were diagnosed after showing a positive resensitization study; in all of them a positive intradermal test to at least a β-lactam precluded the subsequent administration of overall dose of antibiotic (Table 3)
Results of the β-lactam allergy diagnostic workupAs stated in Table 4, 307 patients (78.5% of the group) completed the delabeling procedure without any reaction (delabeled patients).
Demographic and clinical characteristics of the patients, according to main phenotypes obtained.
| Delabeled patientsa | Selective reactors | Cross-reactors | |
|---|---|---|---|
| Number of patients | 307 | 70 | 14 |
| Sex (males/females) | 122/185 | 19/51 | 7/7 |
| Age of study (years±SEM) | 51.33±1.04 | 48.5±1.24 | 45.79±1.14 |
| Age of reaction (years±SEM) | 22.93±1.15 | 40.9±2.53 | 22.5±5.93 |
| Onset in pediatric age (in percent) | 137 (44.5%) | 8 (11.4%) | 6 (42.9%) |
| Types of reaction | |||
| Unknown | 22 (23.5%) | 1 (1.4%) | 2 (14.3%) |
| Skin | 174 (56.7%) | 57 (74.3%) | 8 (57.1%) |
| Systemic | 35 (11.4%) | 16 (22.9%) | 4 (28.6%) |
| Others | 26 (8.5%) | 1 (1.4%) | |
| Percentage of positive β-lactam skin testing (number of patients) | 0% | 45.71% (32 patients) | 28.57% (4 patients) |
| Oral provocation challenges with β-lactam (positive/negative) with negative skin testing | 307 (0/307) | 80 (10/70)b | 10 (5/5) |
| Resensitization studies with β-lactam (positive/negative) | 307 (0/307) | 11 (3/8) | 5 (5/0) |
SEM, standard error of the mean.
Delabeled patients denoted those patients who have presented negative skin test, negative oral provocation challenge and negative resensitization study with a culprit betalactam during delabeling procedure.
Selective reactors denoted those 32 patients with positive skin test to a specific betalactam as well as the 13 patients who reacted during initial o resensitization studies, and the remaining 25 patients with severe reaction with the culprit specific betalactam (mainly amoxicillin), but tolerated other non-structurally any related betalactam during challenge.
Seventy patients (17.9%) were considered who reacted to specific β-lactam (56 to amoxicillin, 13 to a one specific cephalosporin and 1 to meropenem) with tolerance to at least other β-lactam during OPC. Of those patients, 32 (45.71%) had positive skin test to a specific β-lactam. Thirteen patients reacted during OPC in the initial or in re-test allergological evaluation to specific β-lactam, but they tolerated other β-lactam during subsequent OPC. The remaining 25 patients (24 with amoxicillin and 1 with cephalosporins as culprit β-lactams) reported symptoms highly suggestive of an immediate severe allergic reaction, including generalized urticaria, angioedema, shortness of breath, stridor, wheeze, loss of consciousness, or systolic hypotension. When this history of severe reaction was detected, OPC with the culprit β-lactam was avoided and then the patients were exposed to cephalosporin or amoxicillin (when amoxicillin or a specific cephalosporin were historically involved, respectively). If OPC with these alternative β-lactam was negative, these patients was also called selective reactors.
Last 14 patients (3.59%) were considered who reacted to both penicillin and cephalosporin determinants in skin test or when OPC or resensitization studies were carried out for each one penicillin or cephalosporin antibiotics. These patients were called cross-reactors.
Analysis of antibiotic cost pre- and post-β-lactam allergy delabelingThe cost analysis has been carried out between delabeled and truly β-lactam allergic patients, that included selective and cross-reactors patients. The total cost of all types of antibiotics and the days of treatment for each patient-group before and after delabeling were summarized in Table 5.
Antibiotic cost and number of days treatment before and after β-lactam allergy delabeling according to main clinical phenotypes.
| Before delabeling | After delabeling | |||||
|---|---|---|---|---|---|---|
| Total cost antibioticb (range) | Treatment days (range) | EDACb,c | Total cost antibioticb (range) | Treatment days (range) | EDACb,c | |
| Delabeled patients | 21.58 (0–103.6) | 22.78 (0–107) | 0.88 (0–3.4) | 10.02 (0–102)d | 10.67 (0–87)d | 0.62 (0–2.6)d |
| Truly allergic patientsa | 27.73 (0–153.8) | 28.08 (0–108) | 0.87 (0–2.7) | 11.06 (0–75)d | 10.9 (0–70)d | 0.76 (0–2.5)ns |
The cost of antibiotic prescriptions in delabeled patients was less after procedure when compared with previous non-delabeled status (EDAC pre delabeling 0.88 euro vs EDAC post-delabeling 0.62 euro, Wilcoxon signed rank sum test, p value<10−3). EDAC did not show any significant difference (prelabeling 0.87 euro vs post-labeling 0.76 euro, Wilcoxon signed rank test, p value=0.12) when the truly allergic patients were analyzed.
We have also observed a significant trend in the progressive decrease of EDAC post-delabeling during this 4 years-period (p-value for Kruskal–Wallis test=0.001), even taking into account that the predelabeling baseline levels were very similar for each of the years studied (Table 6).
DiscussionLabeling patients with β-lactam allergy in PC significantly affects to their health care, with a higher frequency of visits to the primary care office and higher use of antibiotic drugs, especially those which are considered second-choice antibiotics.2,7 Therefore, we believe that if registering β-lactam allergy label in Primary Care has a wrong real-life impact, it is highly probable that delabeling will have an impact in the opposite direction, i.e. decreasing the cost of antibiotic therapy in PC patients and helping to identify the different phenotypes of patients with β-lactam allergy label who else can benefit from this procedure. And this is precisely what this study has tried to answer.
Firstly, we have shown that about 80% of β-lactam allergy label could be removed using a combined procedure consisted of skin tests, OPC and subsequent resensititization studies. β-Lactam allergy delabelling was safe when both an appropriate skin test with β-lactam were done and the OPC was precluded when convincing severe anaphylaxis was detected throughout clinical history (specially in patients with aminopenicillin and cephalosporin allergy). Reactions occurred around 2 percent and anaphylaxis appeared in 0.25% of the patients. A recent systematic review has also confirmed safety of procedure reporting a low incidence of reactions (4 percent in overall with 0.3% of anaphylaxis) during graded challenge in patients with β-lactam allergy.20
It has been reported that some subjects with a suspected β-lactam allergy and negative SPT/IDT and OPC in the initial evaluation, can present positive SPT/IDT when they were subsequent evaluated or even present clinical reaction to a β-lactam during re-test. This is a biological phenomenon that can be observed in β-lactam allergy and which is called resensitization. Recently, Doña et al. have described that up to 14 percent of patients with β-lactam allergy presented this resensitization phenomenon.21 Based in our results, this resensitization phenomenon was notably lower. In this group, only 8 patients (2.04%) were diagnosed having β-lactam allergy in resensitization studies, although other authors give percentages ranging from 0 to 27%.21 Whatever the percentage, none delabeling will be not completely reliable if not include a resensitization study after the first negative OPC with the β-lactam involved in the reaction. And why? Because it is highly possible that a variable percentage of considered truly delabeled in the first OPC may potentially present a clinical reaction in the next exposure to a β-lactam antibiotic in real-life.21
Our study also suggested that delabeled patients had a lower antibiotic costs per day after performing the delabeling than truly β-lactam allergic patients. The reduction of antibiotic cost during follow-up of patients for at least one year after β-lactam delabelling was the main consequence of this synergy between Primary Care and the allergy Department. The magnitude of the decrease in pharmaceutical spending per day of antibiotic treatment was 29.5% when pre and post delabeling period were compared in the delabeled patients. Several studies carried out have shown that the delabeling procedure, using a combination of skin tests and beta-lactam provocation challenges, decreases the use of alternative antibiotics in the hospital setting, which are generally more expensive, less well tolerated and also have more side effects.8–11
There are several experiences of delabeling in PC that indicate that certain types of reactions, the milder ones, can be safely delabeled at the point of care, by applying simple scoring systems that prevent the possibility of severe reactions, and that this has been shown to be effective.22,23 However, these systems did not come close to the different types of penicillin reactions observed in the real-life. The most frequent patient-group with true β-lactam allergy are those who had a clinical reaction to a β-lactam but have tolerance to all other antibiotics of this group. Amoxicillin is the most commonly β-lactam involved in selective reactions and therefore the recognition of this type of reaction is key to avoid the drug and those with the same lateral chain (ampicillin, cefadroxil and cefprozil) in the whole delabeling process. Selective reactors tend to present an excess of cutaneous and systemic reactions when compared to other groups, they present at an older age usually the period between reaction and study is much shorter and they tolerated other β-lactam with different side chain (Table 2). Interestingly, simultaneous allergy to penicillin and cephalosporins is currently the rarest phenotype, below 4 percent. Taking all these observations together, we can conclude that out of every 100 patients with β-lactam label who consult to their PC physician, 96 of them we will be able to reintroduce some β-lactam and only in 4 will we would have to use another alternative antibiotic group.
Our study has some limitations and strengths. The main limitation of this study stems from the fact that it was designed as a retrospective audit to evaluate patient safety and care involving only a single tertiary hospital center. However, the use of the same study protocol over a long study period with an adequate sample indicated a significative modification in PC behavior with respect antibiotic therapy prescription. Undoubtely, any future research should include long-term prospective data to determine whether the impact of our results is sustainable over the time. In the health-economic assessment, we have only assumed the health services perspective, as we were not able to assess many other costs as those related to transportation, patients and caregiver time, procedure's cost and productivity,24 which is sure to decrease the economic impact of this intervention. Our strength lies in the method used by β-lactam delabeling, always demonstrating tolerance of a graded doses in OPC and with single dose challenges in resensitization studies of all delabeled patients. This is a real-life study that defined the main phenotypes of immediate betalactam allergy found in PC and allows us to identify the patients who will benefit most from this delabeling procedure, in which OPC was the gold standard to evaluate immediate hypersensitivity drug reactions.25,26
In conclusion, delabeling of β-lactam allergy is safe and cost-effective in Primary Care patients. The use of broad spectrum, second-line choice antibiotic increased the antibiotic-resistance and healthcare-associated infections.25 Therefore, to apply systematically a β-lactam allergy delabeling as many as possible patients with suspected β-lactam allergy could be one of the most important steps for decreasing resistance and optimizing medical care in the next future.25,26
- •
Penicillin allergy label has a well-demonstrated impact on Primary Care and this leads to higher costs related with antibiotic usage.
- •
This article identify the main β-lactam allergy clinical phenotypes seen in Primary Care which could mostly benefit of delabeling procedure, and it indicate that delabeling is safe and saves cost in Primary Health Care.
- •
This article contributes to expand the knowledge of β-lactam allergy diagnostic guidelines within health systems incorporating new real-life data.
The work was performed in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki) and it was approved by the Research Ethics Committee approval of Hospitales Virgen del Rocío/VIrgen Macarena de Seville. Informed consent was collected from all patients and their anonymity was guaranteed according to the General Data Protection Regulation 2016/679.
FundingNone.
Conflicts of interestJoaquín Quiralte, María Del Robledo Ávila, Isabel Domínguez, Estela Menéndez, Ana Belén Guisado, José Miguel Cisneros certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.