Allergies are hypersensitivity reactions that occur through specific type Th2 immunological mechanisms characterized by different soluble mediators, as well as specific cells of the immune system. In recent decades, evidence has emerged relating this disease with cancer development. However, most of the results of epidemiology studies have been controversial and contradictory. There are mainly two trends. While the first indicates that allergies can reduce the risk of cancer, the other indicates that they may increase this risk. The first trend can be explained by the immunosurveillance hypothesis, which states that the increased immune surveillance after the immune hyper-responsiveness can inhibit or exert a protective effect against the development of cancer. Similarly, the prophylaxis hypothesis suggests that the physical effects of allergy symptoms can prevent cancer by removing potential carcinogens.
In contrast, the opposing hypothesis propose that there is a deviation of the immune response toward Th2, which favors the development of cancer, or that the process of chronic inflammation favors the generation of mutations, and therefore the development of cancer.
With the purpose of understanding more about these two hypotheses, the main soluble and cellular factors of allergic diseases that could be playing a key role in the development or inhibition of cancer were considered in this review.
Las alergias son reacciones de hipersensibilidad que ocurren mediante mecanismos inmunológicos específicos de tipo Th2. Se caracterizan por distintos mediadores solubles, así como células específicas del sistema inmune. En las últimas décadas ha surgido evidencia que asocia esta enfermedad con el desarrollo de cáncer. Sin embargo, los resultados obtenidos, en su mayoría de estudios epidemiológicos, han sido controversiales y contradictorios. Lo anterior se debe a que existen dos principales tendencias. Mientras algunos estudios han demostrado que las alergias pueden reducir el riesgo de cáncer, otros estudios muestran que puede aumentarlo. Lo primero puede explicarse por la hipótesis de inmunovigilancia, que establece que el aumento de la vigilancia después de la hiperreactividad inmune puede inhibir o ejercer un efecto protector contra el desarrollo de cáncer. Del mismo modo, la hipótesis de la profilaxis sugiere que los efectos físicos de síntomas de las alergias pueden prevenir el cáncer mediante la eliminación de los carcinógenos potenciales. Las hipótesis opuestas proponen que existe un desvío de la respuesta inmune hacia Th2 lo cual favorece el desarrollo del cáncer, o que el proceso de inflamación crónica favorece la generación de mutaciones, y por tanto el desarrollo del cáncer. Con el propósito de entender más acerca de estas dos hipótesis, en esta revisión se consideraron los principales factores solubles y celulares de las enfermedades alérgicas que pudieran estar desempeñando un papel clave en el desarrollo o inhibición del cáncer.
Allergic diseases have been present throughout human development, and are the reason why it has been vital to continue the study and research of a large bouquet of diseases that conform to allergies.
In 1913, Charles Richet and Paul Portier discovered allergies, or anaphylaxis, when they tried to immunize a dog with actinia extracts several times. The animal showed some tolerance and maintained good physical condition after four injections. However, 25min after the final exposure (22 days later), the dog suddenly deteriorated and died. This fact gave rise to the discovery of anaphylaxis because it showed that immunization could acquire, not only protection, but also induce detrimental effects. A significant advancement in understanding anaphylaxis was made through the experiments of Dale and Laidlaw, who showed that histamine was able to induce very similar symptoms.1
At the beginning of the 21st century, both the European Academy of Allergy and Clinical Immunology (EAACI) and the World Allergy Organization (WAO) defined the concepts of anaphylaxis based on a clinical symptom-independent mechanism due to many outstanding contributions.2 Therefore, it is now possible to distinguish between an allergy, and immune anaphylaxis, formerly called ‘pseudo-allergic tm) reactions.
Currently, anaphylaxis prevalence appears to be increasing worldwide, perhaps due to pollen, food allergies, and the increasing prevalence of atopic diseases. Since anaphylaxis describes a syndrome of clinical symptoms involving multiple organ systems with greater or lesser intensity, attempts have been made to classify the severity of this reaction using the severity scales, such as the Mueller3 and the Messmer.1
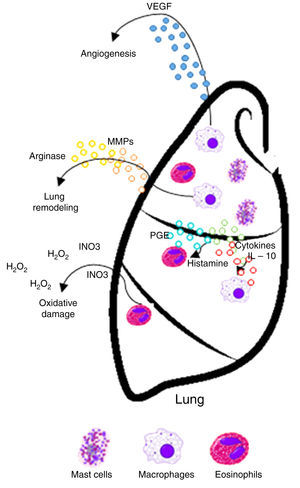
Asthma is defined as a chronic allergic airway inflammation disease leading to various degrees of inflammation. This disease affects millions of people worldwide. Airway Inflammation in asthma is mediated by T-helper type 2 (Th2) cytokines, basophils, eosinophils, and mast cells.4 IL-4, IL-5, and IL-13 cytokines have a prominent role in the inflammatory asthma cascade. Also, they have chemoattractant properties for rapid accumulation of macrophages, granulocytes and other cells to the site of inflammation.5
2AllergoOncologyFor several decades, the biological relationship between cancer and allergies has brought epidemiological, oncological and immunological interest to researchers. Numerous studies show a complex association, which has not been fully elucidated so far due to the varied results. Allergy, or atopy, is considered a hypersensitivity reaction initiated by specific immunologic mechanisms, which involve various soluble mediators such as cytokines, chemokines, specific immunoglobulins (IgE, IgG), as well as the activation and effects of the immune system cells as Th2, Th17, eosinophils, mast cells and others.
Immune hyper-responsiveness is usually assumed to reflect a change in T cell response away from Th1 type to a principal activity of Th2. Even diseases mediated by Th1 and induced Th2 disorders coexist in different pathologies such as diabetes and cancer.6,7
Theoretically, the hyper-reactive state atopy might be accompanied by enhanced immune surveillance, leading to a better detection and destruction of malignant cells, and therefore, a decreased risk of cancer. In contrast, an increased incidence of cancer might result from repeated tissue inflammation in atopic patients, which in turn could be linked to repeatedly damaged tissues.8 To better understand the mechanisms involved in the relationship between cancer and atopy, several studies have suggested various and often contradictory epidemiological findings. On this basis, the following four different immunologic hypotheses have been proposed:
- 1.
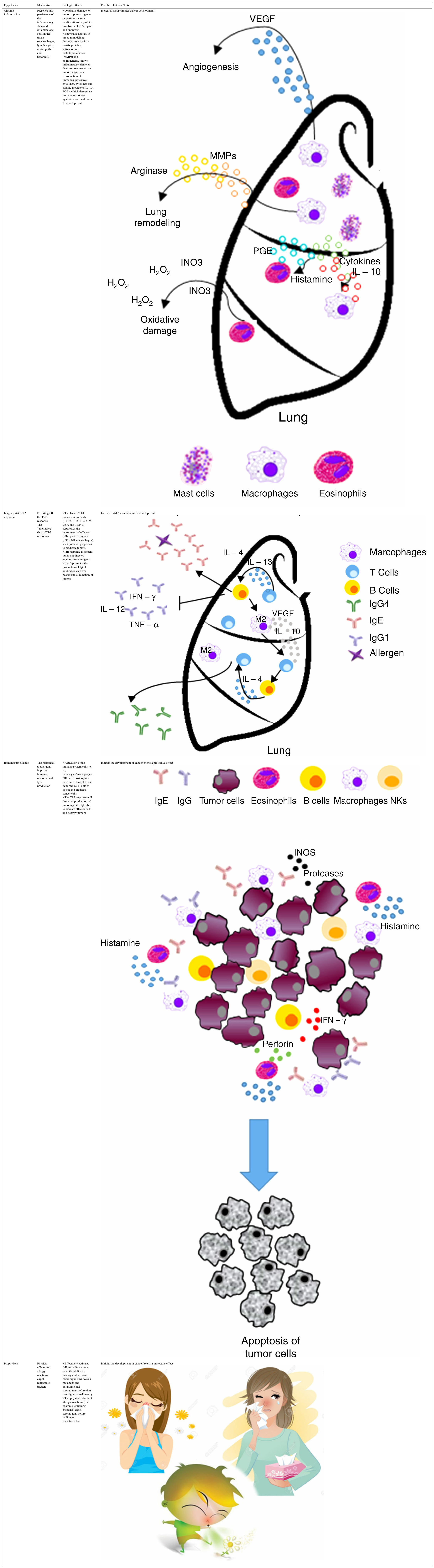
Chronic inflammation or antigen stimulation hypothesis. This hypothesis suggests that the inflammatory conditions associated with•and often secondary to•allergic disease may promote cancer development by inducing oxidative damage, resulting in mutations of tumor suppressor genes or post-translational modifications of proteins involved in DNA repair or apoptosis control.9,10 Therefore, this chronic inflammation hypothesis predicts that allergic inflammation increases the risk of cancer.
- 2.
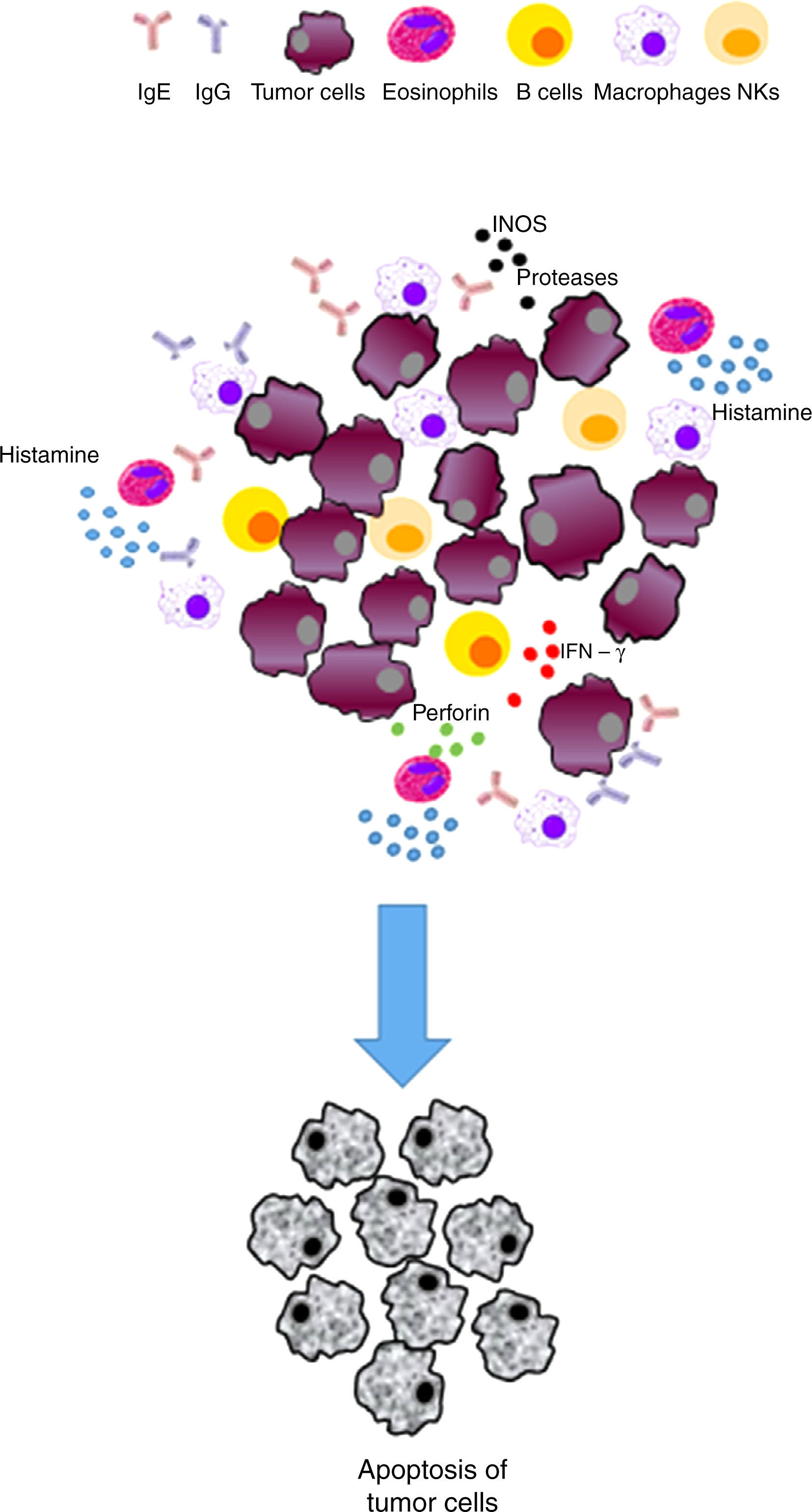
Immunosurveillance hypothesis. In contrast, this hypothesis proposes that allergy is a general consequence of an improved immune response, which can detect deregulated or damaged cells and may eradicate them efficiently before they generate a malignant or cancerous process. Therefore, this hypothesis would predict that allergies might reduce the risk of cancer development. On the other hand, a significant presence of activated IgE has been observed in tumors. IgE is a strong immune cell stimulatory molecule (macrophages and basophils) that promotes the expression of its receptor. The possible production of IgE in specific tumors can support this hypothesis. The functions of these components are key to the Th2 response in the protection against parasites.
- 3.
Prophylaxis hypothesis. In 1991, M. Profet first proposed this hypothesis,11 which suggests that, in some cases, the physical effects of allergic reactions in specific tissues can remove mutagenic triggers before a malignant transformation occurs (e. g. aflatoxin microbial carcinogen B1 produced by the fungus Aspergillus flavus, which may be inhaled and, therefore, cleared by sneezing or coughing).12
- 4.
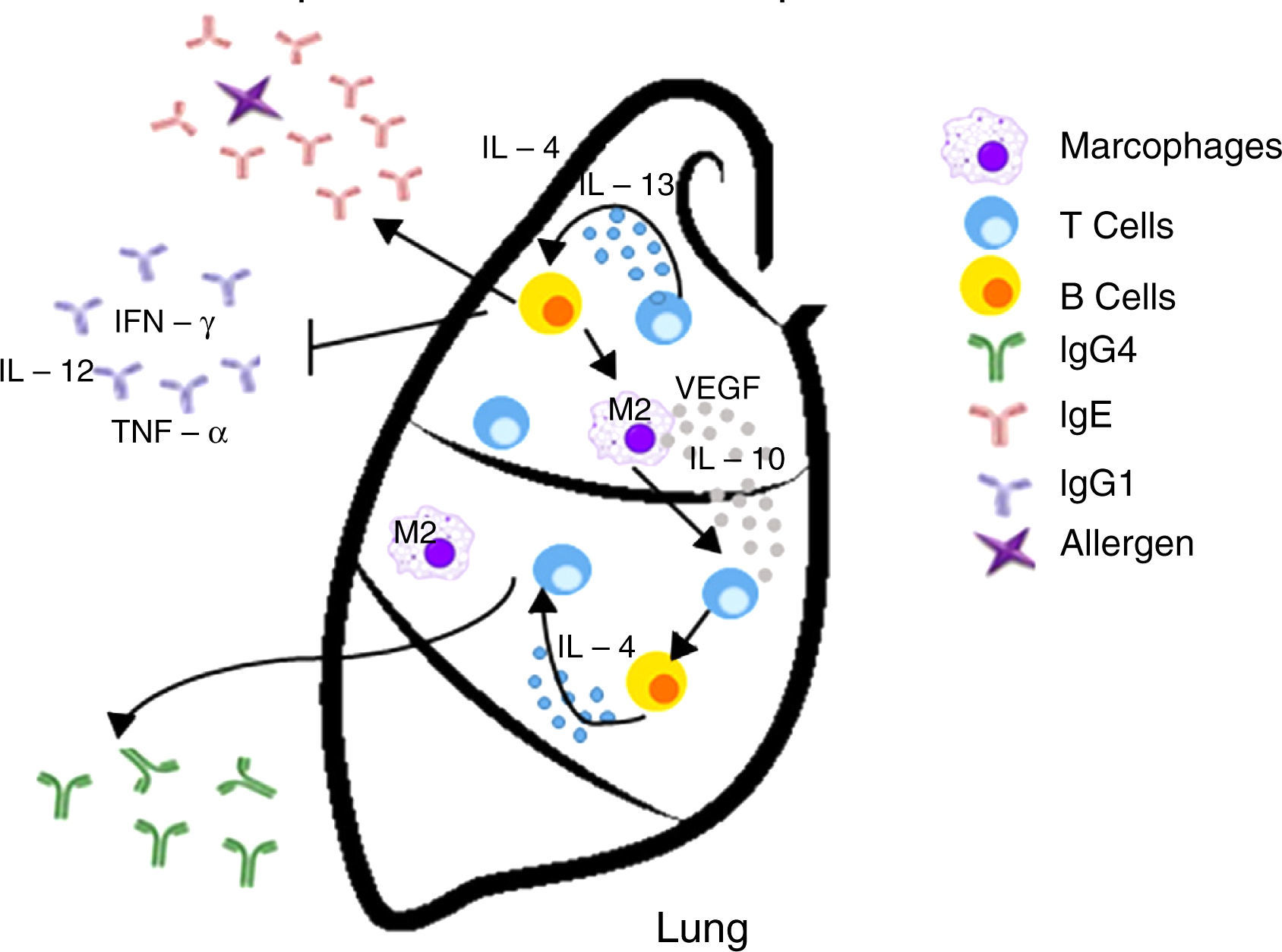
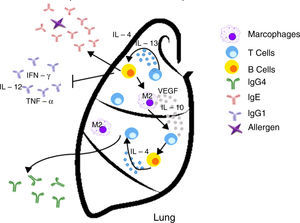
Inappropriate Th2 response hypothesis. Finally, this recent hypothesis suggests that Th2 response present in atopy is not appropriate and may lead to carcinogenesis in two different ways. First, the “classical” Th2 pathway, which is characterized by a prominent IgE response that potentially diverts immunity towards the eradication of the tumor to a Th1-type response (including the production of IgG1 antibodies and cytokines such as IFN-γ, TNF-α and IL-12), focuses on the IgE response to allergens and non-tumor antigens. The second pathway, or the “alternative” route, is dominated by the presence of Th2 and IL-10, and chronic inflammatory conditions in the presence of IL-4 and VEGF, which are redirected by a conventional IgE antibody response towards IgG4 antibody production that has low or no potential to destroy tumors.13
Both chronic inflammation or antigen stimulation hypotheses and inappropriate bias Th2 response hypothesis predict positive associations between cancer and allergies (i. e., allergy sufferers are more likely to develop cancer). In contrast, the immunosurveillance and the prophylaxis hypotheses predict inverse associations (i. e., allergy sufferers are less likely to develop cancer). Consequently, immunosurveillance predicts inverse associations for cancers of all tissues and organ systems, and prophylaxis predicts inverse associations specifically for cancer tissues and organ systems that interact with the external environment (Table 1).
Proposed hypotheses on how the allergic response protects or promotes carcinogenesis.
| Hypothesis | Mechanism | Biologic effects | Possible clinical effects |
|---|---|---|---|
| Chronic inflammation | Presence and persistence of the inflammatory state and inflammatory cells in the tissue (macrophages, lymphocytes, eosinophils, and basophils) | • Oxidative damage to tumor suppressor genes or posttranslational modifications in proteins involved in DNA repair and apoptosis • Enzymatic activity in tissue remodeling through proteolysis of matrix proteins, activation of metalloproteinases (MMPs) and angiogenesis, known inflammatory elements that promote growth and tumor progression • Production of immunosuppressive cytokines, cytokines and soluble mediators (IL-10, PGE), which deregulate immune responses against cancer and favor its development | Increases risk/promotes cancer development |
| Inappropriate Th2 response | Diverting off the Th2 response The “alternative” shot of Th2 responses | • The lack of Th1 microenvironments (IFN-γ, IL-2, IL-3, GM-CSF, and TNF-α) suppresses the recruitment of effector cells cytotoxic agents (CTL, M1 macrophages) with potential properties to eradicate tumors • IgE response is present but is not directed against tumor antigens • IL-10 promotes the production of IgG4 antibodies with low power and elimination of tumors | Increased risk/promotes cancer development |
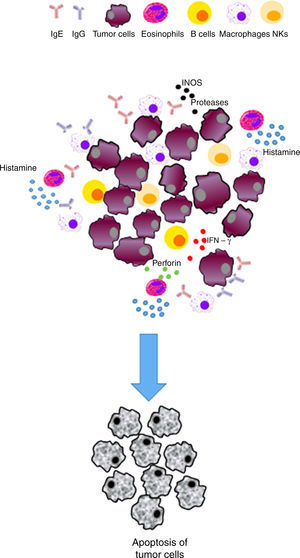
| Immunosurveillance | The responses to allergens improve immune response and IgE production | • Activation of the immune system cells (e. g., monocytes/macrophages, NK cells, eosinophils, mast cells, basophils and dendritic cells) able to detect and eradicate cancer cells • The Th2 response will favor the production of tumor-specific IgE able to activate effector cells and destroy tumors | Inhibits the development of cancer/exerts a protective effect |
| Prophylaxis | Physical effects and allergy reactions expel mutagenic triggers | • Effectively activated IgE and effector cells have the ability to destroy and remove microorganisms, toxins, mutagens and environmental carcinogens before they can trigger a malignancy • The physical effects of allergic reactions (for example, coughing, sneezing) expel carcinogens before malignant transformation | Inhibits the development of cancer/exerts a protective effect |
As a part of the allergic response, inflammation has also been studied. Different elements that are part of this condition could be key for understanding the mechanisms involved in allergy and cancer components.
3Soluble mediators3.1TGF-βThe transforming growth factor β (TGF-β) superfamily belongs to the cytokines, which participate in several processes such as cellular proliferation, migration, inflammation, tissue repair, immune responses, cell differentiation, and apoptosis. In allergic diseases, one of the main roles of this cytokine is to fix the damage or injury that is generated in fibrosis, scarring, autoimmune diseases, parasitic infections, asthma, and cancer.5
TGF-β mediates leukocyte chemotaxis, and acts as a fibrogenic factor and immunomodulator, playing a key role in the airways because it induces significant structural changes in patients with asthma. Both structurally and inflammatory cells that infiltrate the tissue increase the expression of TGF-β in the airways of asthmatic patients. Eosinophils are between 70 and 80% of all cells expressing TGF-β in the airways of these patients and are considered as asthma markers because of their significant role in inflammation and remodeling.14,15 A correlation between the expression of TGF-β and the increase in eosinophils16 and macrophages17 in lung tissue has been observed.
TGF-β can induce the differentiation of TH17 cells, which are capable of producing significant amounts of IL-17 and the perpetuation of an acute inflammatory process. It also promotes the secretion of other inflammatory cytokines and recruits granulocytes, which amplify the immune response.18 Moreover, TGF-β participates as an anti-inflammatory and immunosuppressive molecule, so differentiation (Th1 and Th2 cells and B cells) and cytokine production (IFN-γ and IL-2) is reflected. Finally, TGF-β is essential for the development and differentiation of regulatory T cells (Treg).15
On the other hand, TGF-β plays different roles in other diseases, such as cancer, where it, paradoxically, promotes and suppresses tumor progression. Its suppressive activity is reflected in the antiproliferative and pro-apoptotic effects; however, during tumor progression, TGF-β promotes tumor growth when proteins that participate in the signaling pathway of TGF-β such as its receptors acquire certain mutations. Antiproliferative properties by inhibiting TGF-β in the tumor can induce motility of tumor cells, favoring the epithelial-mesenchymal transition, which in turn produces invasion and the development of metastasis.17
However, there is evidence that suggests that the overexpression of TGF-β, their associated receptors (TβRI, TβRII), and proteins involved in TGF-β signaling pathways increase the survival rate of patients with breast cancer.19 A study revealed that an allergic response to ovalbumin in sensitized mice protected the animals against Ehrlich tumor growth in the footpad; thus, a significant decrease in tumor growth and an increase in apoptosis was observed in these allergic mice. These results suggest that the concomitant allergic condition may decrease tumor progression through increased tumor cells apoptosis.20 Indeed, these results suggest a possible mechanism for the reduction of cancer incidence observed in individuals with allergies.
The importance of TGF-β has also been demonstrated in a mouse model of allergic lung inflammation and urethane-induced lung cancer. Inhibition of TGF-β signaling in lung cells was performed using overexpression of Smad7, an inhibitor protein of TGF-β activity, which increased tumor lesions in the lung.21 Recently, evidence of an experimental model of coexistence of both allergic pulmonary inflammation and cancer demonstrated that tumor growth significantly decreased in an allergy inflammation environment. That occurred, at least in part, via the overexpression of TGF-β, which was able to contain and kill tumor cells through apoptosis and decreased cell proliferation.22
These results demonstrate that TGF-β can control the development of cancer and suggest that a possible interaction between the two diseases might exert a protective effect.
3.2Immunoglobulin E (IgE)Immunoglobulins, also known as antibodies, are specific molecules involved in the immune recognition. Antibodies are composed of two identical heavy (H) and two identical light (L) chains, with a heterotetramer configuration (H2L2). Each chain has both constant and variable regions. The heavy chains can be paired with kappa (κ) or lambda (¿) light chains. In humans, there are five different classes of antibodies that are distinguished by their heavy chain structure, which is indicated by the Greek letters: α (IgA), δ (IgD), ¿ (IgE), γ (IgG) and α/4 (IgM).23
There are four IgG subclasses (IgG1, IgG2, IgG3, and IgG4), whereas IgA has two subclasses (IgA1 and IgA2). IgG is the major class of antibody found in blood and in extracellular fluids that protects the body against infections. IgM is the first response to an antigenic challenge, such as an infection, and exists as a pentamer or hexamer. IgA is secreted by body fluids, while IgD forms the B-cell receptor on the B cell surface. IgE is associated with type I hypersensitivity reactions (anaphylactic/allergic).23
Two IgE receptors exist: the high-affinity receptor FcRI, and the low-affinity receptor Fc¿RII, also known as CD23. The expression of FcRI is abundant in human mast cells (MCs) and basophils; it is expressed at lower levels in dendritic cells (DC), Langerhans cells (LC), monocytes/macrophages, eosinophils, and platelets.24 Additionally, IgE binds to Fc¿RII (CD23), an integral membrane protein. There are two isoforms of the human CD23. The CD23a isoform is expressed in B cells activated by an antigen before differentiation and antibody secretion by plasma cells.25 This isoform is dependent on IgE antibody and is involved in endocytosis of antigen processing and presentation. CD23b is expressed on monocytes and eosinophils, and is dependent on the stimulation of IL-4.26
IgE plays a central role in the activation of allergic immune responses (type I hypersensitivity) when an allergen, such as pollen, is exposed, leading to MCs and basophil degranulation and acute inflammation at the site of allergenic challenge. Allergic sensitization is the result of the production specific-allergen IgE, which in turn coat the surface expressing the FcRI receptor. A re-exposure to the same allergen triggers MCs and basophil degranulation, resulting in the recruitment of inflammatory cells.27,28
Type I hypersensitivity first response reaction occurs within minutes of the allergen exposure: the release of preformed mediators including histamine, heparin, lipid mediators, proteases, chemokines, and cytokines of cytoplasmic granules.28,29 Late phase reaction occurs after the early symptoms have decreased and can last up to several weeks; it recruits inflammatory cells, such as neutrophils, eosinophils, basophils, monocytes/macrophages, and T cells.30
IgE is primarily known for its detrimental role in allergies. However, several studies have pointed a tumor surveillance function of this antibody isotype. IgE class antibodies have certain properties that support the study of these molecules as potential therapeutic agents in cancer immunotherapy. The research focused on the relationship between cancer and allergic disease elements, such as IgE, that belongs to the new field known as AllergoOncology, which aims to reveal the function of the immune responses involved in allergy and IgE against cancer cells. Also, its objective is to elucidate the biology and mechanisms of the two diseases and to develop new treatment options for malignant diseases.31
IgE has been suggested to provide protection against parasitic infections; nonetheless, this feature is controversial.32,33 Allergens that bind to IgE and interact with receptors lead to rapid degranulation of these effector cells, resulting in the release of several factors, including histamine, enzymes, and lipid mediators. The release of all these factors leads to tissue damage and an acute inflammatory response. Therefore, it might be possible that if specific IgE antibodies to a tumor antigen were present in the tumor microenvironment, such hypersensitivity reaction could lead to the death of tumor cells and phagocytosis of dead cells by antigen presenting cells (APC), such as dendritic cells (DC) and macrophages. Furthermore, T cells could then be activated by these APC, leading to an adaptive immune response of T-cells against the tumor. This immune response has the potential to be specific, not only for tumor-specific antigen but to other tumor antigens because of epitope spreading•a phenomenon in which an immune response is triggered to epitopes or antigens other than the epitope or antigen that was initially targeted.34 Importantly, IgE may also mediate antigen presentation through the interaction with APC and expressed Fc¿Rs.24
Several studies describe an inverse or protective association between allergies and levels of IgE antibodies in various malignant tumors, suggesting a natural anticancer effect of IgE.31 Whether or not this is the case, several properties of the IgE antibody make it an attractive option for cancer therapy.
Significantly, many experiments seeking to associate allergies and cancer, using different animals and epidemiological data models have been carried out. However, the results have been contradictory. Only large epidemiological studies have revealed an inverse or protective association between the history of atopic diseases and cancer. In 2005, Turner et al. published a study that included 1.1 million people with a medical diagnosis of asthma without cancer with an 18-year follow-up. The results demonstrated that the relative risk of overall cancer mortality was significantly reduced in this population.31
3.3HistamineHistamine plays a decisive role in immediate and allergic hypersensitivity35,36 as part of the allergic response to antigen antibodies (IgE), which bind to the surface of MCs and basophils through high affinity generated Fc receptors (constant fraction) that are IgE-specific. Allergic patients generate IgE antibodies against antigens commonly inhaled. This is an inherited feature identified a “possible gene product.” Histamine is considered as both humoral and cell modulator that mediates immediate hypersensitivity reactions. Two types of brain cells accumulate histamine: neurons and MCs. MCs from the central nervous system (CNS) contain an extraordinary variety of chemical mediators, including histamine, serotonin (5-HT), kallikrein, and tumor necrosis factor-α (TNF-α), which can increase microvascular permeability,37 facilitate leukocyte chemotaxis and the adhesion and extravasation of inflammatory cells in the brain and spinal cord. These events are important in many CNS inflammatory diseases such as encephalomyelitis and multiple sclerosis. Also, histamine, bradykinin, eicosanoids and free radicals are secreted after acute trauma, ischemia, epilepsy and inflammation.35
Histamine causes a profound drop in blood pressure if it is applied in large doses or released during anaphylaxis. With the dilation of small vessels, significant amounts of blood are trapped, so permeability and plasma out of circulation increases. Therefore, the effective blood volume, venous return, and cardiac output decrease. Histamine is deposited in various cells such as MCs, basophils, macrophages and histaminergic neurons that produce high concentrations of histamine. In adenocarcinomas, like breast cancer infiltrates, MCs and macrophages are abundant.35 These cells accumulate in the stroma in response to chemoattractants and play a double role in tumor biology as secretory factors that can induce tumor cell growth or death, angiogenesis, matrix remodeling, and immunosuppression.38,39 As an endogenous factor, histamine may also influence the interaction between tumor cells and fibroblasts.40 During the last decade, histamine has been used as an immunomodulator in clinical trials phase I and II on renal cells, melanoma, and acute myeloid leukemia. Histamine is well tolerated and shows no side effects.2,41•44 Therefore, the inhibitory effects of histamine on tumor cell proliferation45,46 strongly suggest that it should be considered as a potential agent in the research of new combination therapies. These observations support the idea that histamine, released during allergic diseases, plays a significant role in cancer progression.42
4Cellular components4.1Mast cellsMCs play a major role in allergy and anaphylaxis and develop a critical protective role being intimately involved in wound healing and defense against pathogens. MCs are present in most tissues characteristically surrounding blood vessels and nerves, and are especially visible near the boundaries between the outside and inside, such as skin environment, the lining of the lungs and digestive tract, as well as in the mouth, conjunctiva, and nose.47•50
MCs express a high-affinity receptor for the Fc region of IgE, the least abundant member of the antibodies, which is an essentially irreversible high-affinity binding receptor of IgE molecules. As a result, MCs are coated with IgE, which is produced by plasma cells. MCs release their preformed mediators when they find complement C3a and C5a anaphylatoxins. Microorganisms that attack humans often produce exogenous factors (e. g., bacteria and mites proteases) that induce the release of granular components of MCs through different receptors.51 In addition, degranulation is stimulated by activation of its tyrosine kinase membrane receptor, the c-kit receptor (CD117), through stem cell factor (SCF).52•54 MCs granules are key functional elements characterized by two different discharge patterns: exocytosis and gradual degranulation. Interestingly, the latter mechanism, which is a slow and selective cellular secretion pathway, has been observed infiltrating in areas where a chronic inflammation process is more common, such as tumor tissues. Consequently, a link between MCs, chronic inflammation and cancer has been suggested in time. Thus, MCs are inflammatory cells firstly and mainly recruited in the tumor microenvironment.55,56 MCs are important in allergic and late phase reactions, inflammation, and regulation of adaptive immunity mediated by T cells: MCs mobilize T cells and antigen-presenting dendritic cells. However, the role of MCs in tumorigenesis of cancer is not entirely clear, and data on their benefit or detriment to tumorigenesis have been controversial.57,58
Tumor cells produce inflammatory mediators and pro-angiogenic factors, including SCF. The activation of SCF/Kit is required for the maturation, migration, and survival, and MCs are derived from hematopoietic precursors within the bone marrow and complete their differentiation and maturation within vascularized tissues.59 Through chemotaxis, the microenvironment surrounding tumors promotes SCF infiltration and MCs maturation, which release angiogenic mediators, proteases, and growth factors that promote tumor development.
MCs can directly affect tumor cell proliferation and invasion but also indirectly help tumors as they favor the generation of a microenvironment and modulation of the immune response for tumor cells. The MCs are best known for the control of inflammation and angiogenesis, and its role in shaping the adaptive immune response has become a focus of recent research.58,60,61
The central role of MCs in the control of innate and adaptive immunity gives them the ability to regulate the natural host response against cancer and, ultimately, to influence the outcome of the disease and the fate of the patient with cancer.
MCs can stimulate growth, neo-angiogenesis and metastasis of tumors through multiple mechanisms. MCs are involved in innate immunity by the release of TNF-α and interleukins (IL-1, IL-4, and IL-6) and express both MHC II and the co-stimulatory molecule, which activate the response of T and B cells. Cytokines secreted by stromal cells can exacerbate malignant phenotype of cancer cells. Producing chemoattractant molecules, cytokines that recruit inflammatory cells in tumor sites influence in a way that ultimately promotes cancer progression.37,62•64
4.2EosinophilsEosinophils are immune system granular cells that develop and differentiate in the bone marrow, which are characterized by the presence of specific secondary granules containing toxic cationic proteins in their cytoplasm. Such eosinophil granules contain a core of crystals made of the main basic protein 1 and 2 (MBP1 and MBP2), a compound of eosinophil cationic protein, eosinophilic peroxidase (EPO), and derived neurotoxin eosinophils.65 Under physiological conditions, only a small number of eosinophils are released from the bone marrow. In contrast, the number of eosinophils are increased dramatically as a result of Th2 cell responses associated with allergic helminth infections or diseases such as asthma.65,66 This increase in eosinophil production is driven by the secretion of various cytokines, such as IL-3, IL-5, and granulocyte and macrophage colony-stimulating factor (GM-CSF).65 Among these, cytokine IL-5 is associated with Th2 immune response. This cytokine is unique to the lineage of eosinophils, and it is responsible not only for the expansion of the progenitors of eosinophils in bone marrow but also for its release into the blood and survival after migration.65•67 Once at the site of the injury, eosinophils can release their granular cytotoxic proteins, cytokines, and lipid mediators, which contribute to certain circumstances in the destruction of the parasite, but the exacerbation of inflammation and tissue damage, which are particularly harmful when Th2 responses are directed against allergens.68
On the other hand, the presence of eosinophils has been observed in other diseases such as cancer, including colorectal, breast, ovarian, cervical, oral, and prostate cancer and Hodgkin's lymphoma. The increase in eosinophils as well as their role remain controversial and depend on many factors, including the type of cancer. It is also known that low activation eosinophils have the ability to release their granules quickly, hence their cytotoxic content,69,70 which in turn can induce tissue remodeling and direct death of tumor cells. Eosinophils can also affect carcinogenesis through modulation of the immune response.71
A recent study reported that eosinophils enhance vascularization and improve infiltration of cytotoxic T cells in a murine model, resulting in tumor rejection, which supports the immunomodulatory function of eosinophils.72 Tumor-associated tissue eosinophilia (TATE) or evidence of eosinophil degranulation in various solid tumors improved various prognoses. However, survival of different cancers was examined and differentiated according to the type of cancer.73 Some of the reported outcomes were poor prognosis in cervical cancer74 and Hodgkin lymphoma,75 and a better prognosis of head and neck, bladder, gastric cancer, and esophageal carcinoma.76
This beneficial influence of eosinophils in various tumors appears to be independent of other standard prognosis factors (e.g., stage, age, sex, alcohol intake or smoking, histologic stage, vascularization, vascular invasion, and neural invasion). The study of eosinophil recruitment in solid tumors shows that eosinophil tissue infiltration is mediated by factors released directly from necrotic tumor cells. Principally, one factor is studied: the eosinophil-derived protein HMGB1, which binds to the receptor for advanced glycation end products (RAGE) in eosinophils, and eosinophil degranulation trigger.71•77
4.3BasophilsInitially, basophils were recognized by their rapid release of histamine and synthesis of leukotriene C4 (LTC4) following crosslinking of the IgE bound to its Fc¿ RI and then for the synthesis of IL-4 and IL-13 in response to Fc¿ RI cross-linking.78,79 Basophils contain about one pg histamine/cell and can synthesize more IL-4 and IL-13/cell of other leukocytes.78,80,81 Thus, basophils have the ability to bridge the innate and adaptive immunity, including the capacity to induce and propagate Th2 immune responses. Basophils may be important in the pathophysiology of allergic diseases. The potential of basophils is degranulated for the immediate release of histamine, rapidly generating LTC4, and producing Th2 cytokines to provide the mechanistic basis by which basophils can cause clinical symptoms of hypersensitivity and promote hypersensitivity reactions to contribute to late phase and delayed hypersensitivity reactions.78,82
The recruitment of basophils in response to injury, assault, or infection depends on activation. As chemotactic factors, IL-3 increases basophil activation through expression of CD11b and CD18, thereby enhancing the adhesion to the endothelium. Chemotaxis is mediated by CCR3 eotaxin, predominantly ligands (CCL11) and RANTES (CCL5).83,84 In addition, CCR3 is constitutively expressed. Basophils also express CCR2 and migrate in response to MCP-1.83 Also, basophils can produce chemotactic factors in their microenvironment and further modulate the inflammatory response. Basophils can also release platelet activating factor (PAF) in response to stimulation by IL-3,85,86 which in turn stimulates endothelial cells to increase vascular permeability and allows migration of immune cells.
Th2 immune responses are initiated and amplified in vivo, with important accessory cells and cytokines involved, though is still debated and possibly depends on the model.87 Although the differentiation of Th2 cells may occur in the absence of IL-4, the route of IL4/STAT6 plays a major role in the induction of GATA-3 expression on T cells for stabilizing the Th2 phenotype.29,88 Dendritic cells (DCs) are described as the first cells that are influenced by Th2 cytokines, and favor the polarization of this response comprising thymus stromal lymphopoietin (TSLP).89,90 However, DCs inability to produce IL4 show what has prompted the search for identifying accessory cells, which provide in vivo new innate source of IL4.
Proposals for IL4 included eosinophils, MCs, basophils, NK cells and CD4+ T cells.80 Recently, in different helminth infections in mouse models, it has been shown that basophils contribute to the development of Th2 cells by secreting IL-4 after its temporary recruitment in the draining lymph nodes, where DCs are antigen-presenting cells primarily responsible for the sensitization of Th2 cells.91,92 Therefore, basophils can be the IL4 source necessary to induce GATA-3 expression in Th2 cells induced by DCs,93 functioning as accessory cells during the full development/maintenance of Th2 immune responses generated in allergic diseases.
On the other hand, there is a strong association of basophils with some malignant tumors, particularly chronic myeloid leukemia (CML) and acute myeloid leukemia (AML).94,95 A greater number of basophils circulating and dysplastic basophils are common features of AML, and late phases of CML,96 and the transformation of basophils may rarely occur. It is common for patients with CML to present 70% basophils in the blood.97 They presented over 250/ul of blood basophils, and this has been associated with decreased survival rates. These associations, basophils with myeloid leukemia and poor outcomes have led to the proposal of a clinical trial with an anti-CD123 mAb antibody directed against basophils in patients with CD123+ AML in remission with standard chemotherapy, to delay or prevent recurrence.98
4.4NeutrophilsNeutrophils are the first line of host immune defense against bacterial and fungal infections.99 In humans, they comprise 50-70% of circulating leukocytes in the blood and can enter various tissues by the extravasation of blood circulation.100 They can perform an impressive range of activities that help to eliminate pathogens, which include phagocytosis (and intracellular killing), the ability to generate reactive oxygen species through the phagocyte NADPH oxidase, the release of antimicrobial and cytotoxic compounds previously stored in intracellular granules, the capacity to form neutrophil extracellular traps (NET), and the ability to secrete chemokines and cytokines.101•103
Recently, it has been shown that these cells play important roles in other pathological conditions, including cancer. Neutrophils are a significant portion of inflammatory cell infiltrate in cancer, by showing high functional plasticity and can demonstrate a dual behavior, antitumor, and protumor activity.1 The antitumor effect of neutrophils is related to cytotoxicity and regulation of antitumor immune responses, which has been called neutrophils N1. In addition, the tumor can send signals that induce a pro-tumor phenotype in neutrophils, which supports tumor growth and metastasis (N2 neutrophils). These N2 polarized neutrophils promote proliferation, migration, and invasion of tumor cells, stimulate angiogenesis, as well as maintaining immunosuppression.100,101 Furthermore, the increase in the number of neutrophils in the blood and tumors has been linked to a poor outcome.
Neutrophils produce some mediators, which have a tumoricidal potential activity, including reactive oxygen species (ROS), myeloperoxidase (MPO), hydrogen peroxide (H2O2), and proteases. Neutrophils from healthy donors have potent cytotoxicity against tumor cells.103 The experimental administration of neutrophils from healthy donors reduces tumor growth and increases survival of animals with tumors.102
After cytokine release, neutrophils trigger ROS with oxidative damage and cell death in melanoma cells.104 In addition, neutrophils may inhibit metastatic potential of tumor cells. In an experimental model of breast cancer cells in the lungs of mice,105 it was demonstrated that neutrophils generate H2O2 to suppress metastatic effects, suggesting that neutrophils could prevent metastasis of tumors through the generation of cytotoxic substances.67 However, neutrophils generate and release a broad range of factors to support in vitro and in vivo tumor cell growth.106 Neutrophil elastase (NE) was able to enter tumor cells to degrade the substrate-1 insulin receptor (IRS-1), resulting in greater interaction between PI3K and PDGFR and accelerated tumor cell proliferation.107,108 Neutrophils could promote proliferation through prostaglandin mediated E2 (PGE2) COX-2.109 At the same time, they could induce epithelial-mesenchymal transition (EMT) in tumor cells, which significantly increases the migratory and invasive ability of tumor cells.110
Neutrophils increase cell invasion bladder cancer through the modulation of androgen receptor (AR)/MMP13 signals.111 In addition, neutrophils could promote migration of cells and renal carcinoma invasion through the activation of VEGFa/HIF2α and β-estrogen receptors signals.112 Thus, neutrophils could decrease immune protection to promote metastasis.
Overall, it has become clear that the role of the main components of the immune response of allergic diseases and the association with cancer, exposed beyond as markers for illness or treatment, can have a significant impact on cancer progression. However, at present, the picture of the coexistence of allergy and carcinogenesis is becoming a reality in a rather complex interaction. This is because molecules associated with allergies can have a multitude of anti/pro-tumorigenic effects, either directly or indirectly, depending on the types of cancer and the conditions of the studied cells. In any case, it is clear that these components can affect the process of the onset and progression of cancer in many of its stages, and should be taken into account when considering the effects of treatment. However, further investigation about the delicate balance of the interaction of these diseases is needed, as the elements mentioned above have a dual response capacity, and what initially could be considered as a possible solution or cure, might lead to the onset of cancer.
Conflict of interestThe authors declare no conflicts of interest of any nature.
We acknowledge NIH RO1ES024434 grant; UC-MEXUSCONACyT fellowship and CONACyT fellowship number 263863 for B. Tirado-Rodriguez.