The procurement of pumps/supplies through a tender process is common practice among public services. A report is presented on the feasibility and safety of the transition from one continuous subcutaneous insulin infusion (CSII) system to another within a very short time frame (4-weeks) as the consequence of a public tender.
MethodsThe program consisted of: Session-1 was a system start-up training session. Patient satisfaction was evaluated. Session-2 consisted of a call from technical staff 72h after Session-1 to provide support regarding the programming or the change of infusion set. Session-3 was a training session regarding the use of therapy management software. During and 2 months after Session-2, clinical events, technical issues, and training reinforcement incidents were registered. HbA1c data were collected retrospectively.
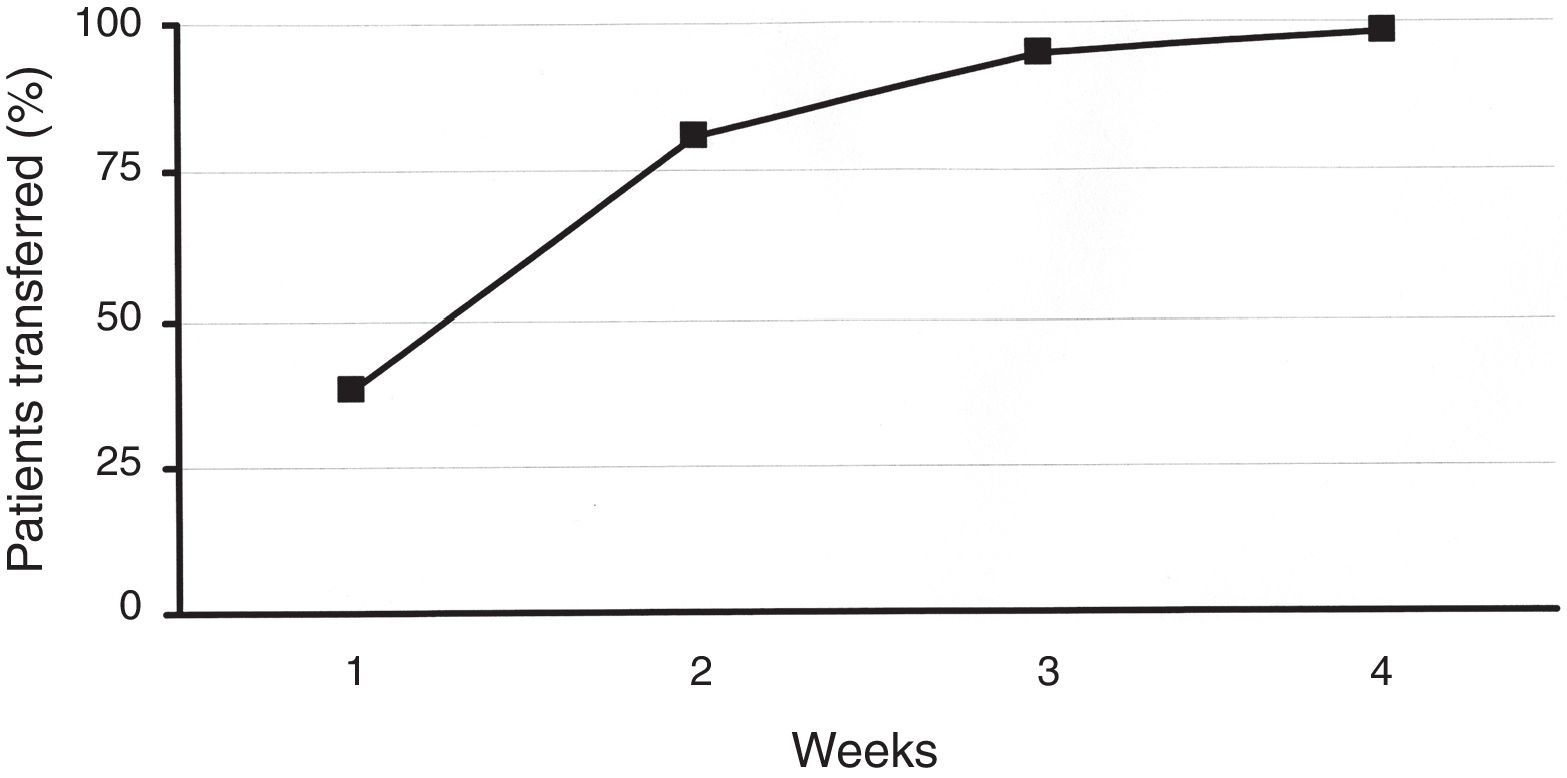
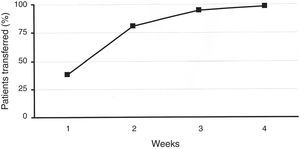
ResultsA total of 219 patients were enrolled. During the second week, 81% of patients were transferred to the new system. Patient overall satisfaction scored 9.4/10 (none <7). There were 30 training reinforcement events and 7 technical issues, with all 37 of them being were sorted out over the telephone. There were 31 additional clinical events (infusion set issues). Twenty-four were considered mild, and were solved by phone technical support. Medical assistance was needed in six (five unexpected hyperglycemia, one ketosis). There was only one severe event (ketoacidosis requiring hospitalization). HbA1c did not deteriorate during the transition process. One hundred twenty-eight patients attended the therapy management software training.
ConclusionsWith the assistance of a specific program, a complete switch to a new insulin pump in a large population of patients with T1D in the context of a public tender in a very short time was carried out safely and without deterioration of metabolic control.
El sistema público de salud financia la utilización de infusores subcutáneos de insulina (ISCI) como tratamiento no convencional en pacientes con diabetes mellitus tipo 1 (DT1). En este contexto, y con el fin de mejorar la eficiencia, es frecuente que los centros encargados de este tipo de terapia utilicen procedimientos de licitación. Nuestro objetivo fue evaluar la eficacia y la seguridad de un proceso de recambio de dispositivos ISCI a llevar a cabo en un breve periodo (4 semanas) en un procedimiento de concurso público.
Pacientes y métodosEl proceso de recambio incluyó 3 sesiones precedidas por la presentación y la justificación del mismo: sesión 1: adiestramiento en la utilización del nuevo dispositivo ISCI y administración de una encuesta de satisfacción; sesión 2: contacto telefónico de soporte a las 72 h de iniciado el programa a la búsqueda de incidencias, y sesión 3: a los 3 meses, sesión de refuerzo/consolidación de los conocimientos y adiestramiento en el uso de programa informático de gestión del tratamiento. Durante 2 meses se recogieron todas las incidencias clínicas y técnicas. Retrospectivamente, se obtuvo la HbA1c más cercana al inicio y la primera una vez finalizado el programa.
ResultadosSe efectuó el recambio en 219 pacientes, el 81% de los recambios se efectuó en las 2 primeras semanas. En la encuesta de satisfacción realizada se obtuvo una puntuación media de 9,4 sobre 10. Se efectuaron un total de 30 llamadas telefónicas extra con el fin de reforzar aspectos educativos y en 7 ocasiones se atendieron incidencias técnicas que fueron resueltas de manera inmediata. Veinticuatro de 31 eventos clínicos registrados fueron considerados de carácter leve. Seis de ellos fueron moderados (5 hiperglucemias simples/1 cetosis). Un evento fue catalogado como grave (cetoacidosis diabética). Todos los eventos se relacionaron con el equipo de infusión (recambio) y en todos se resolvieron de manera satisfactoria. La HbA1c tras el recambio no cambió significativamente. Ciento veintiocho pacientes acudieron al adiestramiento en el uso del programa informático de gestión del tratamiento.
ConclusionesEn el contexto de un proceso de licitación y bajo un programa diseñado específicamente, el recambio de dispositivos ISCI puede realizarse de manera segura y sin deterioro alguno en el control metabólico en un considerable número de pacientes y en un corto periodo.
Continuous subcutaneous insulin infusion (CSII), also known as insulin pump therapy, represents an alternative to multiple doses of insulin (MDI) when this conventional intensive insulin therapy is unable to achieve the major metabolic goals of type 1 diabetes (T1D) treatment, HbA1c as close as possible to normal levels without an unacceptable incidence of hypoglycemia.1 Thus far, meta-analyses indicate that CSII has demonstrated beneficial effects in reducing the number of episodes of severe hypoglycemia, as well as, diminishing HbA1c by 0.3–1.2% depending on the meta-analysis.2–4
There is a huge difference in cost between MDI and CSII therapies. Data regarding the cost-effectiveness of CSII compared with MDI in the delivery of intensive insulin therapy for the treatment of T1D, although positive, are still scarce.5 In this context, national health services and centers that provide CSII therapy employ specific guidelines and indications, including the most suitable target groups of subjects, to sustain the cost of CSII therapy implementation. In addition to this, they may consider procuring pump therapy through a tender process that involves various manufacturers of insulin pump devices.1,6–8
We aimed to describe the feasibility and safety of the transition from one CSII device to another in a very short time frame as the consequence of a public tender.
Materials and methodsThe tender was performed following the local regulatory requirements and all the patients using CSII received information about it. After the resolution of the tender and previously to the start of the transition process, all patients were fully informed about the procedure by the medical team.
The CSII replacement process was based on three main pillars: a properly coordinated human team, a structured and specific training program and a procedure result assessment. The human team was composed of a training group (Medtronic qualified technical personnel), a logistical team (Medtronic) and a medical team (Diabetes Unit, Endocrinology and Nutrition Department, University Hospital Clinic of Barcelona). During the entire procedure, 24h technical (Medtronic) and medical support (Diabetes Unit, Endocrinology and Nutrition Department, University Hospital Clinic of Barcelona) was available. The transition had to be performed in a very short time frame (≤1 month).
A specific training program was designed and proposed by Medtronic and carefully supervised by the medical team. The program was organized in modules and adapted to the necessities of each group of patients. The program was composed of three sessions: Session 1 was a system start-up training session, including customized educational contents of the Medtronic Paradigm® Veo 754™ (Medtronic MiniMed, Inc., Northridge, CA) and new infusion sets for each patient group (4 individuals per group). Educational material was also provided to each patient (Insulin Pump Guide for Patients, clinical video, and telephone contact instructions for technical assistance). Patient satisfaction was evaluated at the end of the initial training session using a 10-point Likert-type questionnaire, with a score of 10 indicating strong agreement and greatest satisfaction. We used documentation for implementation and tracking of training sessions that was specifically designed for this project. It included a patient's data release form, session checklist, session contents guide, incident registry, and visiting schedule. Session 2 consisted of a call from technical staff 72h after Session 1 to provide support and solve any problems regarding the programming or the infusion set change. Session 3 was a training session regarding the use of CareLink® Personal Therapy Management Software for Diabetes (Medtronic MiniMed, Inc., Northridge, CA). This software allows information stored in insulin pumps, continuous glucose monitoring devices, and blood glucose meters to be uploaded and analyzed retrospectively by patients and health care professionals. During this session, patients’ doubts and requests were resolved, and previous training on the new device was reinforced. Sessions included groups of 20 patients and were performed 3 months after Session 2.
During and 2 months after finishing Session 2, three types of incidents were registered: clinical events, technical issues, and training reinforcement incidents. Clinical events were classified as mild (medical assistance was not required), moderate (medical assistance was needed) or severe (hospitalization was required). HbA1c data were collected retrospectively, before (closest value in the previous 3 months) and after (closest value after finishing Session 2, performed at least 4 months later) the training program.
Results are presented as means±SD or percentages. Comparisons between means, if necessary, were performed using Student's paired t-test. A p-value <0.05 was considered statistically significant. All statistical calculations were performed using the Statistical Package for Social Science (SPSS, version 10.0) for personal computers.
ResultsIn total, 219 patients were enrolled in the transition process. Baseline characteristics are described in Table 1. Concerning the previous device, 58 patients had been using an Accu-Chek Spirit (Roche, Roche Diagnostics, Deutschland GmbH, Mannheim, Germany), 67 had been using an Animas 2020 (Animas Corporation, West Chester, PA, US), and 94 subjects had been using the Accu-Chek Combo System (Roche, Roche Diagnostics, Deutschland GmbH, Mannheim, Germany). During the second week of the procedure, 81% of patients were transferred to the new device. This figure rose to 99% after 4 weeks (Fig. 1). Patients’ overall satisfaction with the training process scored 9.4 out of 10.0 (85% scored >8, none scored <7).
Concerning the different incident types described previously, there were 68 events in total. Thirty episodes were classified as training reinforcement, and seven were classified as technical issues. All 37 were sorted out over the telephone. There were 31 clinical events during the transition period, all related to infusion set issues. Twenty-four were considered mild, and were solved by technical personnel using telephone support. Medical assistance was needed in six events (five unexpected hyperglycemia, and one ketosis episode); three of these occurred in the same patient. We performed a specific training reinforcement in infusion set management for this patient. There was only a single severe clinical event, which presented as ketoacidosis requiring hospitalization. In this case, the patient recognized persistent hyperglycemia at home (12h) that did not respond to repeated pump boluses, and was admitted at the hospital and treated using intravenous insulin and fluid replacement. This event was related to kinking on insertion of the cannula which was not recognized by the patient.
Data were available before and after the transition procedure for 109 patients that were clinically non-significantly different from the whole group (age 46.9±11.1 years, 68% women, duration of T1D 26.3±9.9 years, on CSII therapy for 7.4±4.4 years). Metabolic control improved during the transition process (7.9±0.9 before vs. 7.4±0.7 after, p<0.001).
Three months after the initiation of the procedure, 128 patients attended the CareLink personal therapy management software training. Among the reasons explaining the absence of 91 patients from the training process were: medical staff did not consider it necessary, unsolved changes in contact details, and personal unavailability.
DiscussionWe were able to perform the transition of a large population of patients with T1D from previous CSII systems to a new one in the context of a public tender safely and in a very short time using a specific training program.
In 2008, the U.K. National Institute for Health and Care Excellence (NICE) considered that, the use of CSII by patients with T1D is associated with reductions in severe hypoglycemia and HbA1c compared with conventional MDI therapy.1 CSII therapy should be considered a treatment option for adults and children with T1D when attempts to achieve target HbA1c levels with MDI have resulted in a disabling rate of hypoglycemia or when HbA1c levels have remained ≥8.5%. Despite these evidence-based recommendations, the use of CSII therapy is still disparate in Europe. Insulin pumps are used by 15–20% of T1D patients in some Nordic, Central, and Western countries; in other European countries, close to or <5% of patients are treated using CSII.9 Although the vast majority of western European countries have been well covered by full reimbursement for >10 years, pump penetration remains significantly heterogeneous among some of them. In addition to other more country-specific factors that explain differences in CSII therapy penetration, cost effectiveness issues and the economic situation of each nation's healthcare insurance systems, health institutions, and providers also matter.
In this context, we evaluated the possibility and intention to procure pumps for our CSII program through a public tender process. Scrupulously following the local regulatory requirements, a tender was designed to obtain the best possible cost meeting the needs of our program with regard to quality, quantity, time, and location. As a result of the tender process and because we were using different brands of pumps, more than 200 patients switched from their previous pump model to the new one. Even though the transition process was completed in <30 days, the number of significant clinical events (particularly those related to relevant clinical episodes) could be considered small. All events related to relevant clinical episodes, including the only clinically severe event, were related to infusion set issues, and three of these occurred in the same patient. Thus, in our opinion, the transition could be performed safely and in a very short time with the help of a specific training program for that purpose.
Concerning the course of glycemic control after switching to a new pump model, there was a positive impact in HbA1c in the subgroup of patients with available data. The improvement in HbA1c was probably related to increased contact with technical staff and education management regarding pump therapy.
Despite the large number of patients, our study has several limitations. The most relevant one to be addressed is that our tender process and the transition program have been designed and performed locally, and it would be highly speculative to extrapolate the same rate of success to other particular settings. Information from studies that include data after a transition from a previous CSII device to a new system in large populations is very scarce.10 At the time of this writing, we are unaware of any study similar to ours, and this precludes any possible comparison.
In conclusion, with the assistance of a specific training program, we were able to complete the switch to a new insulin pump in a large population of patients with T1D in the context of a public tender in a very short time, safely and without deterioration of metabolic control.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestPR, MG, IV and IC declare no conflict of interest. NC, OM, MM and SI are Medtronic employees.
The authors thank the logistical team (Medtronic) and the medical team (Diabetes Unit, Endocrinology and Nutrition Department, University Hospital Clinic of Barcelona) involved in the transition for their continuous effort throughout the course of the procedure.