Introduction
Policies to promote generic drugs, implemented in Spain and other countries during the last few years as a measure to contain health care costs and favor the rational use of medications, are now well known.1 Since the legal concept of generic drugs2 was introduced in Spain in 1996,2 several structural measures, which have taken into account the legal framework, and the interests of pharmaceutical firms, pharmacies, users and health care professionals, have been used to foment the presence of generics on the market.3
One of the most important legal measures is Real Decreto 1035/1999,4 which established the reference price system. This has led to reductions in the price of brand-name products and the appearance on the market of many generics. From September 1999 to September 2001 the number of active pharmaceutical ingredients available as generics has grown from 57 to 86, which translates as an increase in the number of generic products from 307 to 994.5
Agreements reached with pharmacies have increased their profit margin from 27.9% to 33% for generics, and user information campaigns have been sponsored by the Ministry of Health.5
Among the measures developed for physicians at some health services, two are worth special mention: a system of periodic feedback on prescribing practices (which includes information on costs to the national health system and on the percentage of generics prescribed),6 and incentives (ie, the introduction of concepts such as variable productivity associated with generic prescribing).
The quality, safety and efficacy of generics are assured7,8 by the strict application of current legislation regarding their authorization.2 These products therefore offer the same guarantees as the original pharmaceutical,9 and are made available to prescribing physicians and the health care system as an alternative that is more cost-effective, as long as the original indication for the active ingredient is correct.10
Physicians are increasingly aware of the advantages of generics.11 By December 2000 generics already accounted for more than 5% of the prescription drug market, a figure that surpassed the national Health Administration´s goal for the year 2002. Although regional differences were recorded, the average market share was as high as 7.6% in those parts of the country where health care services were provided by the National Institute of Health (INSALUD)5 as opposed to regionally-administered health services.
Meanwhile, patients too have become better informed about the advantages of using generics. Several surveys showed that 50% of the users know what a generic drug is, and that more than 80% of these patients know that generics are as effective as brand pharmaceuticals.5
However, in daily practice at primary health care centers, it may be difficult to replace a patient's usual drug with a different product.12 This situation is a further example of the key role that family physicians play,13 as their daily contact with patients places them in the position of mediators between the health administration and patients themselves when new strategies are being implemented.
There is increasing awareness of the importance of informed consent14 as a process in the physician-patient relationship. The present study therefore aimed to provide an appropriate volume of understandable information so that patients could decide whether it was in their interest to switch.15 Moving away from paternalistic attitudes and toward more participatory processes is a current quality criterion that accompanies the paradigm shift in relationships between health care providers and users of the health care system.16
The main objective of the present study was to determine which proportion of patients favored changing to a generic after they had received appropriate information, and to what degree the suggestion that they switch caused unease in the patient17 or apprehension in the physician. The secondary aims were to determine whether accepting the switch to a generic was related with specific characteristics of the patient, the medication or the physician, and to analyze some causes of resistance to change.
Methods
This descriptive, cross-sectional study was carried out between July and October 2000 at the Boadilla del Monte Health Center and the Alpedrete Rural Health Center, both part of Health Area 6 in the province of Madrid (central Spain).
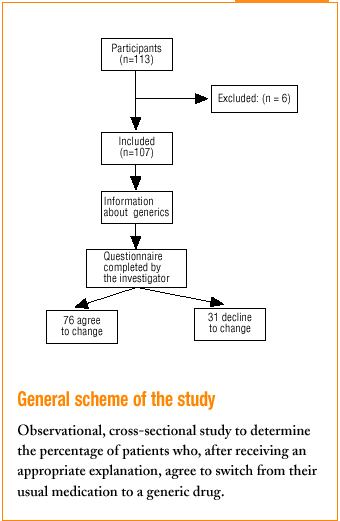
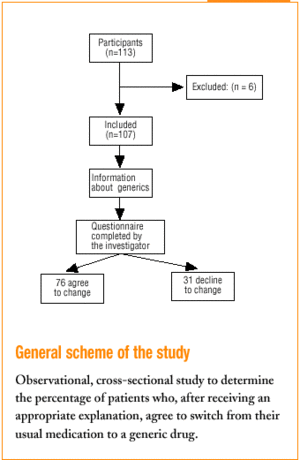
All 113 patients who came to the health centers and who were taking a medication for which a generic substitute was available were included. Patients with communication problems (because of language or hearing) were excluded, as were those who did not come to the center in person and whose relative declined responsibility for the decision to switch. In all, 6 patients were excluded, all for the second reason.
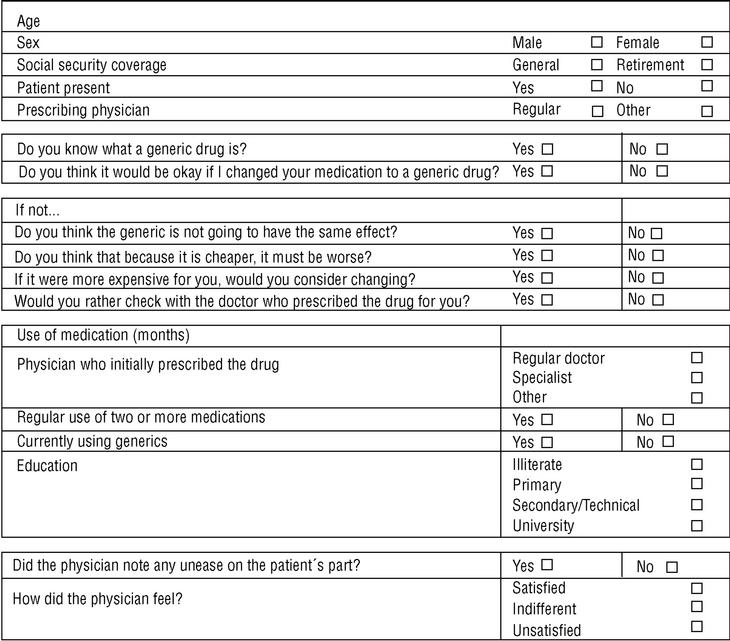
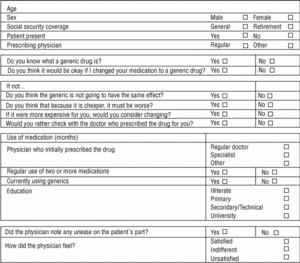
Three family physicians took part in the study; two saw their usual patients, and one saw patients who were temporarily residing (during their vacation) in the area served by the health center. The final sample consisted of 107 patients. All were given the same verbal information about generics in a brief explanation lasting approximately 40 to 60 s (Table 1). This was followed by a verbal questionnaire (Figure 1).
Figure 1. Data sheet
The data were analyzed with the SPSS program. An initial, descriptive (frequency) analysis of the sample was followed by analysis with chi-squared and Student´s t tests to search for relationships between the variable «accepted change» and the other variables we recorded.
Results
Of all patients, 71% (76 of 107) agreed to change to the generic. The physician perceived unease in 23.4% of the cases. Physicians felt satisfied with the interview 83.9% of the times, indifferent 12.9%, and unsatisfied 3.2% of the times.
Slightly more than half of the patients (51%) knew what a generic is, and 12.1% of them used or had used generics before. The patient was present during the physician´s talk in 73% of the cases. Mean age was 66.5 years, and 56.1% of the patients were men (43.9% women). About three-fourths of the patients (77.6%) were receiving retirement benefits; the remaining 22.4% were actively employed. About two-thirds (67.3%) were taking two or more medications. The physician who suggested the change to generics was the patient´s regular general practitioner in 24% of the cases. The medication for which a generic substitute was suggested had been initially prescribed by the patient´s regular primary care physician in 41.1% of the cases, by a specialist in 54.2%, and by a different practitioner in 2.8% of the cases. The educational level of the patients was illiterate in 2%, primary education in 25%, secondary or technical education in 42%, and university-level education in 31%.
Of the 29% of the patients who opted not to switch, 67.4% said that they preferred to consult with the prescribing physician; 45.1% believed that the generic would not have the same effect; 16.1% said that if it was cheaper it must be worse than the brand-name drug; and another 16.1% said that they would accept the change if the alternative product were more expensive.
There was no statistically significant association between agreeing to change to the generic and any of the other variables we studied.
Discussion
Medicine is the art of bringing about small changes in the patient that benefit both the patient and the community. In this study we hoped to evaluate the reasons why different persons hesitate when offered the possibility of changing their habitual treatment. Change is achieved only when the patient is given information and allowed to participate in the decision, without coercion or the imposition of authoritarian criteria. This approach leads to little practitioner apprehension and helps maintain a good physician-patient relationship based on trust and the patient´s decision-making capacity. Informed consent as an ongoing process enhances the quality of medical practice and reinforces the relationship with the user. In recent years, bioethical concerns have clearly illustrated the need to transform paternalistic attitudes toward health care into more participatory approaches.
The fact that 75% of the patients were receiving retirement benefits makes the high percentage of patients who agreed to change to a generic all the more noteworthy, as this population is assumed to be less open to change.
This study did not identify any patient-, medication- or physician-related characteristics that were associated with greater ease or difficulty in switching to a generic. If we had used a larger sample we probably would have found some statistically significant association with at least one of the variables we investigated.
The main reasons patients gave for refusing to change to a generic were doubts about its efficacy and preferring that the physician who originally prescribed the medication recommend the change. These two sources of reticence can be mitigated with additional information campaigns and with a wider use of generics in specialized care. Price was not a factor that favored change: few patients thought that the cheaper product would be less effective. The explanation for this lies in the fact that most of our sample of participants were retired persons, and the advice of a health professional was probably more important to these patients than any possible cost savings.
The main limitation of our results is that they were obtained with a closed questionnaire with four possible answers per item; patients chose one or more option for each question. This approach was used to avoid the risk of influencing the responses. However, an open questionnaire would have made the analysis more complicated.
The accuracy of our results is supported by the fact that the percentage of patients in our sample who knew what a generic is (51%) is similar to the figure published by the Ministry of Health and Consumer Affairs.
The large percentage of patients who were taking two or more medications (67.3%) is a final point worthy of note. This population in particular stands to benefit from the switch to generics, as one of the advantages of this measure is to avoid duplicate treatments, a frequent result of the enormous variety of brand names under which the same active principle is marketed. An added value of this study is the fact that it complied scrupulously with criteria for informed consent. This endows medical practice with greater transparency, and fosters good physician-patient relations.
Conclusion
Our data show that investing less than 1 minute during the physician-patient encounter has considerable advantages: the patient obtains appropriate information on generics, which facilitates the change from a habitually-used medication to a generic product in nearly three-fourths of the patients.
Correspondence: Salvador S. Casado Buendía. C/ Rosalía de Castro, 3. 28420 La Navata, Madrid, Spain. E-mail: scasadob@papps.org
Manuscript accepted for publication 8 April 2002.