Over the past decade, some studies have addressed the therapeutic effects of omega-3 polyunsaturated fatty acids (ω-3 PUFAs) and the opposite effects of omega-6 (ω-6) PUFAs on several diseases, including cardiovascular disorders, diabetes, neurodegenerative diseases, and cancer. Research demonstrates the safety of these naturally occurring ingredients. Of particular interest, several studies have shown that ω-3 PUFAs possess a therapeutic role against certain types of cancer. It is also known that ω-3 PUFAs can improve the efficacy and tolerability of chemotherapy. Previous reports have indicated that suppression of nuclear factor-κB, activation of AMPK/SIRT1, modulation of cyclooxygenase (COX) activity, and up-regulation of novel anti-inflammatory lipid mediators such as protectins, maresins, and resolvins, are the main mechanisms of the antineoplastic effect of ω-3 PUFAs. In contrast, several studies have demonstrated that ω-6 PUFAs induce progression in certain types of cancer. In this review, we discuss epidemiological and experimental studies addressing the relationship between the development of some types of cancer, including colon and colorectal carcinoma, breast cancer, prostate cancer, lung cancer and neuroblastoma, and the ingestion to ω-3 and ω-6 (PUFAs). We also discuss the clinical data, addressing the therapeutic role of omega-3 PUFA against different types of cancer.
Durante las últimas décadas algunos estudios se han enfocado en los efectos terapéuticos de los ácidos grasos poliinsaturados (AGPI) omega-3 (ω-3) y los efectos contrarios de los AGPI omega-6 (ω-6) en diversas enfermedades, incluyendo enfermedades cardiovasculares, diabetes, enfermedades neurodegenerativas y cáncer. Las investigaciones han mostrado la seguridad de estos lípidos naturales. En particular, varios estudios han mostrado que los AGPI ω-3 poseen un efecto terapéutico contra ciertos tipos de cáncer. También se sabe que los AGPI ω-3 pueden mejorar la eficacia y tolerancia de la quimioterapia. En publicaciones anteriores se ha indicado que la supresión del factor nuclear κB, la activación de AMPK/SIRT1, la modulación de la actividad de la ciclooxigenasa (COX) y la regulación positiva de nuevos mediadores lipídicos antiinflamatorios como las protectinas, maresinas y resolvinas, son los principales mecanismos del efecto antineoplásico de los AGPI ω-3. En contraste, otros estudios han demostrado que los AGPI ω-6 inducen la progresión de ciertos tipos de cáncer. En esta revisión se discuten algunos estudios experimentales y epidemiológicos que abordan la relación entre el desarrollo de algunos tipos de cáncer, como carcinoma de colon y colorrectal, cáncer de mama, próstata y pulmón, así como neuroblastoma, y la ingestión de AGPI ω-3 y ω-6. Así mismo, se discuten los datos clínicos sobre el papel terapéutico del AGPI ω-3 contra diferentes tipos de cáncer.
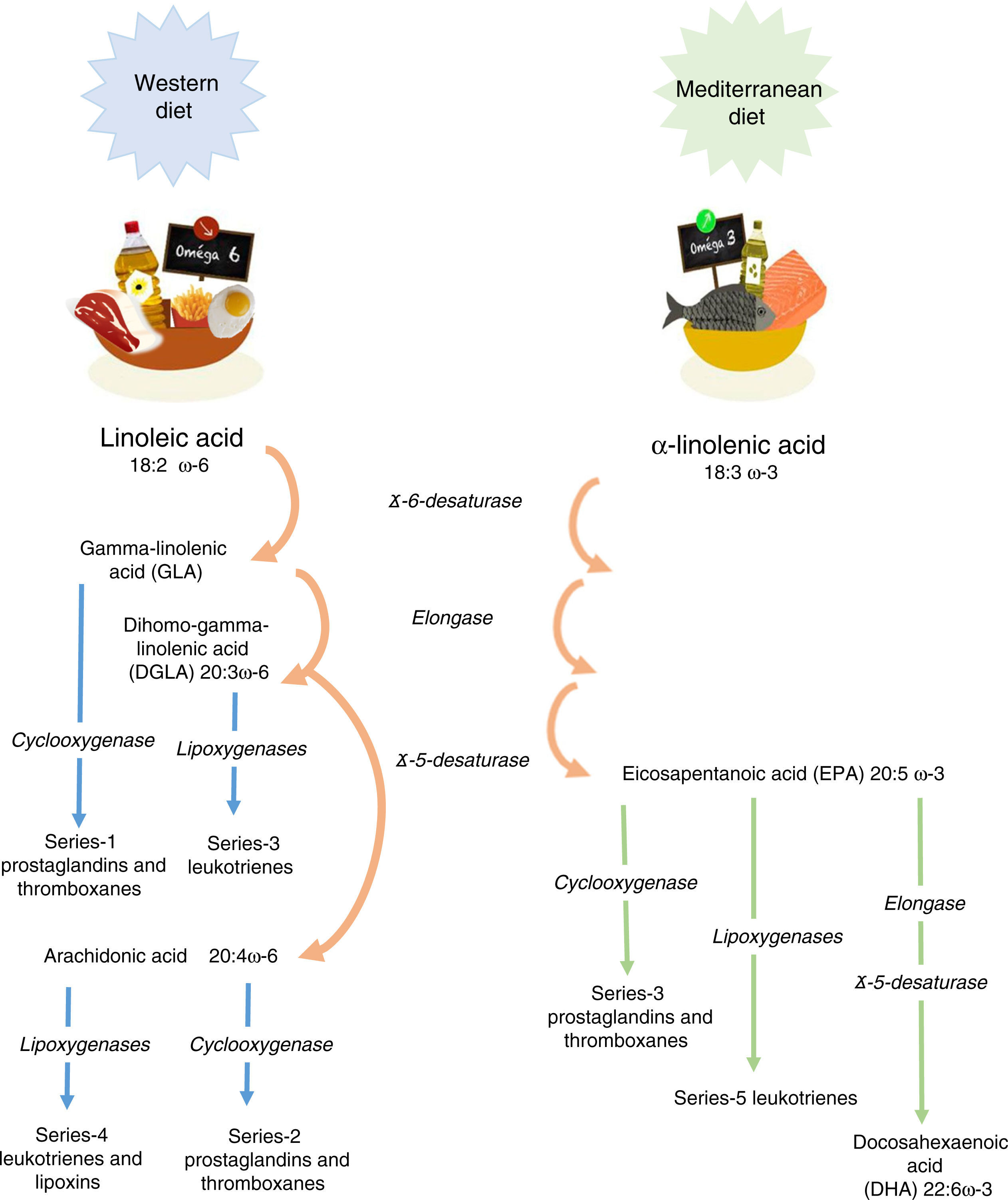
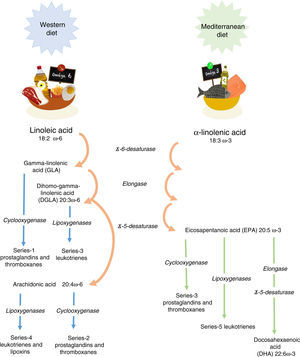
Linoleic acid (LA, 18:2 ω-6) and α-linolenic acid (ALA, 18:3 ω-3) represent the parental fatty acids of the two main classes of long-chain polyunsaturated fatty acids (PUFAs): the omega-6 (ω-6) and omega-3 (ω-3) fatty acids, respectively. Metabolism of LA and ALA generates other PUFAs and a host of other lipid mediators, such as prostaglandins, leukotrienes, and lipoxins (Figure 1). LA is found in the oils of safflower, grape seed, hemp, corn, wheat germ, cottonseed, and soybean. ALA is found at high levels in green leafy vegetables, flaxseed, walnuts, and canola oils.1
A cellular concentration ratio of ω-6/ω-3 is 1:1.5. It represents the most optimal homeostatic levels;2 however, it is complicated to reach this ratio. Some of the reasons are the following:
- •
Enzyme competition. The long-chain derivatives of the ω-6 and ω-3 PUFAs depend on the same enzymes for their production. The ratio of these fats depends on their nutritional intake. Production of the derivatives could be limited by the inefficiency of ¿5 and ¿6 desaturase enzymes. Thus, only 5% to 10% of ALA is converted to eicosapentaenoic acid (EPA) and 1% to docosahexaenoic acid (DHA).3
- •
Western diets. The intake of ω-6 fats is usually at least ten times greater than ω-3 fats. Moreover, many ω-3 fats are lost or oxidized after food processing and cooking. In addition, saturated fats, trans-fats, fat-free diets, glucose-rich diets, alcohol, glucocorticoids, reduced insulin levels, protein deficiency, hypothyroidism, and age also lessen the activity of the desaturase enzymes. For these reasons, it is usually necessary to intake an extra dietary supplementation of ω-3 to achieve a balanced ratio of ω-3/ω-6 fatty acids.
Tissues preferentially metabolize PUFAs in the order: ω-3>ω-6>ω-9. An elevated Mead acid (20:3 ω-9) level suggests a reduced blood level of ω-3 and ω-6 fatty acids. When LA and ALA are not supplied by the diet, oleic acid (18:1 ω-9) serves as the substrate for PUFA generation, creating a Mead acid. In addition, ALA and LA are not easily replaced and are vital to healthy skin.3
It has been shown that ω-3 and ω-6 PUFAs have significant roles in membrane structure and function; in cell signaling and regulation of gene expression. Furthermore, they play important roles as substrates for the synthesis of lipid mediators involved in inflammation, immunity, coagulation, smooth muscle contraction, and many other physiological responses.4 The main dietary ω-6 PUFA, LA, and its derivative, arachidonic acid (ARA), are important in all the roles already mentioned. The main dietary ω-3 PUFA, ALA, as well as its derivatives, EPA and DHA, also participate in the processes mentioned above.5,6 It is worth noting that the conversion of ALA to EPA, and particularly to DHA, appears to be limited to humans.7 Omega-6 and omega-3 PUFAs often compete with one another for metabolism and act in an opposing manner.6 This fact applies in particular for ARA and EPA with respect to inflammatory processes.8,9 Therefore, reaching the correct “balance” between ω-6 and ω-3 PUFA is essential for good health and to improve patients tm) outcomes. Conversely, a disturbed balance may be associated with impaired health and poor outcomes. Since there is a high dietary abundance of ω-6 PUFAs, and a relatively poor abundance of ω-3 PUFAs, ω-6 fatty acids are predominant over ω-3 in blood lipids, blood cells and most tissues.5,6 Nevertheless, the augmented oral intake of EPA and DHA results in an increase in their abundance in blood lipids,10,11 blood cells12,13 and tissues.14•16 Similarly, an intravenous supply of EPA and DHA has been shown to increase the content of those fatty acids in blood lipids and blood cells.17
2Omega-3 and Omega-6 (PUFAs) in cancerHuman health and disease are determined by the interaction between genetic and environmental factors. Diverse studies exploring dietary habits of human ancestors showed that much of the diversity in modern diet involves the types and amounts of essential fatty acids, particularly PUFA,18 that cannot be synthesized by the human body and must be assumed from external sources (food).
As mentioned above, ω-3s are part of the PUFA family that includes EPA, docosapentaenoic acid (DPA), and ALA. EPA and DPA are found in certain fish oils (for example salmon and mackerel oils) and represent the active forms of the ω-3 family. Green leafy vegetables and soybean oil contain ALA which, when consumed, is converted to EPA and then to DHA (although, as mentioned above, inefficiently). Several Cochrane reviews have shown that ω-3 fatty acids are beneficial for many diseases, including cystic fibrosis,19 type II diabetes,20 dysmenorrhea,21 schizophrenia,22 and cardiovascular disease.23
Abundant evidence has shown that ω-3 and ω-6 PUFAs exhibit significant effects in inhibiting various types of tumors. Nevertheless, the mechanisms of their anticancer roles have not been completely demonstrated.
In 1970, the beneficial health effects of ω-3 PUFA gained recognition. Since then, a growing body of evidence from in vitro studies in cell cultures, animal cancer models, and epidemiological and clinical studies has provided evidence to support their use in the prevention of cancer, including those of the colon, breast, and prostate.24 In contrast, evidence indicates that a high dietary intake of ω-6 PUFA is associated with an increased risk for the development of cancer.25
A recent meta-analysis of prospective cohort studies regarding the correlation between PUFAs intake and breast cancer (BC) risk included 527,392 participants and 16,178 BC cases. This analysis showed a 14% reduction with ω-3 PUFA, but no significant association with ALA.26 In contrast, a meta-analysis of prospective studies on prostate cancer revealed that individuals consuming more than 1.5g/d of ALA compared with subjects who consumed less than 1.5g/d had a significantly decreased risk of cancer.27
It is well known that ω-3 exerts anti-inflammatory effects in acute and chronic pathological inflammatory reactions.28 The ω-3 PUFAs are also reported to have anti-cancer effects based on in vitro and in vivo studies.29•31 Several mechanisms have been proposed to explain the anti-cancer effects of ω-3 PUFAs. Thus, ω-3 PUFAs can alter the growth of tumor cells by modulating cell replication, interfering with components of the cell cycle, or increasing cell death via necrosis or apoptosis.27,32 Omega-3 PUFAs are also known to exert anti-angiogenic effects by inhibiting the production of many angiogenic mediators, including vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and prostaglandin E2 (PGE2).33•35
A dietary supplementation is a traditional approach to modifying tissue nutrient composition in animal studies. Animal diets that modify specific nutritional and non-nutritional components can help to distinguish experimental groups; however, sometimes it can be difficult to provide diets that are identical in all but a single or a small, controlled number of variables. J. Kang reported an engineered transgenic mouse that carries the fat-1 gene from the roundworm Caenorhabditis elegans.36 This gene encodes a ω-3 fatty acid desaturase that catalyzes the conversion of ω-6 to ω-3 PUFAs, and induces a block of production of ω-6 in most animals, including mammals. There is a notable difference in the measured ω-6/ω-3 PUFA ratio in the tissue between wild-type and fat-1 transgenic mice,37 which is fat-1 independent of diet. This characteristic allows the performance of carefully controlled studies that contain no confounding factors of diet. This model is very helpful to investigate the biological properties of endogenous ω-3 PUFAs36 in cancer progression.38
Taguchi et al. investigated the role of ω-3 PUFAs in TC-1 tumorigenesis by comparing fat-1 and wild-type mice, with a specific focus on tumor-associated fibroblasts.39 For this purpose, they used TC-1 cells derived from the epithelium of C57BL/6 mice and immortalized by human papillomavirus (HPV) type 16 E6 and E7 oncoproteins, commonly used in vitro and in vivo murine models of HPV-related cancer.40 They demonstrated that a ω-3 PUFAs rich microenvironment suppressed matrix metalloproteinase-9 (MMP-9) secretion from cancer-associated fibroblasts (CAFs), and this was associated with subsequent tumor hypo-angiogenesis. This study proposes a novel anti-tumor effect of ω-3 PUFAs by modulating the tumor microenvironment, especially via effects on CAFs.39
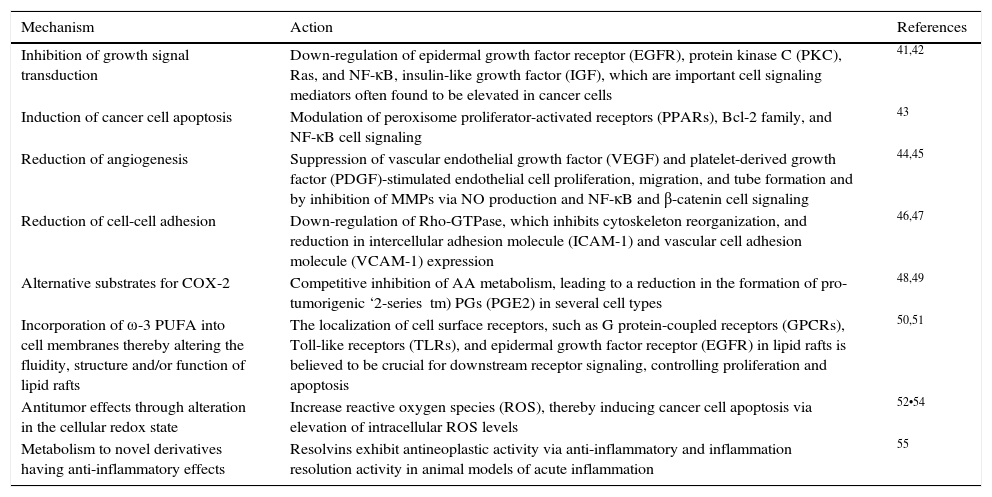
Additional evidence exists that indicates a relationship between fatty acids ingestion and development of cancer. Epidemiological studies have shown a protective association regarding ω-3 PUFAs and cancer risk. In a Japanese population-based study, ω-3 PUFAs (EPA, C20:5ω-3; DPA, C22:5ω-3; DHA, C22:6ω-3) were associated with protection against developing hepatocellular carcinoma.31 Whereas a population-based prospective study undertaken by the Japanese Public Health Center demonstrated an inverse relationship (protector effect) between the intake of marine ω-3 PUFA and the risk of colorectal cancer, even though the association was only significant in the proximal site of the colon.30 Evidence exploring the potential relationship of ω-3 and ω-6 PUFAs effects with cancer is limited, and mostly related to the beneficial effects of ω-3 PUFAs rather than the effects of ω-6 PUFAs. Omega-3 PUFA, especially EPA and DHA, have been shown to present multiple anti-tumor mechanisms of action (Table 1).
The antitumor mechanisms of ω-3 PUFA.
| Mechanism | Action | References |
|---|---|---|
| Inhibition of growth signal transduction | Down-regulation of epidermal growth factor receptor (EGFR), protein kinase C (PKC), Ras, and NF-κB, insulin-like growth factor (IGF), which are important cell signaling mediators often found to be elevated in cancer cells | 41,42 |
| Induction of cancer cell apoptosis | Modulation of peroxisome proliferator-activated receptors (PPARs), Bcl-2 family, and NF-κB cell signaling | 43 |
| Reduction of angiogenesis | Suppression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF)-stimulated endothelial cell proliferation, migration, and tube formation and by inhibition of MMPs via NO production and NF-κB and β-catenin cell signaling | 44,45 |
| Reduction of cell-cell adhesion | Down-regulation of Rho-GTPase, which inhibits cytoskeleton reorganization, and reduction in intercellular adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1) expression | 46,47 |
| Alternative substrates for COX-2 | Competitive inhibition of AA metabolism, leading to a reduction in the formation of pro-tumorigenic ‘2-series tm) PGs (PGE2) in several cell types | 48,49 |
| Incorporation of ω-3 PUFA into cell membranes thereby altering the fluidity, structure and/or function of lipid rafts | The localization of cell surface receptors, such as G protein-coupled receptors (GPCRs), Toll-like receptors (TLRs), and epidermal growth factor receptor (EGFR) in lipid rafts is believed to be crucial for downstream receptor signaling, controlling proliferation and apoptosis | 50,51 |
| Antitumor effects through alteration in the cellular redox state | Increase reactive oxygen species (ROS), thereby inducing cancer cell apoptosis via elevation of intracellular ROS levels | 52•54 |
| Metabolism to novel derivatives having anti-inflammatory effects | Resolvins exhibit antineoplastic activity via anti-inflammatory and inflammation resolution activity in animal models of acute inflammation | 55 |
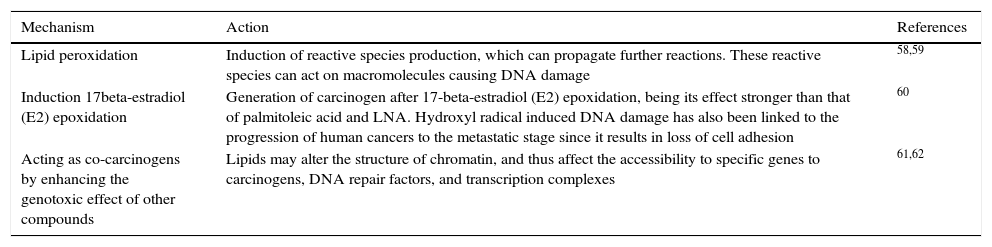
In contrast, the ω-6 PUFAs, especially ARA, are much more abundant in our daily diet and are associated with many adverse effects on the human body, including cancer promotion. For instance, a high intake of ω-6 has been found to correlate with a high risk of breast, prostate, and colon cancer incidence in many animal and human studies, and the ratio of ω-6 to ω-3 was suggested to be a predictor of cancer progression.44,56,57 Different mechanisms are proposed regarding the relationship of ω-6 PUFA tumor progression in diverse types of cancer (Table 2).
The pro-tumor mechanisms of ω-6 PUFA.
| Mechanism | Action | References |
|---|---|---|
| Lipid peroxidation | Induction of reactive species production, which can propagate further reactions. These reactive species can act on macromolecules causing DNA damage | 58,59 |
| Induction 17beta-estradiol (E2) epoxidation | Generation of carcinogen after 17-beta-estradiol (E2) epoxidation, being its effect stronger than that of palmitoleic acid and LNA. Hydroxyl radical induced DNA damage has also been linked to the progression of human cancers to the metastatic stage since it results in loss of cell adhesion | 60 |
| Acting as co-carcinogens by enhancing the genotoxic effect of other compounds | Lipids may alter the structure of chromatin, and thus affect the accessibility to specific genes to carcinogens, DNA repair factors, and transcription complexes | 61,62 |
There is evidence indicating that the ω-6 PUFA, LA, can be involved in both pro- and anti-cancer processes. For example, ω-6 PUFA increases the proliferation of the breast carcinoma cell line BT-474 and the human lung cancer cell line A549 in vitro, and promotes colon and prostate tumorigenesis and tumor growth in animal models.57,63 On the other hand, a high dose of LA inhibits the proliferation of the colon cancer cell line Caco-2,41 while a high intake of LA also can show a protective effect against cancer development.64
The accelerated cancer growth due to ω-6 PUFA could be the result of both increased ω-6 levels and decreased ω-3 levels. The ratio of ω-6 to ω-3 is often believed to be of greater importance than the absolute levels of a particular fatty acid. Interestingly, increasing evidence suggests that unlike the downstream ω-6 PUFA, which have been associated with cancer development, the upstream ω-6s such as LA, γ-linolenic acid, and dihomo-γ-linolenic acid, may possess anti cancer effects, and thus could represent promising dietary sources for cancer prevention and therapy.41,64•66 The ω-6 upstream PUFAs have been shown to exert their anti-cancer proliferation effects by disrupting the cell cycle in the G1 phase; up-regulating the protein expression of the cell cycle inhibitor p21; and decreasing the expression of cyclins A and D. Moreover, downstream ω-6 PUFA have been reported to induce apoptosis by triggering cytochrome c release and increasing caspase 3 activity, and probably by increasing the expression and activity of pro-apoptotic proteins (e.g. caspase 3, caspase 9, and Fas), while decreasing the expression of pro-growth and anti-apoptotic proteins (e.g. ErbB3, phosphorylated Akt, bcl-2, c-myc, and Ki-67). Importantly, through free radical-mediated lipid peroxidation, COX can catalyze ω-6s to produce two types of PGs, the 1-series, and 2-series PGs, which have been shown to possess diverse activities and are proposed to be responsible for the bioactivities of ω-6s.41 Given these differences, the bioactivities from ω-3 have been extensively studied for health improvement purposes, whereas the potential beneficial effects of ω-6s have received less attention.66
3Omega-3 PUFA in colon and colorectal cancerDuring the last decades, several studies have been conducted to evaluate the effects of ω-3 and ω-6 (PUFAs) on colorectal cancer (CRC). This type of cancer is one of the most common causes of death in Western industrialized countries and represents one of the most prevalent cancers in men after lung and prostate cancer.67
Many factors can be responsible for the development of CRC; genetic predisposition is considered an especially important risk factor. Furthermore, several epidemiological and experimental studies have suggested that the consumption of a typical Western-style diet, high in fat and proteins, significantly increases CRC risk.68 On the other hand, a high intake of fruits, vegetables, and whole grains has been shown to be protective against CRC.69 For example, CRC significantly increased in Japanese people who have migrated to the United States and adopted a more Western-style diet/lifestyle compared with those living in Japan.70 This strongly suggests a significant role of the environment in the development of CRC. More recently, the incidence of CRC has dramatically increased in Japan, coinciding with an overall increased consumption of the Westernized diet.71,72
In some in vitro and animal studies, ω-3 PUFAs have been suggested to play a protective role in CRC development mediated by inhibition of cyclooxygenase-2 (COX-2) and a resulting decrease in the production of ARA-derived eicosanoids.73 A long-term prospective study of U.S. men reported a significantly reduced CRC risk in cohorts ingesting the highest versus the lowest levels of ω-3 fatty acids. Certain fish represent the main dietary source of the long-chain ω-3 PUFAs. Some observational studies found an inverse association (protective effect) between fish consumption and CRC risk, while others did not.74,75 However, an important meta-analysis reported a 12% reduction in CRC risk contrasting high vs. low fish consumption.74 In contrast, a multi-center randomized controlled trial investigating the effect of a 6-month intervention with oil-rich or lean fish on apoptosis and mitosis within the colonic crypt found no marked change in these parameters.76
Some studies have found a stronger association between ω-3 PUFA and reduced CRC risk in analyses with longer duration of follow-up, suggesting a protective role of ω-3 PUFA against colorectal carcinogenesis.77,78 It is important to consider the site of carcinogenesis. With regards to one prospective study, researchers separately examined proximal, distal colon and rectal cancer risk in relation to PUFA intake, and reported a stronger inverse association of ω-3 PUFA with CRC in the proximal regions than in the distal regions.78
Song et al. performed a detailed analysis of the association between fish, PUFA, and CRC risk in two large prospective cohorts: the Nurses tm) Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). In this report, prospective studies with repeated measurements of diet over 24-26 years were performed. Moreover, an association between these dietary factors and the risk of CRC was investigated in a large sample taking into account sample site, levels of fatty acid intake, and time of sampling, among other parameters. The results of these studies did not support a strong association between fish, ω-3 and ω-6 PUFA intake, and overall CRC risk. However, some findings did suggest an association of ω-3 PUFA with increased risk of distal colon cancer and reduced risk of rectal cancer.79
Considering all the relevant data, and despite the lack of a clear effect in some of the studies, overall the studies indicate a likely beneficial effect of PUFA supplementation on colorectal carcinogenesis, especially with high amounts of ω-3 PUFAs.
Recently, Huang et al. have demonstrated that CRC was much lower in a ω-3 treated group of rats than in the untreated group, indicating a significant antitumor role for ω-3 PUFAs. A decrease in DNA 5-methylcytosine (5 mC) adducts is associated with poor prognosis of tumors. Interestingly, these adducts were higher in ω-3 PUFA-treated rats than in the CRC model, suggesting ω-3 PUFAs promotes 5 mC synthesis. Therefore, ω-3 PUFAs probably inhibited tumor growth via regulating DNA methylation, which represents a novel epigenetic anticancer mechanism of ω-3 PUFAs.80
An oral supply of ω-3 PUFAs increases their levels in plasma and cell membranes, often at the expense of the ω-6 PUFAs, ARA and LA. This increase produces an altered pattern of lipid mediator production to one that is less pro-inflammatory. Al-Taan et al.81 demonstrated that short-term intravenous supply of ω-3 PUFAs could change the levels of EPA, DHA, ARA, and LA in plasma and erythrocytes in patients with hepatic colorectal metastases. They studied patients awaiting surgery for removal of colorectal liver metastases while giving them a 72-hour infusion of total parenteral nutrition containing (treatment group) or not containing (control group) ω-3 PUFAs. Then they measured EPA, DHA, ARA, and LA levels in phosphatidylcholine (PC) plasma and erythrocytes at different time points. In the results, the treatment group showed increases in plasma PC, EPA, and DHA, as well as erythrocyte EPA, and decreases in plasma PC and erythrocyte LA. These findings suggest that infusion of ω-3 PUFAs could be used to induce a rapid effect, particularly in targeting inflammation.
4Omega-3 PUFA in prostate cancerProstate cancer is the most commonly diagnosed cancer among men in the U. S.82 Dietary factors are thought to play a role in prostate cancer.83 There is limited evidence that total fat is a risk factor for prostate cancer;84 however, the evidence for an association between specific fatty acids and prostate cancer development or progression is inconsistent.85•89
Animal and in vitro studies suggest that ω-3 and ω-6 PUFA have opposite effects on cancer development.90 However, epidemiologic studies remain inconclusive.87,88 One explanation for inconsistent findings among these studies is that the ratio of ω-3 and ω-6 PUFA levels can be more important for prostate cancer risk than the total intake of these fatty acids.91,92 Several reports indicate that the recommended dietary ratio of ω-6/ω-3 fatty acids for health benefits is 1.1 to 2.1.3 Nevertheless, the typical Western diet regularly contains ten or more times the amount of ω-6 relative to ω-3 PUFA.93 Alternatively, the relationship between diet and prostate cancer may differ according to race and ethnicity. Prostate cancer incidence and mortality rates are the highest among black men,82 and very few studies have focused on race-specific associations between diet and prostate cancer risk. Dietary factors may also have stronger associations for more aggressive prostate cancers,94 and this finding would be missed when all prostate cancers are combined.
Williams et al.95 examined the association between ω-3 and ω-6 PUFA and prostate cancer risk, and determined if these associations differ by race or disease aggressiveness. The results showed no significant associations between specific ω-3 or ω-6 PUFA intakes and overall prostate cancer risk. However, the highest dietary ratio of ω-6/ω-3 was significantly associated with elevated risk of high-grade, but not low-grade prostate cancer. In race-specific analyses, an increasing dietary ratio of ω-6/ω-3 fatty acids correlated with a higher prostate cancer risk among white men, but not black men. Those findings emphasize the importance of examining the ratio of ω-6 and ω-3 PUFA when assessing the relationship between PUFA intake and prostate cancer risk, and the need to examine these associations in subgroups of tumor grade and race/ethnicity.
5Omega-3 PUFA in breast cancerBreast cancer (BC) is the most common cancer among women worldwide.67,96 While it has yet to be determined what initially causes the onset of BC, research suggests potential links to dietary habits and fat intake, and specific dietary fatty acids have been proposed to play an important role.97
Epidemiological studies have found significant differences in BC incidence between populations consuming Western diets and those consuming Asian diets. Westerners typically consume more ω-6 polyunsaturated fatty acids (ω-6 PUFA) such as LA, which is metabolized to ARA, and much less ω-3 PUFA. In contrast, Asians typically consume higher amounts of ω-3 PUFA, such as ALA, EPA, and DHA.98 Animal studies suggest a beneficial effect of ω-3 PUFA for BC; however, the relationship in humans is more complex. Many human studies fail to differentiate between ALA, EPA, and DHA when reporting effects of ω-3 PUFA on cancer risk, preventing evaluation of their individual effects. Despite these challenges, important mechanistic insights are continually being identified that will eventually help to elucidate the individual effects of ω-3 PUFA in one of the most common forms of cancer worldwide.
Recent observational studies have assessed BC risk and breast adipose tissue fatty acid composition. Two case-control studies compared women with invasive nonmetastatic breast carcinoma and women with benign breast disease,99,100 and assessed the inverse correlation between breast adipose tissue ALA and BC risk. One study noted a significant decrease in risk for women in the highest of ALA intake, while another reported a reduced risk for women in the high versus low of ALA intake.101
In rodent models, a trend towards a protective effect of ALA on mammary tumorigenesis has been observed. High ALA diet significantly inhibited spontaneous mammary tumorigenesis in mice,102 and in mice fed with ALA-rich linseed oil it reduced mammary tumor growth and metastasis.61 Similar reductions in tumor growth rate and metastasis was observed when a basal diet supplemented with ALA-rich flaxseed was fed to nude mice injected with human BC cells.103 Reduced tumorigenesis was accompanied by downregulation of insulin-like growth factor and epidermal growth factor receptor expression, presenting a potential mechanism for the effects of ALA.
Few studies have reported the effect of varying the ω-3/ω-6 ratios on BC. The in vitro effect of different ratios of ω-6/ω-3 fatty acids on cellular mechanisms in cancerous and non-cancerous cells was studied. The results showed that different ratios of ω-6/ω3 fatty acids in the diet regulate tumor proteins MARBPs (matrix attachment region binding proteins), SMAR1 (scaffold/matrix attachment region binding protein 1), and Cux/CDP (CCAAT-displacement protein/cut homeobox). Thus, the ω-6/ω-3 fatty acid ratio can modulate intrinsic signal transduction mechanisms that in turn can regulate cell growth, and suggests that by reducing the proportion of ω-6/ω-3 fatty acids in our diet many aspects of cancer cell metabolism could be regulated. Thus, the risk of cancer would be reduced by restricting the intake of ω-6 fatty acids in our diet.104 The results suggest that dietary PUFAs and also total fat may be associated with the risk of BC. Thus, the use of the correct diet balance could potentially prevent the development of BC.
6Omega-3 PUFA in lung cancerLung cancer is the leading cause of cancer-related mortality worldwide.67 Approximately 85% of lung cancers are non-small cell lung cancer (NSCLC), which include squamous cell carcinoma, adenocarcinoma and large cell carcinoma. The main treatment for NSCLC involves the utilization of chemotherapy agents, including cisplatin and paclitaxel. However, side effects of chemotherapy are usually difficult to tolerate. Therefore, new drugs that are safe and efficient should be developed. Natural dietary agents consist of numerous bioactive compounds that have demonstrated significant potential in preventing and treating a wide variety of diseases, including lung cancer.29
Several studies have reported inconsistent results for the existence of an association between PUFA intake and risk of lung cancer. In 2004, Yu-Fei et al.60 summarized the evidence regarding this relationship using a dose response meta-analytic approach. The authors searched PubMed, Embase, and Cochrane Library electronic databases for relevant articles published through July 2013. Only prospective studies that reported effect estimates with 95% confidence intervals (95% CI)>2 of lung cancer incidence regarding PUFA intake were included. They did random-effects meta-analyses of study-specific incremental estimates to determine the risk of lung cancer associated with a 5g/d increase in PUFA intake, including eight prospective cohort studies reporting data on 1,268,442 individuals. The dose-response meta-analysis suggested that a 5g/d increase in PUFA has no significant effect on the risk of lung cancer. However, findings of dose response curve suggested that an increased PUFA intake from 5 to 15g/d seemed to increase the risk of lung cancer.
An experimental study showed that DHA and EPA significantly suppressed proliferation and induced apoptosis of A549 lung cancer cells in a dose- and time-dependent manner. Furthermore, the formation of autophagosomes was clearly enhanced in DHA-or EPA-treated cells.105 Most toxic effects in mammals caused by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), an environmental pollutant, are mediated by the aryl hydrocarbon receptor (AHR).106 After binding TCDD, the AHR activates the transcription of several genes. Some of the most important are CYP1A1, CYP1A2, and CYP1B1. It has been shown that those enzymes have the ability to metabolize polycyclic aromatic hydrocarbons to genotoxic derivatives. In addition, human CYP1A1, CYP1A2, and CYP1B1 can also metabolize ARA and other PUFAs in studies in vitro. ARA and other PUFAs are mainly metabolized via three pathways: the cyclooxygenase, lipoxygenase, and cytochrome P450 epoxidation/hydroxylation pathways. The immediate products of the metabolism of ARA by the P450's include four cis-epoxyeicosatrienoic acids (EETs) and certain hydroxyeicosatetraenoic acids (HETEs). The epoxide metabolites can be further metabolized mainly by soluble epoxide hydrolase, which converts them to the dihydroxyeicosatrienoic acids (DHETs). Other PUFAs, including LA (C18:2 ω6), α-LA (C18:3, ω3), EPA (C20:5 ω3), DHA (C22:6 ω3) are metabolized in a similar fashion.107,108 Recently, Bui et al.109 analyzed the levels of more than twenty-five eicosanoids in different organs of wild-type, and Ahr-null mice treated or untreated with TCDD. They demonstrated that TCDD increased the levels of many metabolites of the cytochrome P450 epoxidation/hydroxylation pathway in the serum, liver, lung, and spleen. In addition, TCDD treatment increased the levels of several eicosanoids that are categorized as lipoxygenase products in the serum, liver, and spleen but not in the lungs or heart. Interestingly, TCDD did not induce an increase of eicosanoid levels in Ahr•/• knockout mice demonstrating that AHR mediates the effects of TCDD on the eicosanoids. They also observed that the levels of the CYP1A1 and CYP1B1 mRNAs were increased by TCDD treatment in the wild-type mice in the liver, lung, and heart. The CYP1A2 mRNA increased only in the liver. Moreover, no induction of any of these enzymes occurred in the Ahr•/• mouse. These cytochromes P450 are likely to be responsible for most of the increases in these metabolites in the various organs after TCDD treatment.
Based on those findings, they demonstrated that TCDD also increases the levels of many metabolites of the ω-3 PUFA in mice liver and lungs, as they previously observed for several of the equivalent ω-6 metabolites.110 Those results are significant if we consider that several epidemiological and preclinical evidence supports that a diet rich in ω-3 fatty acids is correlated with reduced risks of several diseases, including heart diseases and cancer.111 Of interest, the epoxides of ω-3 PUFA have the opposite effects from the epoxides of ARA on tumor growth, angiogenesis, and metastasis.112 The increased levels of the epoxides and hydroxyl derivatives of the PUFAs elicited by TCDD in the liver and lung are likely to have biological consequences. This study opens a new field for future investigations.
7Omega-3 PUFA in neuroblastomaNeuroblastoma is the most common extracranial solid organ tumor in infancy.113 It accounts for ∱/47•8% of all childhood cancers and nearly 15% of pediatric oncology deaths, making it the deadliest extracranial malignancy of childhood. As stated before, the Western diet contains a disproportionally high amount of ω-6 PUFA and low amount of ω-3 PUFA, and the resulting high ω-6/ω-3 ratio is thought to contribute to different diseases like inflammation and cancer. In particular, evidence that ω-3 PUFA may be inhibitory on neuroblastoma has been observed both in vitro and in animal studies.24
A recent study evaluated the effect of the ω-3/ω-6 PUFA ratio on neuroblastoma tumor growth in an orthotopic and subcutaneous murine xenograft tumor model. Since vascular endothelial growth factor (VEGF) expression and a vascular phenotype correlate with metastasis and clinical outcome in neuroblastoma,114 this study evaluated the effect of the FDA-approved agent sunitinib (Sutent; SU11248; Pfizer, New York, NY), in combination with ω-3 FA-enriched diet.115 The authors demonstrated an antitumor effect of dietary ω-3 that was enhanced in the presence of sunitinib. Furthermore, when combined with an enriched-FA ω-3 diet they found a decrease in tumor microvessel density compared with single-agent therapy.115 In other tumor models, it has been suggested that the in vivo antitumor effects of ω-3 FA may be mediated by inhibition of tumor cell proliferation and induction of apoptosis.116 Significantly, it has been shown in mice that the same effect may be due to changes in the metabolism of local eicosanoid-induced FA ω-3 diet or may be related to a reduction in PLA-2 expression, an alteration in the inflammatory response, or induction of mitochondrial dysfunction.
On the other hand, a recent study showed that the anti-proliferative effect of DHA and EPA on a human neuroblastoma cell line LA-N-1 apparently depended on a G0/G1 cell cycle arrest in the LA-N-1 cells as well as a decrease in the expression of CDK2 and cyclin E proteins. Also, DHA and EPA may have also induced apoptosis in LA-N-1 cells as revealed by increased DNA fragmentation, phosphatidylserine externalization, and mitochondrial membrane depolarization. Upregulation of Bax, caspase-3, and caspase-9 protein activation, and down-regulation of the Bcl-XL protein could explain the occurrence of apoptotic events.116 Therefore, DHA and EPA are potential anti cancer agents that might be used for adjuvant therapy or combination therapy with conventional anti cancer drugs for the treatment of some forms of human neuroblastoma with minimal toxicity. Since the prolonged administration of high levels of ω-3 FA in children is safe,117,118 it is proposed that the use of ω-3 FA should be incorporated as a part of a combination treatment.
Conflict of interestThe authors declare no conflicts of interest of any nature.
We acknowledge NIH grant RO1ES024434 and UC-MEXUS-CONACyT fellowship and CONACyT fellowship number 263863 to Ana B. Tirado-Rodriguez.