Continuous Glucose Monitoring (CGM) is among the most important recent advances in diabetes technology for better diabetes management. The CGM provides patients with real-time information about glucose levels, direction and rate of change, and glucose trends. There are currently four continuous glucose monitoring devices (sensors) in clinical use which measure interstitial fluid to calculate blood glucose levels using several algorithms. These devices include sensors developed by GlucoWatch®, DexCom™ (DexCom SEVEN® PLUS), Medtronic (MiniMed Paradigm® REAL-Time and Guardian® REAL-Time), and Abbott Diabetes Care (FreeStyle Navigator®). Continuous Glucose Monitoring is currently approved as adjunctive to Self-Monitoring of Blood Glucose (SMBG), and the CGM data should be confirmed using SMBG for treatment decisions. The use of CGM, both in research and clinical settings, has been documented to decrease blood glucose excursions, lower HbA1c values, and reduce hypoglycemic episodes, which together diminish the risk of complications associated with diabetes. In addition, use of CGM helps in reducing glucose variability. The CGM is also useful associated with special patient populations such as pregnant women with gestational diabetes or type 1 diabetes, children, and subjects with unexplained hypoglycemic unawareness, even though it is not approved by the Food and Drug Administration (FDA) for many of these special circumstances.
Certified Diabetes Educator
continuous glucose monitor/monitoring
continuous subcutaneous insulin infusion
durable medical equipment
European Medicines Agency
Food and Drug Administration
Health Maintenance Organizations
Juvenile Diabetes Research Foundation
multiple daily injections
near infrared
soluble insulin basal analogue
self-monitoring of blood glucose
within the target range.
Diabetes currently affects over 23 million people in the United States and its prevalence rate is increasing globally.1 Uncontrolled diabetes may lead to long term complications including retinopathy, neuropathy, nephropathy and cardiovascular disease.2 Improved glycemic control reduces the risk of long term complications of diabetes, however studies have shown that the number of severe hypoglycemic events tripled.2-6 Continuous Glucose Monitoring (CGM) is among the most important recent advances in diabetes technology that improves glucose control without adding medication. The CGM provides information about glucose concentrations, direction of change, rate of change, and overall glucose trends, whereas self-monitoring blood glucose (SMBG) only provides a single blood glucose measurement at the time of the test.7,8 Because of these features, numerous studies have indicated that the CGM can improve glycemic control, which may reduce both the micro- and macrovascular complications associated with diabetes, while not increasing the risk of hypoglycemia.3,4
Current CGM devicesThe United States Food and Drug Administration (FDA) has approved five continuous glucose monitoring devices, four of which are currently in clinical use.7 All four devices measure interstitial fluid glucose to calculate blood glucose levels using a mathematical algorithm.3 These devices include the Gluco- Watch® (Redwood City, California, USA), the DexCom SEVEN® PLUS (San Diego, California, USA), the Medtronic MiniMed Paradigm® REAL-Time and the Guardian® REAL-Time (Northridge, California, USA), and the Abbott Diabetes Care FreeStyle Navigator® (Alameda, California, USA).7
GlucoWatch Biographer (figure 1)The GlucoWatch2 Biographer was the first real-time system to be approved by the FDA.7 The biographer was worn like a wristwatch and detected interstitial glucose using reverse iontophoresis3 (figure 1). The biographer had a 2-hour warm-up period during which no glucose readings were displayed.9 After the warm-up period, the biographer provided glucose readings as often as every 10 minutes, which was an improvement from the first-generation device which only produced readings every 20 minutes. Adjustable high and low blood glucose alerts were additional features of the device. The biographer obtained painless, automatic, noninvasive glucose measurements.3 Despite this, the device had problems, including skin irritation related to reverse iontophoresis and tape issues and frequent skipped readings. Readings were often skipped due to bumping the device, perspiration, or rapid changes in temperature.3 Further, the device only lasted for 14 to 15 hours before the AutoSensor needed to be changed.3 Therefore, the biographer is not currently being marketed or used in clinical practice. The product was originally developed by Cygnus (Redwood City, California, USA), later bought by Animas (Philadelphia, Pennsylvania, USA), and now, intellectual property is owned by LifeScan (Johnson & Johnson Milpitas, California, USA). This device paved the way for future developments.
DexCom Continuous Glucose Monitor (figure 2)The DexCom SEVEN® PLUS CGM is a wireless device with a sensor, which is inserted into subcutaneous tissue of the abdomen. The sensor uses interstitial fluid to evaluate blood glucose levels using an algorithm. There is a 2-hour start up period before the device will begin to transmit blood glucose values. The transmitter sends the data from the sensor to the receiver, giving real-time glucose values to the patient every 5 minutes, with alarms to alert the patient of hypoglycemia or hyperglycemia. The new receiver screen shows information such as glucose rate of change arrows and graphs/trends of blood glucose over 1, 3, 6, 12 and 24 hours.10 Additionally, the SEVEN PLUS provides the patient the ability to track meals, insulin dose, and physical activity.10 The device requires calibrations every 12 hours of wear. These calibrations are preferably performed at relatively stable blood glucose values to ensure accuracy (rate of change of glucose <2 mg/dl/min).11 The positive aspects of the DexCom sensor, in particular, are its small size and increased sensor life. The sensor wear time was originally 3 days and now, after being approved by the FDA in January 2009, is 7 days.10 However, many patients wear the sensors for longer periods of time, averaging 10-14 days (off-label).11 The rationale for extended stability of the DexCom sensor is the use of a patented membrane that does not clog the sensor. The size of the sensor is also relatively small, making it easier and more convenient to wear (figure 2b). One of the problems with the SEVEN PLUS is that acetaminophen (Tylenol) will cause inaccurate readings as it interferes with the glucose signal, and therefore, should not be consumed while wearing the sensor.12
Medtronic MiniMed Continuous Glucose Monitoring System (figure 3)The Medtronic MiniMed Continuous Glucose Monitoring System (CGMS®) consists of a subcutaneous sensor and an external monitor3 (figure 3). Like the DexCom, this sensor measures interstitial fluid to evaluate blood glucose. There is a 2-hour initialization period with no glucose readings, after which blood glucose levels are displayed every 5 minutes. The new device must be calibrated with a SMBG reading a minimum of every 12 hours. The MiniMed CGMS includes 3 and 24 hour blood glucose graphs, high and low alerts, and trend arrows with rise and fall rates.13 The MiniMed Paradigm REAL-Time was created for patients using continuous subcutaneous insulin infusion (CSII). Only one device is necessary, acting as both an insulin pump and a CGMS device. However, subcutaneous insertion sites for the CGM sensor and pump catheter to deliver insulin must be separated by at least a minimum of a few inches to avoid faulty glucose data. Medtronic also has a device available for non-CSII users called the MiniMed Guardian® REAL-Time. The Guardian offers additional features to the Paradigm, including predictive alarms and the ability to enter meal and insulin events. One of the disadvantages of these CGMS devices is that sensor wear has only been approved for 3 days, compared to the longer sensor life of other devices. However, most patients use the sensor for extended periods of 5-6 days after recalibration of the same sensor (off-label). One of the MiniMed pump systems, the Paradigm VEO, has a sensor approved for up to six days wear.
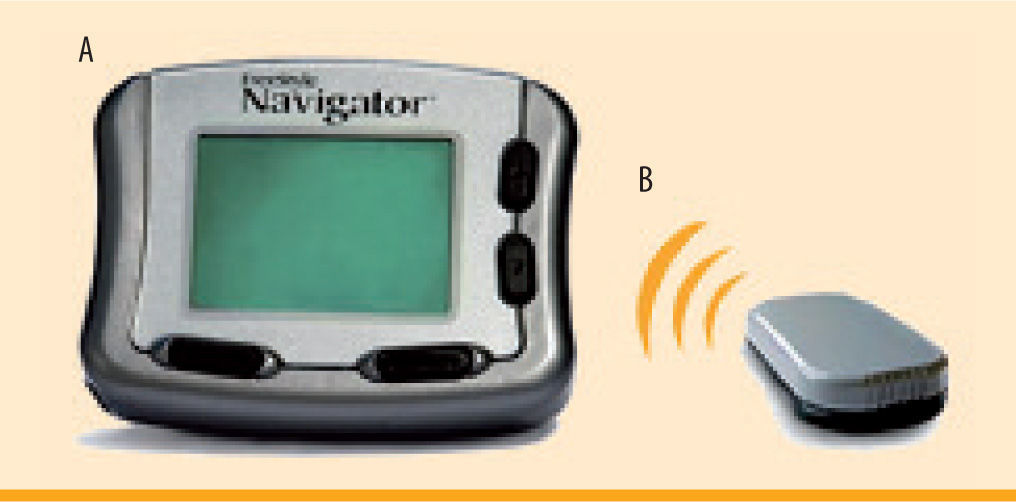
Freestyle Navigator Continuous Glucose Monitoring System (figure 4)The Freestyle Navigator is the most recent continuous glucose monitoring system to be approved by the FDA in March of 2008. Like the other devices, the Navigator consists of a subcutaneous sensor and an external monitor. The calibrations occur at 10, 12, 24, and 72 hours after sensor insertion. Although there are fewer total calibrations, the readings do not display on the receiver until the first calibration has been completed. Therefore, the first 10 hours of sensor wear will have no glucose readings. However, more recently, a 1-hour warm-up period was approved by the FDA and the European Medicines Agency (EMEA), although the version of the Navigator with the 1-hour warm up period is not currently available in the United States. Features of the Navigator include low and high alerts, predictive alarms, 2, 4, 6, 12, and 24 hour glucose trend graphs, and trend arrows.14 Meal, insulin, exercise and other events can also be entered into the device. The Navigator is currently the only CGM to display a blood glucose value every minute. In addition, this is the only device that has a built in blood glucose meter, which allows the unit to auto calibrate and also eliminates the need to carry around a separate blood glucose meter. The sensor is currently only approved for 5 days of sensor wear,14 however, many patients are able to extend use for 7-10 days (off-label). Furthermore, the sensor is larger and bulkier, and therefore tends to fall off (sooner than 5 days) more often than the other sensors on the market (figure 4). It has been observed that the Navigator sensor causes more skin area reactions as compared to the DexCom sensor.15
Advantages of CGM UseThe CGM provides an abundance of glucose readings, leading to improved patient care through observation of trends in glucose values.8 Not only does CGM provide real-time blood glucose values to the patients, but the devices can also be downloaded by health care providers and patients themselves at home to view glycemic trends and patterns.8 These results can be used to modify insulin doses and provide insight as to the potential causes for glucose excursions.8 Furthermore, with the use of the CGM, the patients are able to see their glucose values at times when they would not normally test with SMBG, such as overnight.3 With a CGM, a patient is given additional information rather than a single blood glucose value from SMBG including, for example, glucose trends and rate of change. The patient also receives information such as the direction of change by providing glucose trend arrows. This information assists the patients in making lifestyle modifications to more effectively manage their diabetes,16 leading to decreased blood glucose excursions, reduced HbA1c, and less hypoglycemic episodes.8,17-20 The availability of real-time glucose monitoring has been demonstrated to help reduce glucose excursions and decrease the time spent in hypo- and hyperglycemic ranges.21
The CGM alerts patients when they enter a hyperglycemic or hypoglycemic range, often manually set to alarm when blood glucose is <70 mg/dl or >200 mg/dl. In addition, two of the CGM devices (Freestyle Navigator and MiniMed Guardian REAL-time) can be programmed to alarm for projected hyperglycemic or hypoglycemia with predictive alarms. This occurs when the CGM senses the rate of change of glucose will lead to a high or low event within a given time period, such as 10-30 minutes.22 All of the CGM devices have rate of change alerts, which will alarm the patients when their blood glucose levels are changing at a certain rate, usually >2 mg/dL/min. This can help to prevent hypoglycemic and hyperglycemic episodes before they occur as the patients will undertake the necessary action.
Disadvantages of CGM UseContinuous glucose monitoring is currently approved as an adjunct to SMBG.8 The patients need to confirm low and high blood glucose values before making any treatment decisions. The accuracy of current CGM devices is still not as good as currentday glucose meters, and therefore, the sensor data must be verified by SMBG before a decision to take insulin or eat a meal is made.8 Additionally, some patients experience erythema, edema, or skin irritation due to the sensor adhesive8. Mild erythema has been observed in approximately 5% to 20% of patients.8,17,19
Another concern with CGM is a possible time lag with glucose readings given by the sensor,8 especially when the rate of change of glucose is greater than 2 mg/dL/min. The time lag consists of a physiological lag between blood and interstitial fluid (5-20 min) due to excessive rate of change of glucose values (greater than 2 mg/dl/min) in both directions (hyperglycemia and hypoglycemia). In addition, the time lag may fluctuate between different CGM systems. In one recent study, different time lags were observed between the SEVEN PLUS and the Navigator.23
As a result of the knowledge gained from receiving continuous glucose values, a patient's behavior may be modified negatively. Patients are more likely to overcorrect hyperglycemia by stacking their bolus insulin, which can lead to hypoglycemia.8 Conversely, patients may also overcorrect their hypoglycemia by stacking carbohydrate intake, resulting in hyperglycemia.8 To prevent these issues, patients need to be instructed properly on the pharmacokinetics of different insulins, the effects of insulin stacking, and proper treatment of hypoglycemia. For example, food intake does not result in instant absorption and rising of blood glucose levels, and instead, may take 20-40 minutes depending on the type of food ingested. Proper education is the key to success of using this new technology.
Many physicians have difficulty finding the time and resources necessary to provide proper training on the CGM. Certified Diabetes Educators (CDEs) are needed to demonstrate proper insertion of the sensors and connection of the transmitter. In addition, the patient must be informed on how to use the information the CGM provides as well as the proper calibration schedule. These issues, along with poor reimbursement by health maintenance organizations (HMOs), make it challenging to incorporate the CGM into routine practice for many physicians.16 In addition, health care cost in the short period is likely to increase with the use of the CGM, but long term health care cost may go down as a result of better glycemic control and thus a reduced risk for complications.
Many patients initially increase the number of times they check their blood glucose with SMBG while wearing the sensor. However, over extended periods, the patients reduce the number of SMBG values taken.16,24 The CGM is not currently approved as a stand alone device and thus, verification of hyperglycemia and hypoglycemia with SMBG (adjunctive) needs to be done before treatment.
Recent continuous glucose monitoring studiesMany studies have been conducted using CGM in the past several years using different outcomes, for example, time spent in euglycemia, hypoglycemia or hyperglycemia, and percentage of glucose readings within the target range (WTR), A1c levels, and the frequency of hypoglycemic episodes. A study demonstrated that the group of patients using a CGM decreased the time spent in hyperglycemic (>240 mg/dl) and hypoglycemic (<55 mg/dl) range by 23% and 21% respectively while simultaneously increasing the time spent within euglycemic range (81-140 mg/dl) by 26% as compared to the control group.17 In a different study, the number of glucose values WTR increased by an average of 6.5% while the number of glucose values above target range decreased by an average of 5.6%.4 These changes resulted in a decrease of A1c by 0.4%.4 Lastly, CGM devices have been shown to improve 29 of 48 indices of glycemic control and glycemic variability including mean glucose and the percentages of values within hyperglycemic, euglycemic, and hypoglycemic ranges.26
In 2006, Deiss et al. conducted the first randomized, controlled trial; a 12 week long study done in Europe, which demonstrated that continuous use of a CGMS can improve A1c values.18 The study population was divided into three groups: continuous sensor wear, biweekly for 3-day periods every 2 weeks, or the use of SMBG without sensor wear (control).18 The A1c reduction was over 1% for 50% of the patients and at least 2% in 26% of the patients using the CGM.18 However, the mean A1c at baseline was high (greater than 9%) in the subjects enrolled. In the same year, Garg et al. showed that patients using both continuous subcutaneous insulin infusion (CSII) and multiple daily injections (MDI) improve their time spent within the target range by the same amount with the use of a CGM.25 However, Bailey et al. demonstrated in 2007 that MDI patients reduce their A1c values more than patients using CSII.27
One of the most recent studies being conducted on CGM compares the effectiveness of continuous glucose monitoring in patients using CSII versus MDI. The study will determine if there is a difference in benefit between the two groups. The study group includes 29 patients using CSII and 30 patients using MDI (personal communication).
The CGMs are also being commonly used as tools in many clinical research studies in the blinded mode, where patients are unable to see any information about their blood glucose values. In this way, the patients' blood glucose values are able to be measured constantly without the patients changing their behavior based on the glucose data. The blinded CGM information can be used to help determine the effectiveness of an experimental medication. The CGM has been used in studies on immunomodulatory drugs for type 1 diabetes and type 2 diabetes and GLP-1 analogs. Research studies involving new insulin analogs have also incorporated CGM into their protocol, including insulin analog studies on SIBA (soluble insulin basal analogue) and studies on rapid acting analogues of insulin glulisine, insulin lispro, and insulin aspart.
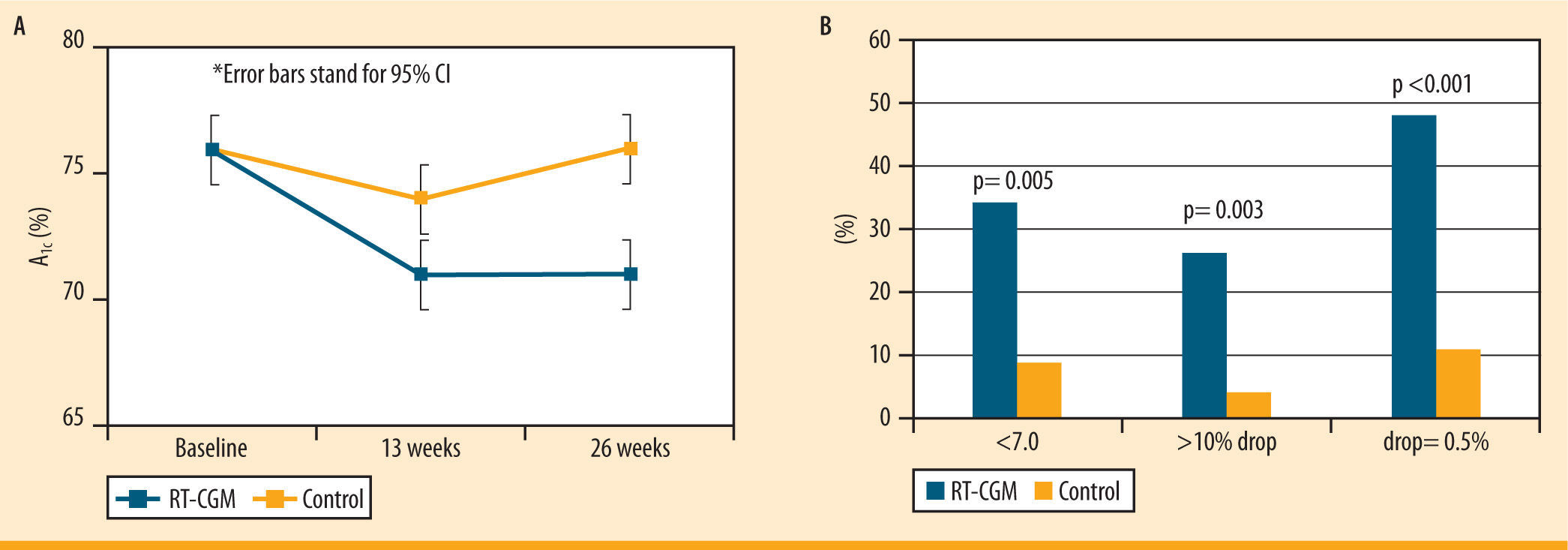
JDRF studiesThe Juvenile Diabetes Research Foundation (JDRF) has perfor- med several studies testing the efficacy of CGM devices. A significant decrease in the HbA1c values was demonstrated in adult patients (25 years and older) from baseline in a 26 week long trial.28 However, no improvement in A1c was shown in the younger patients in the study using CGM.28 This may be a result of the increased CGM use in the adult group compared to younger age groups.28 Furthermore, there was a greater number of patients with A1c levels less than 7.0% (without having a severe hypoglycemic episode) in the CGM group compared to the control group28 (figure 5). The decrease in A1c of the adult group was not related to a higher frequency of hypoglycemic events, which demonstrates that the adult patients in this study improved their A1c values without the risks associated with low blood glucose.28
Changes in A1c in >25 year olds in the JDRF study. Multi-center RCT; 6 months; n= 142. A) The graph shows the overall decrease in A1c in adult subjects in the CGM group compared to the control group over 26 weeks. B) The graph shows the percent of people after 26 weeks who had an A1c <7.0%, had a >10% drop in A1c, and had a drop of A1c = 0.5%, in subjects using the CGM vs. the control group. Data from: Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464-76
Another JDRF trial demonstrated that a greater decrease in A1c without the increased risk of hypoglycemia resulted from increased CGM use in all age groups.29 This study also revealed that those subjects who monitored their blood glucose more than 6 times a day were more likely to use the CGM consistently, potentially because they already closely monitored their diabetes.29 One further study was designed to demonstrate the efficacy of CGM devices in patients with A1c values <7.0%.30 The trial showed that the patients in the CGM group were able to maintain their low A1c values with less hypoglycemia.30 Contrarily, the A1c values increased in the control group, indicating that CGM is advantageous for patients with well-controlled type 1 diabetes.30 A 6-month extension study evaluated the long-term benefits of CGM.31 The follow-up data concluded that in intensively-treated adults with type 1 diabetes, the benefits of CGM can be sustained for one year.31
Continuous glucose monitoring in pregnancy and childrenThere is a higher prevalence of maternal and fetal complications in women with diabetes, despite having adequate A1c values <7.0%.32 Tight glycemic control, especially in the postprandial phase, reduces the risks for complications.33 The CGM allows the opportunity to observe frequent blood glucose readings making it easier to achieve blood glucose levels within target range. Many of the studies on CGM during pregnancy (off-label) have suggested that CGM is an accurate tool for additional glucose monitoring in pregnant women with type 1 diabetes mellitus.34,35 More prospective, randomized studies need to be done in this area to further document the effect that CGM has on reducing the risk of complications during pregnancy. Similarly, most CGMs (sensors) are not approved for use in children but are commonly prescribed by the providers in this population because of the obvious benefits (off-label).
Insurance coverage of continuous glucose monitorsWhile CGM devices are not currently always covered by insurance companies, the progress over the last two years has been substantial in the United States. The positive feedback in research is allowing CGM to become a standard of care in diabetes, and insurance companies are allowing better coverage for the devices. Unfortunately, many HMOs reimburse the sensors under Durable Medical Equipment (DME) or have higher deductibles. The JDRF studies and lobbying have helped increase coverage of CGM devices by the HMOs.
The future of continuous glucose monitoringFor the CGM to be more widely used among patients, the continuous glucose monitors need to have improved accuracy, comfort, convenience, and integration with other diabetes technologies.36 It is imperative that CGMs be approved by the regulatory agencies as stand-alone devices, rather than being adjunctive to SMBG.36 Only then will the sensor reimbursement improve. The continuous glucose monitors need to become more affordable and more ubiquitously covered by insurance to allow more patients to utilize them, as occurred with SMBG.36
There are other devices currently in development which will utilize different methods for measuring glucose values. DexCom, in collaboration with Edwards Sciences (Irvine, California, USA), has begun developing and studying an intravenous sensor for use in a critical care environment. The sensor resides in a peripheral IV catheter and has a life of 72 hours.37 The use of the system is safe and well-tolerated, and shows potential for real time monitoring in a hospital setting.37 Further, there is a device currently in clinical use in Europe called the GlucoDay (Menarini Diagnostics, Florence, Italy), which utilizes a minimally invasive microdialysis system to continuously monitor blood glucose levels every 3 minutes.38 In addition, in-patient use of CGM for intensive glucose management is currently being evaluated at many centers around the world.
Several companies have also been researching true non-invasive monitoring of blood glucose. One of these is Raman Spectroscopy which uses a transcutaneous technique for measuring blood glucose.39 Different wavelengths of near infrared technology (NIR) are currently being studied as true non-invasive systems. Sensys Medical Inc. (Chandler, Arizona, USA) has been trying to develop a non-invasive system which utilizes NIR technology.40 No devices using NIR have been FDA approved. There has also been research using saliva and lacrimal fluid for measuring glucose continuously but all have been unsuccessful.
The future of research in CGM will focus on developing a closed-loop system for insulin requiring patients. This type of system would encompass an insulin pump and a continuous glucose monitor working together to control blood glucose levels, which would create an artificial 'bionic' pancreas. The CGM would continuously monitor blood glucose and trigger the release of insulin based on the glucose levels. This type of system could reduce time spent in hyperglycemia. One of the major problems that researchers are facing with this type of system, however, is preventing hypoglycemia.41 Clearly, there are numerous factors that play a role in blood glucose levels including exercise, stress, and food composition and intake, which can not be accounted with the current level of technology in development for closed-loop systems. Also, algorithms need to be further developed so that they are proactive and not just reactive for this type of system to work and imítate physiological release of insulin. Although closed loop systems may be many years away, the advantages of current CGM devices are clear, and continued research will hopefully lead to improved glycemic control with better technology and algorithms in patients with diabetes.
- •
Continuous glucose monitoring (CGM) provides patients with real-time information about glucose levels, direction and rate of change, and glucose trends.
- •
The use of CGM has been documented to decrease blood glucose excursions, lower HbA1c values, and reluce hypoglycemic episodes.
- •
CGM is currently approved as an adjunct to self-montoring of blood glucose. The patients need to confirm low and high blood glucose values before making any treatment decisions.
Emily Moser and Lauren Crew have nothing to disclose. Dr. Garg received honoraria from research grants (through the University of Colorado) from, and is on the advisory board for Abbott Laboratories; DexCom; and Medtronic MiniMed, Inc. Dr. Garg is not a share holder of any of these companies.






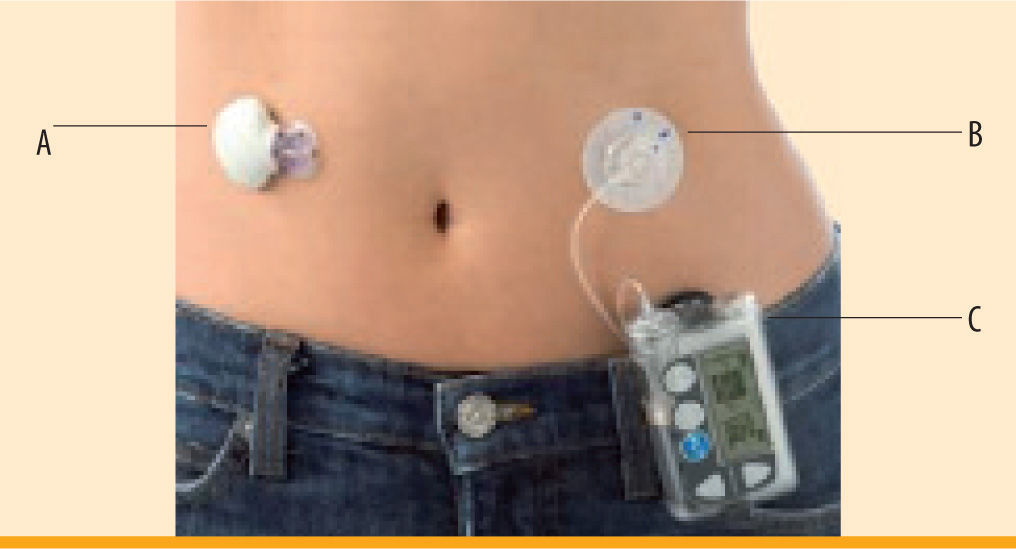


 CGMS, dimensions 4.8 × 7.6 × 2 cm. Courtesy of Medtronic, Northridge, CA' title='MiniMed Paradigm® REAL-Time. A) MiniLink Transmitter, dimensions 3.6 × 2.8 × 0.8 cm. B) Pump catheter insertion. C) Insulin pump and Real-Time
CGMS, dimensions 4.8 × 7.6 × 2 cm. Courtesy of Medtronic, Northridge, CA' title='MiniMed Paradigm® REAL-Time. A) MiniLink Transmitter, dimensions 3.6 × 2.8 × 0.8 cm. B) Pump catheter insertion. C) Insulin pump and Real-Time 
 JDRF study. Multi-center RCT; 6 months; n= 142. A) The graph shows the overall decrease in A1c in adult subjects in the
JDRF study. Multi-center RCT; 6 months; n= 142. A) The graph shows the overall decrease in A1c in adult subjects in the 