To estimate the effectiveness of a Medication Discrepancy Detection Service (MDDS), a collaborative service between the community pharmacy and Primary Care.
DesignNon-controlled before-and-after study.
SettingBidasoa Integrated Healthcare Organisation, Gipuzkoa, Spain.
ParticipantsThe service was provided by a multidisciplinary group of community pharmacists (CPs), general practitioners (GPs), and primary care pharmacists, to patients with discrepancies between their active medical charts and medicines that they were actually taking.
OutcomesThe primary outcomes were the number of medicines, the type of discrepancy, and GPs’ decisions. Secondary outcomes were time spent by CPs, emergency department (ED) visits, hospital admissions, and costs.
ResultsThe MDDS was provided to 143 patients, and GPs resolved discrepancies for 126 patients. CPs identified 259 discrepancies, among which the main one was patients not taking medicines listed on their active medical charts (66.7%, n=152). The main GPs’ decision was to withdraw the treatment (54.8%, n=125), which meant that the number of medicines per patient was reduced by 0.92 (9.12±3.82 vs. 8.20±3.81; p<.0001). The number of ED visits and hospital admissions per patient were reduced by 0.10 (0.61±.13 vs 0.52±0.91; p=.405 and 0.17 (0.33±0.66 vs. 0.16±0.42; p=.007), respectively. The cost per patient was reduced by €444.9 (€1003.3±2165.3 vs. €558.4±1273.0; p=.018).
ConclusionThe MDDS resulted in a reduction in the number of medicines per patients and number of hospital admissions, and the service was associated with affordable, cost-effective ratios.
Estimar la efectividad del servicio de detección de discrepancias de la medicación, un servicio de colaboración entre la farmacia comunitaria y la atención primaria.
DiseñoEstudio de intervención antes-después, sin grupo control.
EmplazamientoOrganización Sanitaria Integrada de Bidasoa, Gipuzkoa, España.
ParticipantesEl servicio fue ofrecido por un grupo multidisciplinar que incluía farmacéuticos comunitarios (FC), médicos de atención primaria (MAP) y farmacéuticos de atención primaria a pacientes que presentaban discrepancias entre la medicación prescrita en la hoja de tratamiento activo y lo que realmente estaban tomando.
Mediciones principalesLas variables principales del estudio fueron el número de medicamentos, tipo de discrepancia y la decisión del MAP. Las variables secundarias fueron tiempo invertido por el farmacéutico, visitas al servicio de urgencias, ingresos hospitalarios y los costes.
ResultadosEl servicio se ofreció a 143 pacientes, y el MAP resolvió las discrepancias de un total de 126 pacientes. El FC identificó 259 discrepancias de las cuales la mayoría fue que el paciente no estaba tomando un medicamento prescrito (66,7%, n=152). En la mayoría de los casos, la decisión del MAP fue suspender el tratamiento (54,8%, n=125); el número de medicamentos que tomaba el paciente se redujo en un 0,92 (9,12±3,82 vs. 8,20±3,81; p<0,0001). El número de visitas al hospital y los ingresos hospitalarios se redujeron en 0,10 (0,61±0,13 vs. 0,52±0,91; p=0,405) y 0,17 puntos (0,33±0,66 vs. 0,16±0,42; p=0,007), respectivamente. El coste por paciente se redujo en 444,9€ (1.003,3±2.165,3 vs. 558,4€±1.273,0; p=0,018).
ConclusiónEl servicio redujo el número de medicamentos que tomaba el paciente e ingresos hospitalarios y esto se relacionó con unos ratios de coste-efectividad positivos.
Medication errors (ME) are among the top 10 causes of death worldwide.1 Such errors can cause patient safety incidents, which are associated with a higher rate of hospitalisation and increased morbidity and mortality, accounting for more than 1% of total global health expenditures.2 ME is the single most common preventable cause of adverse events in medication practice and a major public health burden, with an estimated annual cost in Europe of €4.5 billion to €21.8 billion.3 Due to the health and economic impact of ME, the World Health Organisation (WHO) has included the reduction of ME in the Global Patient Safety Challenge.4
ME has been defined as ‘any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient, or consumer’.5 Contributing factors may be associated with health care professionals, patients, the work environment, medicines, computerised information systems, and/or primary–secondary care communication.6 Reducing the frequency and impact of preventable harm related to medicines as the consequence of error, accident, or communication problem will contribute to the achievement of medication safety for patients.7 Statistics show that these strategies will lead to 95,000 fewer deaths per year in Europe.2
Various strategies to reduce ME in the community setting have been proposed in recent years; they include medication review and reconciliation services, the use of automated information systems, education, and multicomponent interventions.8–10 The effectiveness of clinical pharmacists in identifying ME has been demonstrated, but data from primary care are relatively scarce and few studies have included community pharmacists (CPs).11–13 This lack of research among CPs and the previous experience that these professionals have in other services14 have led the WHO to consider the involvement of CPs in the prioritisation of strategies to reduce ME in primary care.6
In this context, to meet the need for high-quality and cost-effective identification of medication discrepancies, a medication discrepancy detection service (MDDS) was designed. To ensure patient-centred care, collaboration among different health professionals is needed.15 The MDDS is offered by a multidisciplinary team including CPs and general practitioners (GPs) in collaboration with primary care pharmacists and primary care nurses. The identification of medication discrepancies is a way to detect ME, and CPs in Spain are ideally positioned to do so, as they have access to electronic medical records and are responsible for dispensing medicines. Therefore, the aim of the present study was to evaluate the impact on the number of medicine intake and the cost effectiveness of the MDDS as implemented collaboratively in the community pharmacy and primary care services settings.
MethodsStudy design and ethical approvalThis non-controlled before-and-after study.was undertaken between October 2015 and September 2016 in the Bidasoa Integrated Healthcare Organisation, Spain, which is comprised of one regional hospital and three primary care units. The multidisciplinary professional group that provided the MDDS consisted of CPs, primary care pharmacists, GPs, and hospital specialists. All the CPs of the pharmacies located in the municipalities attended by the Integrated Healthcare Organisation, were invited to participate in the project. CPs and GPs attended a 2-hour workshop that presented and described the study protocol. The protocol for this study was approved by the Ethics Committee for Clinical Research of the Basque Country (PI2015080 EPA-SP) and was in line with the Helsinki Declaration. All participants provided written informed consent at the time of their enrolment, and CPs delivered information sheets explaining the study to patients who met the study criteria.
PatientsPatients were recruited according the following criteria: patients that had a discrepancy between their active medical charts and the medicines they were actually taking. CP identified this patients with discrepancies like (i) patients not taking medications that appeared in their charts, (ii) taking medications that did not appear in their charts, (iii) not following the prescribed dosage regime, (iv) not following the prescribed posology and (v) duplicated treatment.
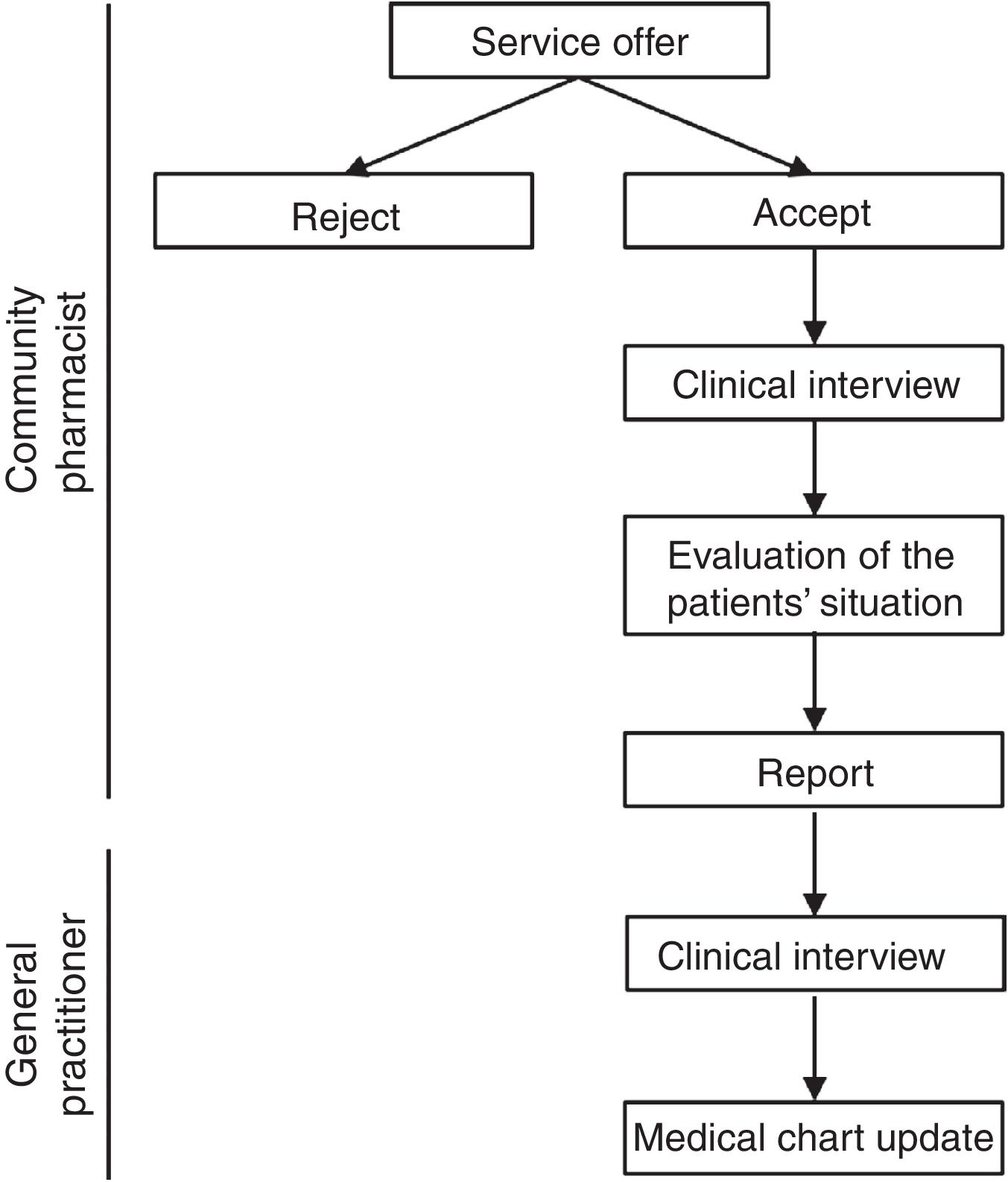
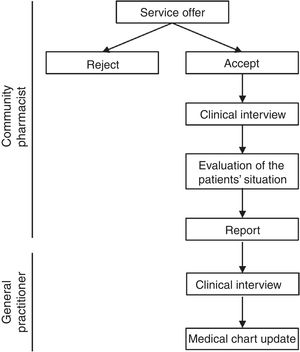
Study procedure and health outcomesCPs offered the MDDS service to patients in whom at the time of dispensing they identified a discrepancy between their active medical chart and the medicines they were taking. CPs registered each participating patient's name, health identification number, willingness to participate in the study, and date of first appointment (record 1). Patients were asked to bring all current medications, including dietary and other products, to the pharmacy. The CPs performed a clinical interviews and checked brown bags For the interview, the pharmacist used a guide consisting of structured questions that allowed to collect as much information as possible about taking prescribed medications, other medications, supplements, creams or other products. The brown bag checking consisted of checking an inventory of the medications taken by each patient based on the medication packages. At the time of the clinical interview, each patient provided written informed consent. If a patient did not return for the scheduled appointment, it was recorded as “rejected”. After the clinical interview, the information was compared with the patient's medical chart and the CP prepared a report in which all detected discrepancies were registered. Once the CP evaluated the patient's situation, the CP completed the report and sent it to the primary care pharmacist. Time invested in the clinical interview and report preparation was also registered.
Upon receiving the report, the primary care pharmacist contacted the corresponding primary care nurse, who cited the patient with the GP. The GP conducted a clinical interview and was responsible for making any necessary changes to the medical chart in the electronic prescribing system. If a medical specialist was responsible for the prescription, the primary care pharmacist contacted directly by telephone to solve the problem. Pharmacotherapeutic changes were made in agreement with the patient, and the GP made sure that the patient understood the new treatment (Fig. 1). Discrepant medications were classified using the Anatomical Therapeutic Chemical system.
Primary care pharmacists compiled and recorded all data, and were responsible for registering discrepancies and for ensuring that the flowchart was followed correctly. Emergency department (ED) visits and hospital admissions 6 months before and after the intervention were registered at the end of the study period using hospital records.
Economic outcomesAn economic evaluation was conducted from the National Health System (NHS) perspective. The cost and effectiveness of the service was analysed. The direct costs of medications (including discrepant medications), ED visits and hospital admissions 6 months before and after the intervention, and interventions costs were included. The numbers of medicines, ED visits, and hospital admissions served as effectiveness variables. Costs were estimated using posology and the prices of the medicines. The costs associated with ED visits were estimated based on the Basque Health Service (BHS) rates.16–18 The diagnosis-related group (DRG) was identified for each hospital admission. DRGs make up an established payment system for groups of patients with similar clinical characteristics who are expected to have similar health resource consumption.4 The cost for each DRG was determined using BHS rates.16–19 The total cost of each intervention included costs associated with: (i) the time spent by the CP on the clinical interview, (ii) the time spent by the CP to complete the report, (iii) the cost of GP consultation (iv) the cost of hospital telephone specialist consultation and (v) the cost of the time spent by primary care pharmacists. Costs (i) and (ii) were estimated using collective CP bargaining data. Costs (iii) and (iv) were estimated using BHS rates.16 All costs were expressed in euros and updated to 2017 using the Spanish Retail Price Index. The incremental cost-effectiveness ratio (ICER) was calculated to compare costs before and after the intervention.
Statistical analysisChanges in the numbers of medicines, ED visits, and hospital admissions were evaluated and compared before and after MDDS implementation with the paired t test or Student's t test for parametric variables. The chi-squared test and Fisher's exact test were used to analyse the frequency distributions of the study variables. A one-way sensitivity analysis was conducted to examine the impacts of the study variables on the results of the economic evaluation. General data are expressed as means±standard deviations. Statistical analyses were performed using the SPSS software (version 18.0 for Windows XP; Microsoft Corporation, Armonk, NY, USA). Two-tailed p values<0.05 were considered to be statistically significant.
ResultsTen of the 30 community pharmacies located in the municipalities attended by the Integrated Healthcare Organisation participated in the project and offered the MDDS to a total of 240 patients. CPs identified 259 discrepancies in 143 patients, leading to 228 medication reconciliations for 126 patients by GPs and other medical specialists. The majority (72.3%) of participants were women and the mean age was 72.3±13.1 years. The mean number of prescribed medicines take was 9.1±3.8 per patient and the mean number of medication interventions was 1.8±1.3 per patient.
The main type of discrepancy registered by CPs was that patients were not taking medicines listed on their active medical charts (58.7%, n=152).
In more than half (54.8%, n=125) of discrepancy cases, GPs decided to withdraw the treatment. In other cases, the treatment was not modified (24.6%, n=56), it was modified (13.6%, n=31), or new treatment was initiated (7.0%, n=16). The groups of medicines with the most discrepancies were drugs for obstructive airway diseases (R03; 8.3%, n=19), psycholeptics (N05; 8.3%, n=18), and non-steroidal anti-inflammatory and antirheumatic products (M01A; 7.5%, n=17).
After the intervention, a significant reduction in the number of medicines in patients’ active medical charts (−0.92±1.09, p<0.0001)was seen. CPs invested an average of 11.8±4.1min performing each initial patient interview and 13.8±5.0min drafting the report. They thus spent a mean total of 25.5±7.4min per patient providing the service. Thirteen cases were transferred to medical specialists who had prescribed discrepant medicines.
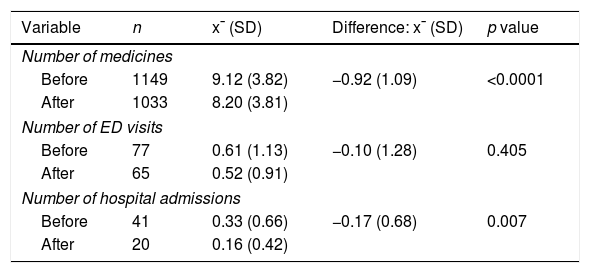
The number of hospital admissions decreased (−0.17±0.68, p=0.007) after MDDS implementation compared with baseline (Table 1). The number of ED visits also decreased, but this difference was not significant.
Numbers of medicines, emergency department visits, and hospital admissions 6 months before and after the resolution of medication discrepancies (n=126).
| Variable | n | x¯ (SD) | Difference: x¯ (SD) | p value |
|---|---|---|---|---|
| Number of medicines | ||||
| Before | 1149 | 9.12 (3.82) | −0.92 (1.09) | <0.0001 |
| After | 1033 | 8.20 (3.81) | ||
| Number of ED visits | ||||
| Before | 77 | 0.61 (1.13) | −0.10 (1.28) | 0.405 |
| After | 65 | 0.52 (0.91) | ||
| Number of hospital admissions | ||||
| Before | 41 | 0.33 (0.66) | −0.17 (0.68) | 0.007 |
| After | 20 | 0.16 (0.42) | ||
x¯, mean; SD, standard deviation; ED, emergency department.
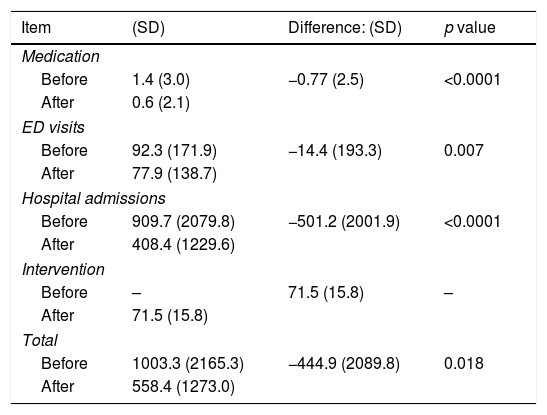
The mean cost of the intervention was €71.5±15.8. GP consultations were the costliest components (€55 each) followed by the telephone specialist consultation (50€ each); the average costs of CP and specialist consultations were €11.3±3.3 and €5.2±15.3, respectively. The costs of medication, ED visits, and hospital admissions were lower after the intervention (Table 2). Even taking into account the cost of the intervention, all costs were lower thereafter (p<0.05).
Mean costs per patient (€, 2017; n=126).
| Item | (SD) | Difference: (SD) | p value |
|---|---|---|---|
| Medication | |||
| Before | 1.4 (3.0) | −0.77 (2.5) | <0.0001 |
| After | 0.6 (2.1) | ||
| ED visits | |||
| Before | 92.3 (171.9) | −14.4 (193.3) | 0.007 |
| After | 77.9 (138.7) | ||
| Hospital admissions | |||
| Before | 909.7 (2079.8) | −501.2 (2001.9) | <0.0001 |
| After | 408.4 (1229.6) | ||
| Intervention | |||
| Before | – | 71.5 (15.8) | – |
| After | 71.5 (15.8) | ||
| Total | |||
| Before | 1003.3 (2165.3) | −444.9 (2089.8) | 0.018 |
| After | 558.4 (1273.0) | ||
SD, standard deviation; ED, emergency department.
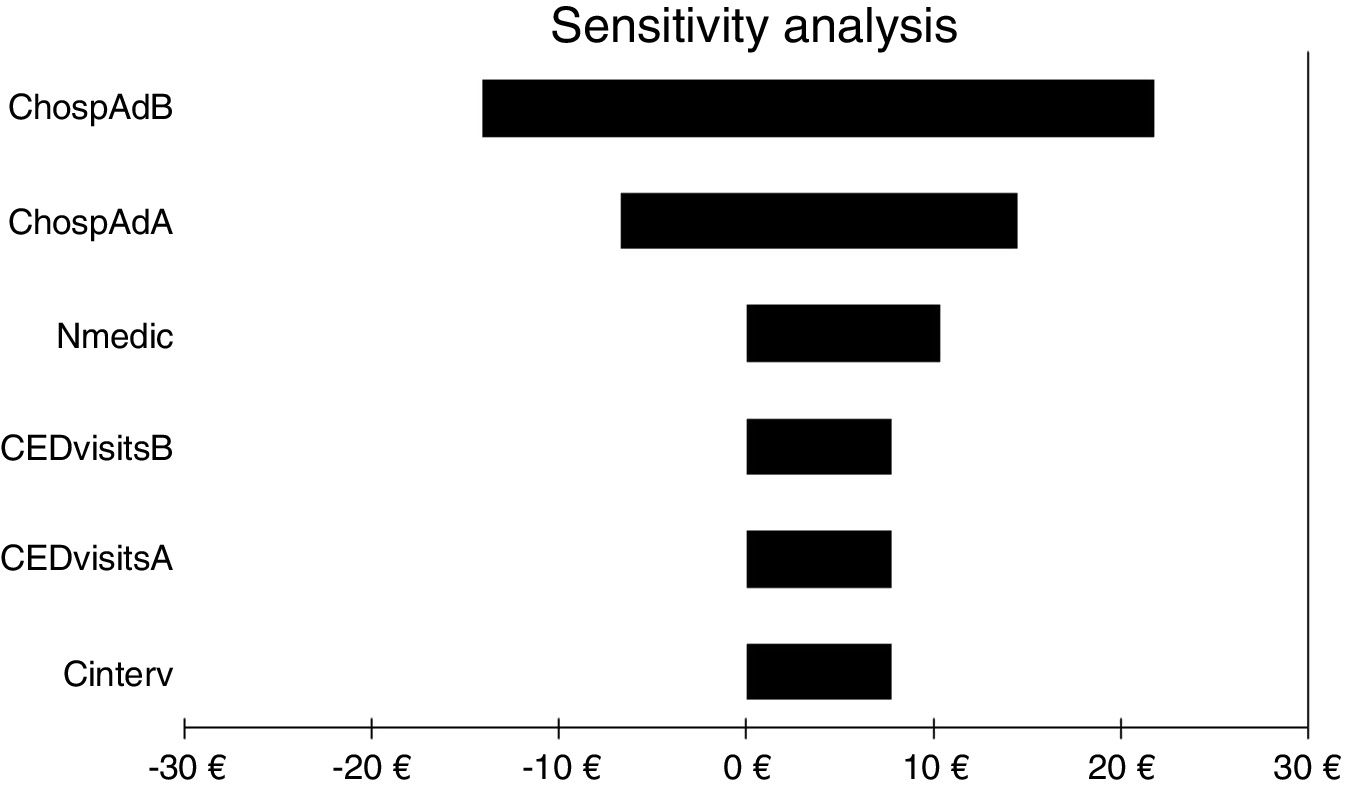
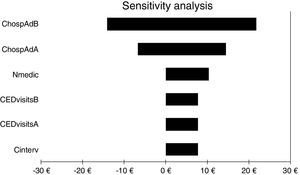
For all three cost-economic variables, the intervention was cost effective because health outcomes were better and costs were lower. The sensitivity analysis showed that the variable with the greatest impact was the number of hospital admissions, as it was the only variable that could invert the cost. All other variables analysed slightly increased or decreased the benefits obtained with the service (Fig. 2).
Results of one-way sensitivity analysis including variables critical to the economic evaluation. CHospAdB, cost of hospital admission before intervention; CHospAAB, cost of hospital admission after intervention; Nmedic, number of medications; CEDvisitsB, cost of emergency department visits before intervention; CEDvisitsA, cost of emergency department visits after intervention; Cinterv, cost of intervention.
This study showed that the MDDS is an effective and innovative way to detect medication discrepancies in community pharmacies and to resolve them with the collaboration of diverse health professionals, such as CPs, GPs, other medical specialists and primary care pharmacists. The high percentage (88%) of resolved discrepancies and the reduction in the number of drugs taken (by almost one per patient) suggest a significant improvement in patient safety.
CPs identified 240 patients with medication discrepancies, of whom 143 accepted study participation. The majority of these 143 patients had single discrepancies, and the rest had discrepancies in more than one medication. Medication discrepancies can be detected at different levels. Several systematic reviews have shown that pharmacist-based interventions are effective in the community setting.20,21 The MDDS identifies and reduces discrepancies being the particularity of this study the involvement of all health agents, especially community pharmacists, in the control of medication errors. Our data suggest that CPs are ideally positioned to detect medication discrepancies, in agreement with the WHO's strategy to include CP in plans to detect ME.6
Removing a medication from the medical chart was the most common intervention performed by the GP. It has been demonstrated that after the MDDS intervention, each patient in this study used, on average, almost one fewer medication than at baseline. Polypharmacy is related to poor adherence, interactions and ME,22 and reducing this condition is included in the WHO's third Global Patient Safety Challenge.7 Thus, the MDDS could provide a strategy for the reduction of polypharmacy-related problems. Furthermore, this service represents that it could be an efficient way of improving patients’ medication-related safety and a strategy to prevent and manage patients’ frailty.23
One problem associated with medication reconciliation interventions for CPs is the difficulty of contacting physicians.24 Several authors have stated that future initiatives should focus on collaboration between health care professionals, and such collaboration is also essential when designing services.25,26 Therefore, CPs and primary care pharmacists participated in the design of the MDDS. Primary care pharmacists served as intermediaries between CPs and GPs, and this strategy was effective.
The numbers of hospital admissions and ED visits were 45% and 16% lower, respectively, after the intervention than at baseline. Similar reductions have been observed after clinical pharmacists-based interventions.27,28 Due to the use of a wide range of methods to calculate the cost of ME, calculation of the worldwide health care expenditure associated with hospital admissions and ED visits due to such error is difficult.29 However, authors agree that this cost is high.30 A study conducted in the Netherlands showed that the cost of hospital admission due to preventable medication-related events increased to €3171 per patient, and ED visits accounted for €30,896, or 5.3% of the total health costs, during the study period.31 One objective of the Organisation for Economic Co-operation and Development in 2014 was to identify good practices in managing health care budgets.32 Therefore, reducing hospital admissions and ED visits with the MDDS could contribute to improving the sustainability of the health system.
Our analysis supports the hypothesis that the MDDS is a dominant intervention, as it improves clinical outcomes with lower costs than usual care, regardless of the cost of the intervention itself. The sensitivity analysis showed that only the cost related to hospital admissions could invert the ICER. The variability in the cost of such admissions is greater than variabilities for other health outcomes. Some authors have stated that use of the DRG system may lead to inequities in associated costs.26 To reduce this variability, the identification of hospital admissions related to medicines and exclusion of unrelated admission from analysis could be useful.33 Previous economic evaluations have focused on transitional care programmes that included interventions to prevent ME among settings, and they have produced variable results.34–37 Recent evaluations have shown that the services provided by CPs tend to be cost effective.38,39 The implementation of professional pharmacy services like the MDDSmay be an efficient way to improve patient safety.
The groups of medicaments with the most discrepancies in this study were drugs for obstructive airway diseases (R03), psycholeptics (N05), and non-steroidal anti-inflammatory and antirheumatic products (M01A). Considering that most discrepancies detected in this study were due to patients not taking medicines included in their medical charts we could state that patients’ more frequently have adherent problems. Patients with medicines prescribed for obstructive airway diseases, psycholeptics and non-steroidal anti-inflammatory and antirheumatic products are one of the most prevalent groups of patients to have adherent problems.40 Although CPs should be aware of discrepancies in all types of medication, special attention must be given to these medication groups when providing the MDDS.
The present study has several limitations. Firstly, it was conducted within the Bidasoa Integrated Healthcare Organisation, and a relatively small number of patients participated. To increase the external validity of our findings, the study should be replicated in other regions. Sencondly, only patients in the NHS are eligible for the MDDS, as they are the only ones for whom CPs receive electronic prescription information. However, the authors do not believe that the inclusion of the entire target population would alter the results. Thirdly, the present study included no random assignment or control group, and the modifications observed could be attributed to factors other than the intervention. To increase the reliability of the MDDS and our finding that it is cost effective compared with usual care, the results of this study should be compared in studies conducted with control groups. Finally, all hospital admissions and ED visits were included in analysis, with no evaluation of cause. To minimise possible bias, future analyses should include only hospital admissions and ED visits associated with ME. Future health policies must provide support for the development and implementation of evidence-based services to prevent ME and improve patient safety.
- •
The reduction of preventable harms related to medicines will reduce patient safety incidents.
- •
Community and primary care pharmacists are useful health professionals in efforts to reduce medication errors in primary care.
- •
The MDDS might reduce the numbers of medications prescribed, emergency department visits, and hospital admissions.
- •
The MDDS might be a cost-effective service that could contribute to improving the sustainability of the health system.
- •
The MDDS might be effective as a collaborative approach between the community pharmacy and ambulatory settings.
The authors received no specific funding for this work.
Conflict of interestThe authors declare no conflict of interest.
Authors would like to thank the patients, community pharmacists, in special to Ainhoa Ayerza and Heillen Mora pharmacists in Martin Ezcurras’ pharmacy, general practitioners and primary care nurses who shared their time for the purposes of this project.