Introduction
The coumarols have been known since the 1960´s due to their capacity to control blood coagulation.1 This action is secondary to the reduction of procoagulant factors linked to vitamin K and have an antithrombotic effect which, due to the reduction in prothrombin concentrations and, consequently, thrombin, makes the clot more friable.2,3
Their absorption, when administered orally, is very rapid, about 90 minutes, and their half-life is around 40 hours; they circulate mainly bound to albumin, and they accumulate in the liver, where they are transformed metabolically.4 The dose-response relationship is influenced by genetic and environmental factors, including mutations in the gene that codes P450.5-7 The pharmacological effect of oral anticoagulant therapy (OAT) can be altered by any process or drug that interferes with its absorption,8 its binding to proteins, bioavailability of vitamin K or the metabolism of the oral anticoagulant system by cytochrome P450.
From a pharmacological point of view, the list of oral anticoagulant promoters and inhibitors is long and always growing.9-11
From a practical point of view, any foreseeable pharmacological reaction in the long term is preventable by using more frequent controls and the subsequent dose adjustment to balance out or decrease the interaction. Their strong binding to proteins, their metabolism dependent on cytochrome P450, the easily measurable pharmacological effects using the prothrombin time and a narrow therapeutic margin make oral anticoagulants (OAC) the model of the drug interaction.12-14
There are many drugs that can interact with OACs15 but there are few studies carried out in the primary care setting. For this reason, we propose to study the situation of our patients on anticoagulant therapy by assessing the therapeutic quality and possible pharmacological interactions they have, based on the recommendations regarding pharmacological interactions with OACs established as quality criteria in the Performance Guide and in the semFYC Therapeutic Guide.16
Given that the list of drugs with possible interactions with oral anticoagulants is very extensive, we restrict ourselves to the anatomical therapeutic groups most commonly used in primary care, particularly those regarding the locomotor system (non-steroidal anti-inflammatory drugs [NSAIDs], allopurinol and analgesics), cardiovascular drugs17 (antiarrhythmics and antihypertensives), lipid lowering drugs, antidiabetics and digestive system drugs.
We were also interested in establishing the possible association between haemorrhagic complications of patients on anticoagulation therapy and drugs which can interact with OACs.18-20
Methods
The present study was carried out in the Colmenar basic health area, in the La Axarquia Health District (Malaga, Spain), where there is one health centre and 4 local clinics, which look after a population of 10 600 inhabitants. The monitoring of anticoagulated patients has been carried out since summer 2001 and is now established in all the centres of the basic health area.
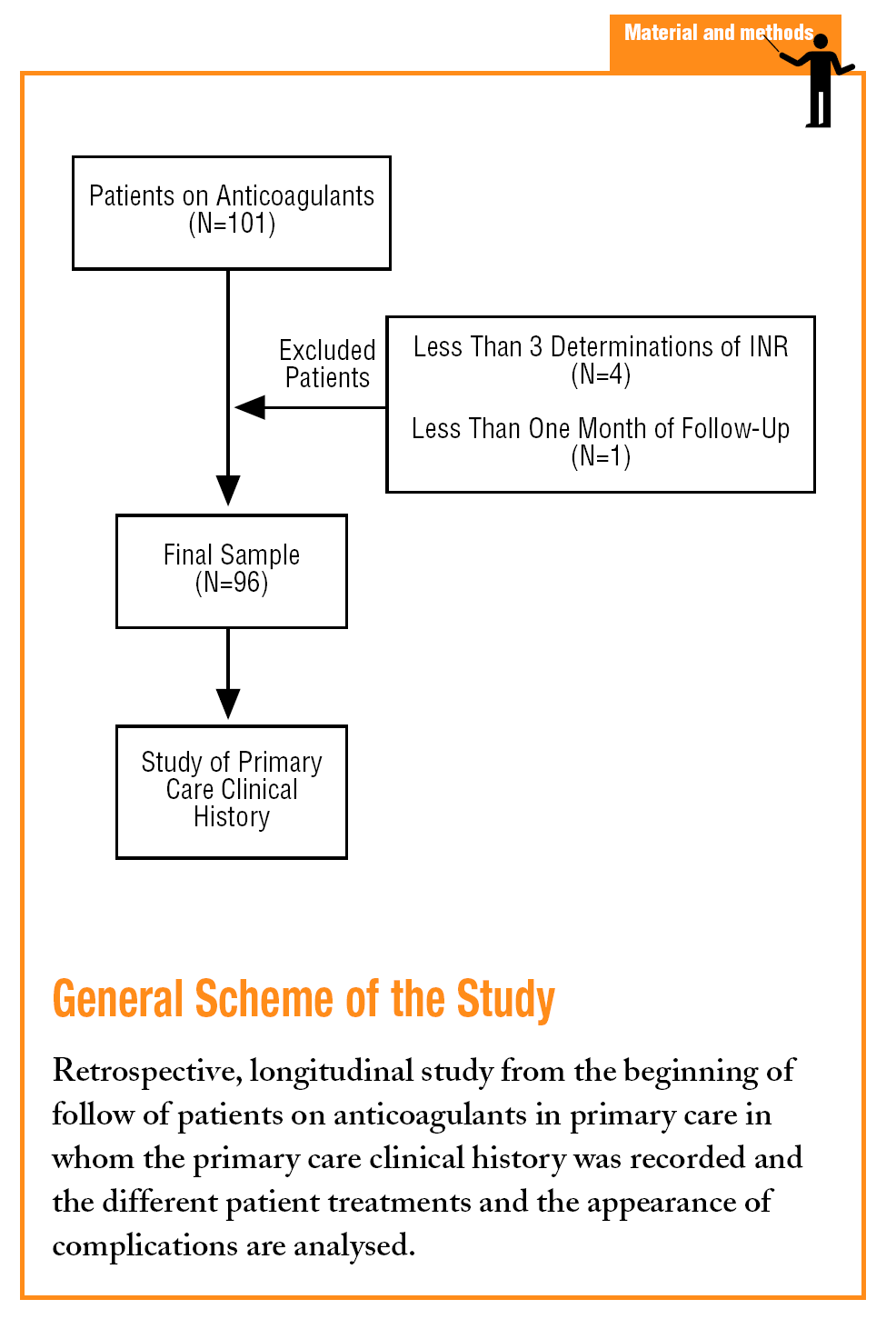
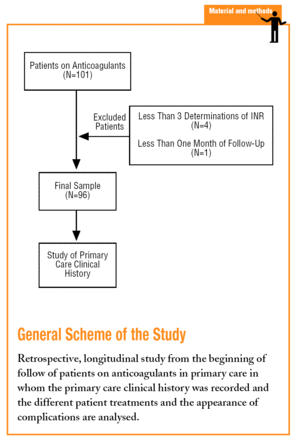
A longitudinal retrospective study was carried out. The information was obtained from the beginning of the follow-up of each patient in the health centre until the time of carrying out the study (March 2004). In the study we included the population who received OAT and were monitored in the Colmenar basic health area, and who complied with the inclusion criteria on having at least 3 prothrombin time (INR) determinations performed in primary care in the least 6 months.
Patients were excluded when their OAT control in primary care was less than one month or less than 3 INR determinations were performed in the health centre. The primary care computerised clinical history (TASS) was used as a source of information and the sociodemographic variables, the indication for OACs, time of treatment, the number of INR determinations, the values of the determinations, major haemorrhagic complications (which placed the life of the patient in danger, required transfusions or hospital admission), the minor haemorrhagic complications, and the number and type of drugs used by the patients.
A descriptive analysis was performed on all the variables collected, with the calculation of the absolute and relative frequencies for the categorical variables, and the point estimation and the 95% confidence interval (CI) of the certainty of the mean and standard deviation (SD) for quantitative variables. A bivariate analysis was also performed using #X2 tests for categorical variables tables and the Student t test for continuous variables.
With the objective of determining in what way the use of the pharmacological groups studied is associated with the appearance of minor haemorrhagic complications, it is adjusted to a multiple logistic regression, with the objective of controlling confounding factors and effect modification.
Results
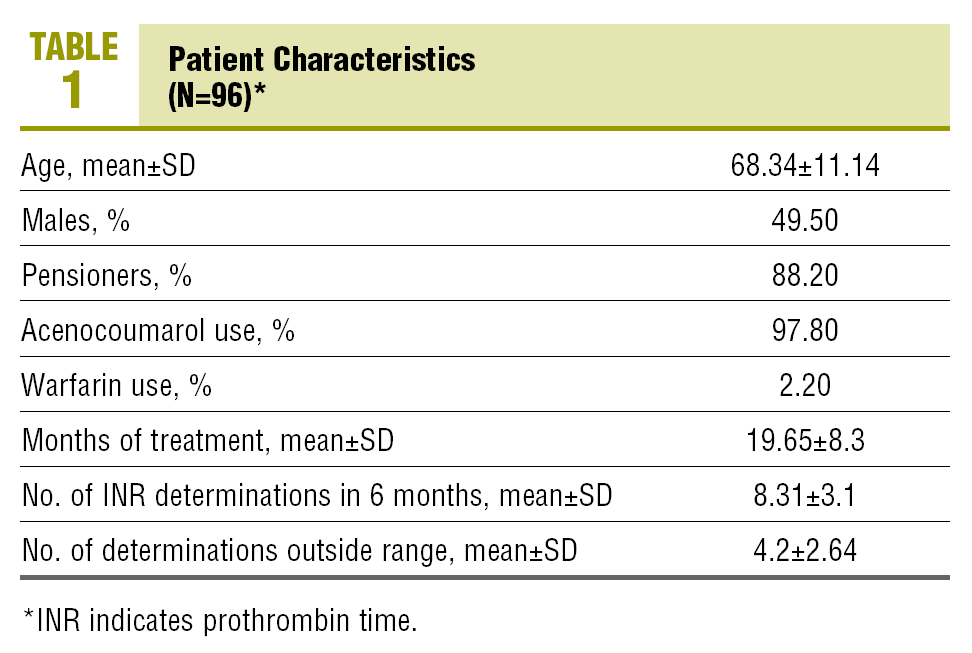
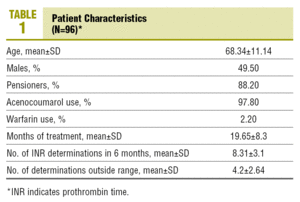
The number of patients who complied with the inclusion criteria at the time of the cut-off was 96, with 50.5% females. The mean age was 68.3 years.
97.8% used acenocoumarol and 2.2% were on warfarin. The mean time of treatment with OACs was 19.6 months (Table 1). The indications for starting treatment were: atrial fibrillation (51.6%), mechanical valve prosthesis (17.2%), prevention and treatment of venous thromboembolism (15.1%), ischaemic stroke (6.5%), valve diseases (4.3%), peripheral arterial diseases (3.2% and others (2.2%).
The mean number of INR determinations in the last 6 months was 8.3, with an average of 4.2 controls outside the range.
In the analysis of the last INR determined, 74.2% of the patients were within the therapeutic range (INR, 2-3±0.2 or 2.5-3.5±0.2, depending on the anticoagulation indication). 17.2% had an INR below the range (under-treated), while 8.6% of the patients had an INR above the therapeutic range (over-treated).
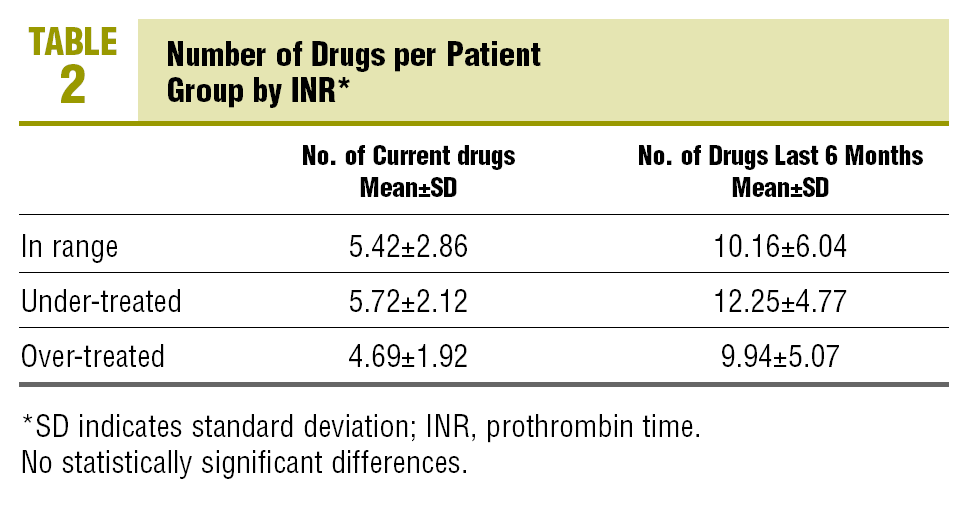
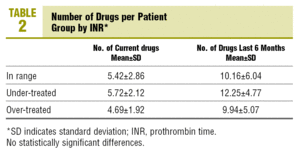
60.2% of the patients complied with the criteria of multiple medication (concomitant use of more than 5 different drugs, excluding topical medication, eye drops and syrups) at the time of collecting the data, and 88.2% were using multiple medication in the last 6 months. There were no statistically significant differences between the means of the number of drugs used currently and in the last 6 months among patients in the therapeutic, the over-treated and the under-treated range (Table 2).
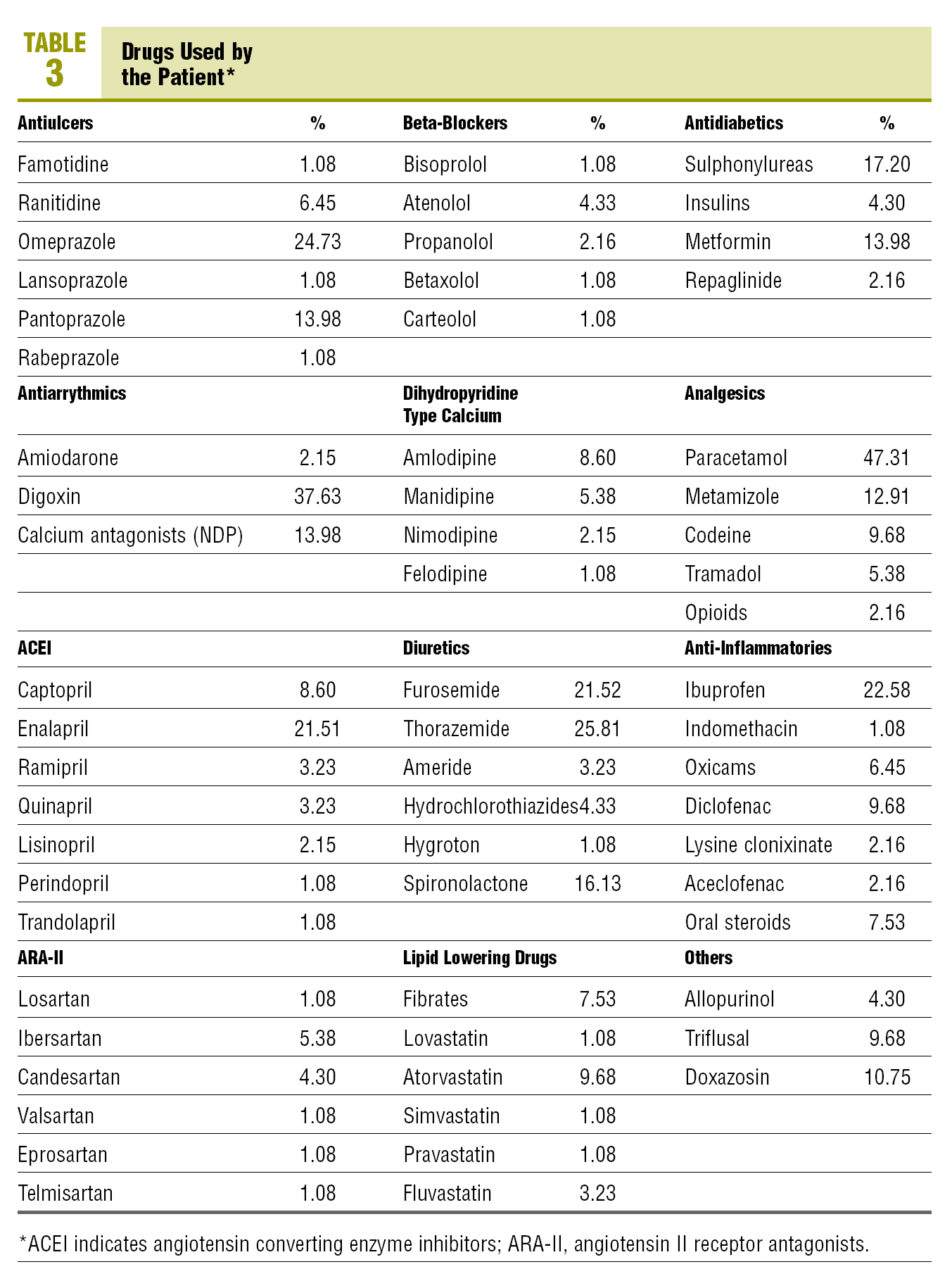
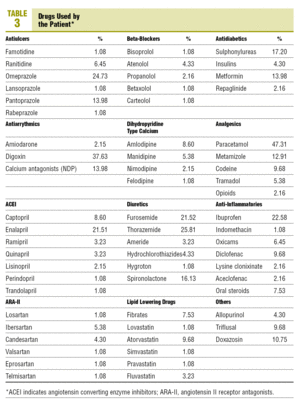
The drugs, not recommended due to having more interactions with OACs and used by the patients are: antiulcer drugs (26.9%), locomotor system drugs (10.7%), cardiovascular drugs (2.2%), lipid lowering drugs (8.6%), and oral antidiabetic drugs (17.2%) (Table 3).
None of the patients had a major haemorrhagic or a thromboembolic complication. 16.1% of the patients had minor haemorrhagic complications.
There were no statistically significant differences on comparing the minor haemorrhagic complications with consensus standard for the control of patients treated with anticoagulants, which is established as 13% (P=.37).
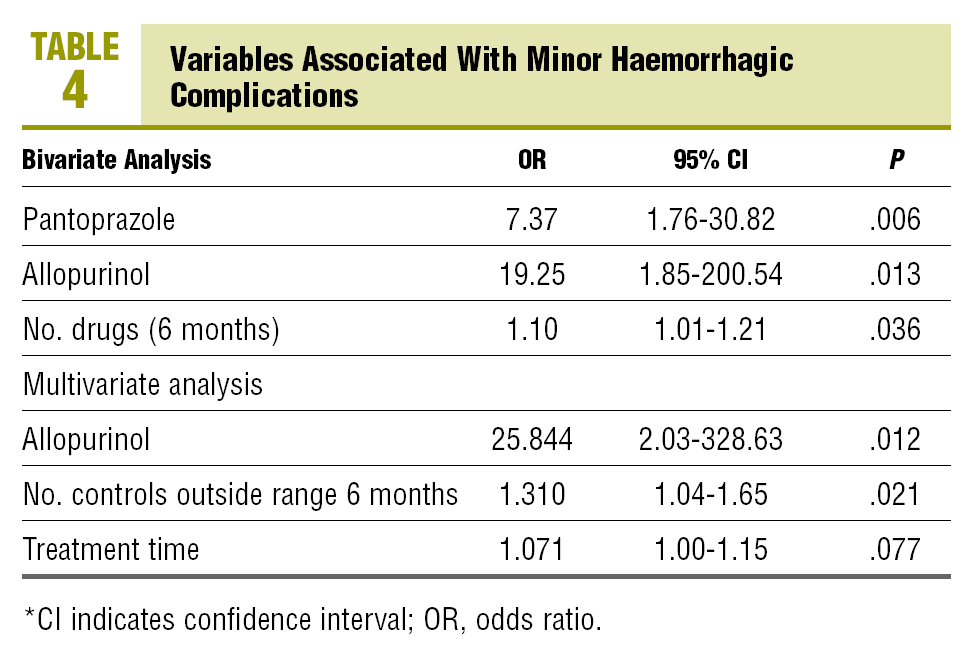
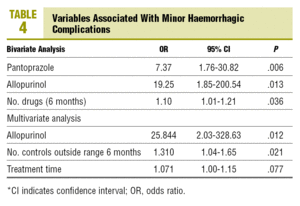
In the bivariate analysis, statistically significant differences are observed between, the presence of minor haemorrhages and the number of drugs used in the last 6 months, the current use of allopurinol and the current use of pantoprazole (Table 4). For each additional drug used by the patient on anticoagulants in the last 6 months, the risk of a minor haemorrhage is increased by 10%. The current use of allopurinol and pantoprazole
by patients multiplied the risk of a minor haemorrhage by 19 and 7 times, respectively. In the multivariate analysis, the variables associated with the presence of minor haemorrhages are the current use of allopurinol (odds ratio [OR], 25.8), the number of INR controls outside the range in the last 6 months (OR, 1.3), and the time of treatment with OACs (OR, 1.07).
Discussion
The study is set in a basic health area with some specific characteristics, therefore we cannot guarantee that the results can be extrapolated to the whole of the population of the province.
The percentage of patients within the therapeutic range in the last INR analysed was above the standard criteria (70%) of the recommendations of follow-up quality of patients receiving anticoagulants in primary care which has been agreed by the Andalusian haematologists.
The number of minor haemorrhagic complications exceeded the consensus standard for the control of patients receiving anticoagulants (13%), although there were no statistically significant differences between our results and expected values, therefore this difference could be due to chance. The presence of major haemorrhagic complications in many cases involves hospital admissions, which in some cases leads to a lack of information in the primary care clinical history, a fact that has to be taken into account on evaluating the results of our study.
The use of drugs with a higher capacity for interacting with OACs is high in the antiulcer and antidiabetic groups.
In the widely used antiulcer therapeutic group, the drugs that increase the oral anticoagulant effect are the antacids with magnesium, cimetidine, omeprazole, and lansoprazole. Sucralfate and bismuth carbonate inhibit the anticoagulant effect. In our study, the percentage of drugs in this group used by anticoagulated patients was 26.9% of the total antiulcer drugs. The drugs recommended to be used with anticoagulant treatment are: almagate, magaldrate, ranitidine, famotidine, and pantoprazole, which made up 73.1% of the treatments in this group.
The statistical relationship between the use of pantoprazole and the presence of minor haemorrhages has to be analysed in detail, since in the clinical histories of these patients the prescribing of this drug is checked after the presence of the complication, and different antiulcer drugs had been used previously without finding any association between these and the presence of complications. This statistical relationship has little relevance as a clinical interaction, since the option in these cases would be to monitor the INR concentrations.
The first and second generation sulphonylureas increase the oral anticoagulant effect, and 17.2% of the patients in our study are using drugs from this group. Metformin is recommended for diabetics on oral anticoagulant therapy (OAT), as well as insulin and repaglinide which is used by 14%, 4.3%, and 2.2% of these patients, respectively.
Allopurinol is a drug that reduces uric acid concentrations in blood by inhibiting its production by acting on xanthine oxidase and the conversion of hypoxanthine to xanthine, and then to uric acid.
It has a high potential for interacting with other drugs.21
It produces a severe hypersensitivity syndrome and increases its concentrations in the elderly,22,23 due to lower renal excretion. Allopurinol increases the anticoagulant effect, therefore the INR values need to be monitored. Blann et al24 pointed it out as a drug that must be taken into account in the follow up of anticoagulated patients, due to its capacity to increase the anticoagulant action of the coumarols. In our study, the risk of a minor haemorrhage is increased by a factor of 19 when the patient uses this drug together with OACs.
The multivariate model that we have used in our study shows that, in the presence of the rest of the variables, the use of allopurinol, the time out of the therapeutic range and the duration of anticoagulant treatment are associated with the presence of minor haemorrhages. The use of allopurinol increases the risk of having a minor haemorrhage by a factor of 25; for each INR control out of range in the last 6 months, the risk of having a minor haemorrhage increases by 31%, and for each additional month on OAT, the risk of minor bleeding is increased by 7%, the same as in the rest of the variables (P=.077).
The increasing use of allopurinol, and the possibility of it being used even more due to the greater number of indications being attributed to it, not only its capacity to reduce uric acid, but also as a cardioprotector, vascular protector and even as adjuvant therapy in cancer of the colon,25 makes the use of this drug very significant, therefore it will be necessary to carry out a more thorough control and stricter monitoring of the INR values in anticoagulated patients who are being treated with allopurinol.
The percentage of patients on multiple medication, that is, using 5 or more different drugs at the time of the study cut-off, amounted to 60.2%, If we analyse the previous 6 months, the percentage reaches 88.2%. Pharmacological control needs to be carried out on these anticoagulated patients to prevent multiple medication, as well as evaluating the accumulative effect of using different drugs at the same time, since for each different drug used by the anticoagulated patient in the last 6 months, the risk of a haemorrhage increases by 10% due to interfering in the metabolic pathway.
The data shown must make us reflect on the importance of patients on multiple medications in all health care settings, but even more so in primary care. This is particularly so for those on anticoagulants, as they are more likely to have pharmacological interactions.
In the search for drugs that theoretically have less interactions, we must not forget the need for the careful and thorough control of these patients, to those who in this situation, as in others, we must consider as delicate.
What Is Known About the Subject
* Treatment with oral anticoagulants has meant an improvement in the prognosis of patients with thromboembolic disease and has increased expectations and the quality of life.
* The increase in life expectancy has caused an increase in associated morbility, with the higher use of new drugs with the potential capacity of interacting with oral anticoagulants.
* The list of anticoagulant promoters and inhibitors is long and ever increasing. From a practical point of view, any foreseeable pharmacological interaction in the long term is monitored by means of more frequent controls and the subsequent adjustment of the dose.
What This Study Contributes
* The number of drugs used by patients treated with anticoagulants is associated with the probability of presenting with haemorrhagic complications.
* The time outside the prothrombin time therapeutic range and the concomitant use of allopurinol increases the risk of haemorrhages in the anticoagulated patient.
* Monitoring the INR of patients on anticoagulants and multiple medication should to be intensified to ensure that the coumarins are in the therapeutic range, maintaining the treatment.
Correspondence:
R. Sánchez-Garrido Escudero.
Obispo, 44. 29200 Antequera. Málaga. España.
E-mail: rsanchezgarrido@tiscali.es
Manuscript received June 29, 2005. Manuscript accepted for publication January 16, 2006.
Spanish version available at www.atencionprimaria.com/135.014
A commentary follow this article (pág. 432)