Introduction
Many patients who consult their doctor, regardless of the level of care involved, are smokers. According to the 1997 National Health Survey in Spain,1 35.7% of all Spaniards older than 16 years smoke. In addition, smoking causes 56 000 deaths yearly in Spain.2 This means that for many patients, the reason for seeking medical help is likely to be related with smoking.
The use of primary care by the general population in Spain is increasing, possibly because of its accessibility. (It is estimated that 75% of all Spaniards visit their doctor at least once a year.) The mean number of visits per year per person is 5.5, a number that provides practitioners and the health care system itself with multiple opportunities to help those who wish to quit smoking.3 Many other persons seek help from the second level of care, ie, from specialists. As a result, a very large percentage of persons in Spain seek health care and may thus be reachable through interventions to quit smoking.
The favorable cost-benefit ratio of smoking cessation treatments, especially in comparison to other preventive measures often used in primary care, is well known. Programs to quit smoking are possibly the procedures that most efficiently improve the health of the population.4 However, systematic intervention for smoking is not yet a reality in the Spanish health system, and we may still be far from such intervention.
Despite the frustrating slowness with which smoking prevention is becoming part of clinical practice, the situation is changing. It is revealing in this connection to reread the medical training texts used in the 1970s, which contained statements such as «Quitting is very difficult if the inveterate smoker does not cooperate or lacks will power...The decision to quit smoking once and for all is of prime importance in getting through the first three days without smoking, and the following may be of help: 3 tablets per day of belladenal or bellergal, taking a walk before bedtime, candies, exercising, and showering in the morning.»5
Fortunately the current concept of smoking has changed, and the problem is now considered one of the main public health issues and the most frequent cause of preventable deaths in developed countries.6 However,
as noted above, we are still far from the day when anti-smoking therapy forms part of the daily activities of primary care physicians and nurses. At most, patients are asked to provide a history of their smoking habit, and this history is usually incomplete, lacking information on which phase of the quitting process the patient is in, or on the degree of nicotine dependence. Moreover, the health advice given is sometimes not accompanied by printed supporting material or plans for follow-up, which are part of systematic minimal intervention for smoking cessation. Pharmacological treatment has been relegated in most cases to specialized anti-smoking units, which are scarce and hence cover only a small proportion of the population. These units are therefore unlikely to have a substantial influence on the public health problem that smoking creates.
Minimal intervention is undoubtedly a measure that ought to be implemented by primary care physicians, but what is to be done about pharmacological treatment? Should it be restricted to specialized units, or could it also succeed if offered at primary care centers? The aim of this study was to compare the efficacy of smoking cessation treatments based on systematic minimal intervention and nicotine replacement therapy (NRT) offered in the setting of primary care (PC) and specialized care (SC).
Material and methods
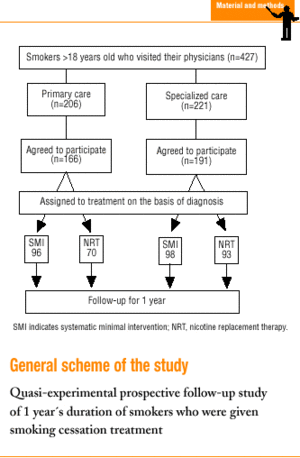
This quasi-experimental, longitudinal, prospective study7 formed part of a larger research project.8 Some of the findings for the predictive power of abstinence 2 months after quitting have been reported previously.9
Subjects
The population we studied consisted of all smokers older than 18 years who came for any reason to the family medicine service at the San Juan Health Center in Salamanca (northwestern Spain) or to the pneumology out-patient clinic at the University of Salamanca Hospital. Exclusion criteria for patients on NRT were the same as those for any pharmacological treatment: recent history of myocardial infarction, severe cardiac arrhythmia, unstable angina, pregnancy, breastfeeding, active gastroduodenal ulcer, and severe mental illness. In both treatment groups addiction to other drugs besides tobacco was considered an exclusion criterion.
Interventions
For each smoker we recorded name, age, sex, and phone number. Disease antecedents were noted, and information was obtained about disease antecedents and smoking habits: number of cigarettes smoked/day, nicotine consumption/day, packs/year ratio, phase of the quitting process, degree of nicotine dependence (as measured with the Fagerström test), and carbon monoxide concentration in exhaled breath (measured with a Bedfont Micro Smokerlyzer).
The patients were classified on the basis of the phase of the quitting process, and all were offered stage-appropriate oral and printed medical advice. Those in the precontemplation phase were given an information sheet about smoking, and those in the contemplation, preparation and action phase were given in addition a 10-item list of steps for quitting smoking, and a practical guide to quitting. All advice was given at each visit by the same person, and a talk lasting approximately 3 min was given to explain the damage caused by smoking, and the short- and long-term advantages of quitting. The same information was provided in both settings, and was developed in accordance with the recommendations of the Section on Smoking (Área de Tabaquismo) of the Spanish Pneumology and Thoracic Surgery Society (Sociedad Española de Neumología y Cirugía Torácica, SEPAR).10 All physicians in both settings were trained to follow exactly the same procedures.
Patients with high (score of 7 or more on the Fagerström test) and moderate nicotine dependence (score of 5 or 6) who also smoked more than 10 cigarettes/day or reported previous attempts to quit which failed because of nicotine withdrawal symptoms, medical advice was accompanied by pharmacological support with nicotine patches as recommended by the SEPAR.10
The patients were divided into two groups:
Group 1. Patients who smoked ¾20 cigarettes/day, with low nicotine dependence (score <5 on the Fagerström test), those with moderate nicotine dependence (Fagerström score of 5-6) and low cigarette consumption, and those with moderate or high dependence who declined NST with nicotine patches (19 patients). All participants were given printed material and medical advice, psychological support and follow-up during the quitting process. This group consisted of 194 persons: 75 in the precontemplation phase, 65 in the contemplation phase, and 54 in the preparation phase.
Group 2. The members of this group were in the preparation phase, and were candidates for NRT either because they smoked more than 20 cigarettes/day or because their nicotine dependence was high. This group consisted of 163 persons: 16 with low dependence, 49 with moderate dependence and 98 with high dependence.
Follow-up
Patients in both the systematic minimal intervention and NST group were seen on day 15, and 1, 2, 6 and 12 months after starting the program, and abstinence (as the main outcome variable) was evaluated after 2, 6 and 12 months. When the participant missed an appointment he or she was contacted by telephone to determine the reason and to reschedule the appointment.
At each follow-up appointment progress in quitting was recorded as self-reported abstinence, which was verified with exhaled carbon monoxide measurements. A value <10 ppm was considered the cutoff value for distinguishing between smokers and nonsmokers, and between nonsmokers and light smokers.11 For patients who were unable to quit we recorded the number of cigarettes/day, nicotine dependence, exhaled carbon monoxide concentration, phase of the quitting process, and whether the phase had changed since the start of the program. Patients in both groups were offered additional information aimed at achieving abstinence.
Outcome measures
The main outcome variables were:
Success rate, measured on the basis of intention to treat, ie, patients who quit smoking 2, 6 or 12 after the intervention started were considered successes. The main outcome variable was abstinence after 6-12 months. Patients who had not given up smoking after 12 months, and those who skipped the appointments, were considered failures.
Number of patients who significantly reduced the number of cigarettes consumed during the year of follow-up, or who progressed in the quitting process.
Statistical analyses
Chi-squared tests were used for comparison of the proportions; Fisher´s exact test was used when appropriate. Student-Fisher´s t test was used for comparison of the means.
To investigate the changes in the number of cigarettes consumed per day during the follow-up period and the variables related with these changes, we used multifactorial analysis of variance of repeat measures. In the initial model the dependent variable was number of cigarettes smoked per day, the intrasubject factor was the number of follow-up visits, and the intersubject factor was the level of care (primary or specialized) that provided treatment. After possible differences were identified, multiple comparisons were done with the Bonferroni test.
Results
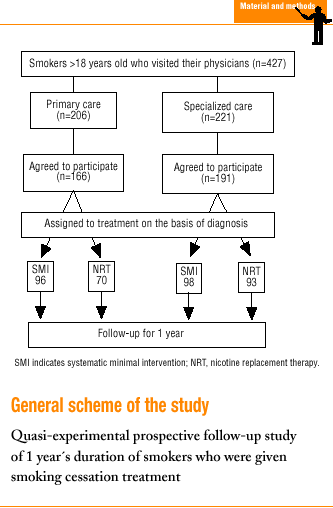
In all, 427 patients were seen during the study period, 221 in SC and 206 in PC. Of these patients, 357 (83.6%) agreed to participate in the study (191 in SC and 166 in PC); 194 received systematic minimal intervention, and 163 received NRT.
Thirty-two patients (9%) did not attend scheduled appointments (19 [8.6%] in SC and 13 [6.3%] in PC), and were considered cases in which therapy failed. Of these patients, 17 received systematic minimal intervention (12 [10.5%] in SC, and 5 [4.9%] in PC), and 15 received NRT (7 [6.5%] in SC and 8 [7.6%] in PC). Seventeen patients were men and 15 were women; 10 were younger than 30 years. Of the 32 patients who missed appointments, 23 had moderate or high nicotine dependence.
Of the total sample, 200 patients (56%) were men and 157 (44%) were women. The distribution according to level of care differed significantly: men predominated in SC (66% vs 44.7%; P<.0001).
Mean age for the entire sample was 39.9 years (95% confidence interval 38.7-41.2 years); mean age was 45.1±12.9 years in SC and 34.2±12 years in PC. In men, mean age was 44.5±13.8 years, and in women the figure was 34.3±10.9 years (P<.0001).
Among patients seen in the pneumology service a significantly higher percentage (63.4%) had some underlying disease in comparison to patients seen at the PC center (34.9%; P<.0001). The most common diagnoses were asthma (24.6%), chronic bronchitis (23.5%) and emphysema (9.5%); together these three accounted for more than 57% of all the diseases detected. These diseases were present in 76% of the patients followed at the pneumology service, but in only 20.7% of those followed at the PC center, where they were seen in 48.2% and 7.2% of the patients who kept their appointments. Of the patients with some underlying disease, 67% were men and 31.8% were women (P<.0001).
The mean number of cigarettes smoked per day at the beginning of the intervention was 20.8±10.2 in SC and 25.1±12.9 in PC (P<.05). However, the packs/year ratio was significantly higher in SC patients (29.2±20.9) than in PC patients (22.5±20.9; P<.01).
The degree of nicotine dependence at the beginning of the study was greater in the PC (mean Fagerström score 6.3) than in the SC group (mean score 5.8; P<.01).
Mean concentration of exhaled carbon monoxide was 25.5 ppm in PC and 23.6 ppm in SC (P>.05).
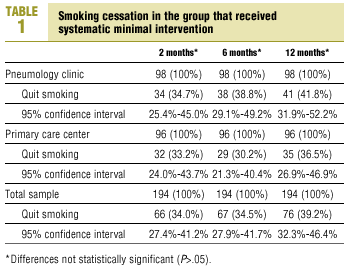
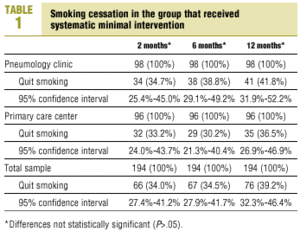
The percentage of participants who had quit smoking after 12 months of systematic minimal intervention was 41.8% in SC and 36.5% in PC. This difference was not statistically significant, nor were the differences seen at any of the intermediate follow-up visits (P>.05) (Table 1).
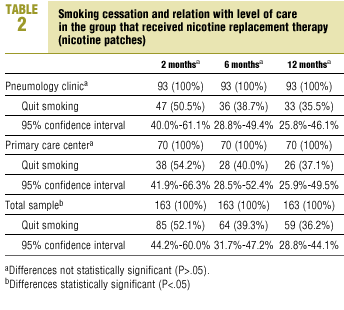
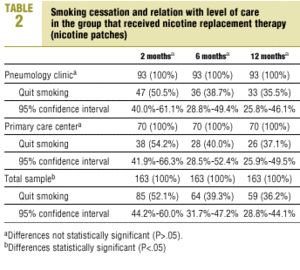
After one year of NRT the percentage of smokers who had quit was 35.5% in SC and 37.1% in PC (P>.05) (Table 2). Although abstinence after treatment with systematic minimal intervention showed no large changes during the follow-up period (P>.05), abstinence in patients who received NRT decreased steadily with time, although the differences from one follow-up visit to the next were not statistically significant.
The percentage of patients in the systematic minimal intervention group who were able to quit at the beginning of the intervention and who remained abstinent throughout the 12-month follow-up period (sustained abstinence) was 29.6% in SC and 27.1% in PC. In patients who received NRT the figures were 33.3% in SC and 31.4% in PC (P>.05).
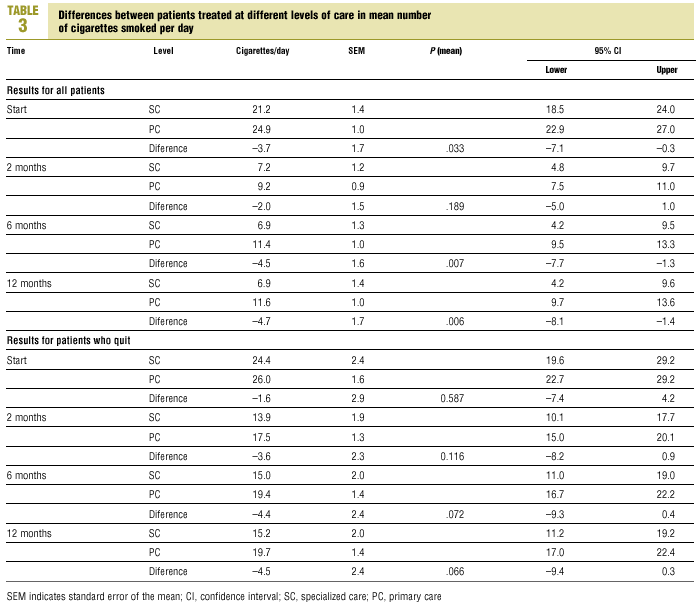
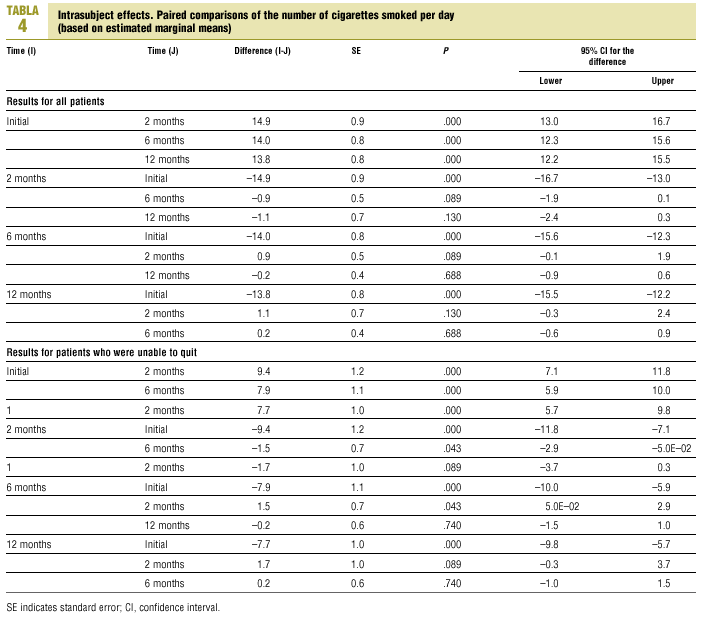
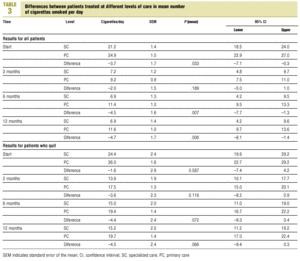
Throughout the follow-up period, mean daily cigarette consumption (Table 3) was always lower in SC patients (10.6; 95% CI, 8.3-10.2) than in PC patients (14.3; 95% CI, 12.7-15.9; P=.008).
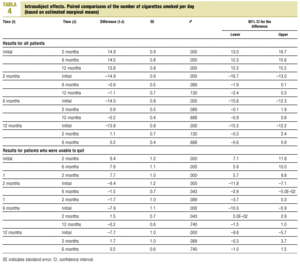
Multivariate analysis was used to determine the effectiveness of the intervention in reducing the number of cigarettes smoked per day. After 2 months the number had decreased significantly in comparison to the number at the start of the study. This reduction was maintained with little change in subsequent follow-ups, and there were no significant differences in the number of cigarettes smoked per day after 2, 6 and 12 months (Table 4).
Multivariate analysis showed that there was no interaction between duration of follow-up and level of care: the changes during follow-up in the numbers of cigarettes smoked per day were similar in SC and PC patients.
The decrease in the number of cigarettes was reflected in mean values of exhaled carbon monoxide concentration at the beginning of the study and during follow-up. Statistically significant differences between the results at each follow-up visit were found for the sample as a whole when participants who were able to quit and those who were unable to quit were considered together. Mean carbon monoxide concentration at the start of the study was 24.3±10.5 ppm, as compared to 12.1±10.2 ppm at the end of the study. In participants who were unable to quit smoking by the end of the 12-month study period, the final concentration was 19.9±8.9 ppm. When we compared the results for the two levels of care we found similar differences between participants who quit and those who did not. In the SC group the initial and final concentrations were 23.9±11.2 ppm and 11.7±10.9 ppm respectively, and the final concentration for those who were unable to quit was 20.6±10.1 ppm. In the PC group the figures were 24.7±9.7 ppm and 12.3±9.5 ppm, respectively, and the final concentration in those who were unable to quit was 19.3±7.8 ppm.
Discussion
The differences in the results between the primary care and specialized center resulted from the particular characteristics of the patients managed at each level of care. The population followed by the pneumology clinic was older on the average, and the main reason for consulting was chronic respiratory disease related with smoking. These problems take longer to appear than do the acute processes normally seen in primary care.
The predominance of men in the specialized clinic was related not with an actual difference in the prevalence of smoking between men and women, but with the fact that women have begun smoking relatively recently, and hence the manifestations of the resulting damage have not yet appeared. However, this situation is changing in accordance with the classical epidemiological curve of smoking.12
Patients followed at the specialized center had more severe respiratory disease and may therefore have smoked fewer cigarettes per day. However, the packs/year ratio was higher in these patients. A logical finding was that the degree of nicotine dependence and carbon monoxide concentration, which are linked to current smoking habits, were lower in patients followed at the pneumology clinic.
A number of studies have shown minimal intervention to be effective13-22 in both PC and SC settings, and have found NRT to be effective in SC,23-28 but there are no studies that used the same method to compare the efficacy of these two interventions for smoking cessation at both levels of care.
We found no significant differences in the results between groups at any time during follow-up, in terms of change of phase, abstinence or smoking reduction. This leads us to note that despite the limitations related to the different populations treated at the two levels of care we compared, and foregoing any attempt to undertake a rigorous comparison of the two centers, smoking cessation can and should be undertaken in primary care, a setting with the added advantages of greater accessibility and coverage, and thus greater benefits in terms of public health as shown in the classic study by Russell et al.13
The percentage abstinence rates in the present study contrast with earlier results reported by other authors.13-29 The better results obtained in the present study are probably due to the fact that our patients sought medical help for health problems, and smoking cessation treatment was offered to them within the wider context of treatment for their underlying illness. This made more prolonged, systematic interventions possible (with periodic follow-up examinations scheduled regularly and also taking place during visits to the center for any other health problem). The results, as shown in other studies,30-32 were thus better than if the interventions had been attempted in isolation. Another possible factor is the greater need in the general population to quit smoking, as also reported in a recent study by Torrel et al.33, in which a high percentage of participants ceased smoking. This factor has been noted and discussed in a previous article in Atención Primaria.34
It should be recalled that our participants were assigned to receive minimal intervention or NRT on the basis of their degree of nicotine dependence and cigarette consumption. This might explain, in part, the high percentages of abstinence in the group that received systematic minimal intervention, as it might be assumed that it would not be difficult for these patients to quit.
An important task for health care professionals is to develop activities aimed at fomenting healthy attitudes in patients, and smoking cessation treatment is one way to favor such attitudes. The appearance of new drugs35 along with personal factors that might give some indication of the patient´s course in the quitting process,36,37 as well as more reliable predictive factors--such as the results of cessation intervention after 2 months,9 can help enhance the efficacy and efficiency of measures to help the patient quit.
On the basis of the results of the present study, we believe that systematic minimal intervention and NRT should be used by health professionals and included in all medical contacts regardless of the level of care.
In conclusion, NRT is effective, with success rates ranging, according to a meta-analysis by Silagy et al.,38 from 15% to 24% depending on the mode of treatment. This therapy should be used at all levels of health care and not be limited to specialized centers. Because it is recommended for patients with higher levels of dependence, it has been found effective in primary care, as the patients seen at this level of care smoke more cigarettes and have higher levels of dependence than patients seen by specialists.
It may be useful to identify the limitations of these interventions in primary and specialized care, especially now that the appearance on the Spanish market of bupropion means that there are more options for pharmacological treatment, and now that the future holds expectations for gene therapy.39,40 The limitations of available treatments will probably be determined by specific situations that require more specialized interventions such as those offered by anti-smoking units. One aim should thus be to define the criteria for referral to such units,41 having accepted that systematic minimal intervention and pharmacological treatment can and should be used at all levels of care within the health system.
Correspondence: Miguel Torrecilla García. Centro de Salud San Juan. C/ Valencia 32. 37005 Salamanca. España. E-mail: mtorrecillag@papps.org
Manuscript accepted for publication 4 February 2002.