Tumor cell resistance to chemotherapy agents is one of the main problems in the eradication of different neoplasias. One of the mechanisms of this process is the overexpression of anti-apoptotic proteins such as Bcl-2 and Bcl-XL; blocking the activity of these proteins may contribute to the sensitization of tumor cells and allow the adequate effects of chemotherapeutic drugs.
Methods and resultsThis study adressed the transfection of prostate cancer cells (PC3) with a plasmid encoding a recombinant protein with an antagonist peptide from the BH3 region of the Bax protein fused to the GFP reporter protein (BaxGFP).
This protein induced apoptosis of these tumor cells; further, selective transport of this plasmid to the tumor cell with Salmonella enterica serovar Typhimurium (strain SL3261), a live-attenuated bacterial vector, can induce sensitization of the tumor cell to the action of drugs such as cisplatin, through a process known as bactofection.
ConclusionsThese results suggest that Salmonella enterica can be used as a carrier vector of nucleotide sequences encoding heterologous molecules used in antitumor therapy.
La resistencia a los agentes quimioterapéuticos por parte de las células tumorales es uno de los principales problemas para la erradicación de distintas neoplasias. Uno de los mecanismos involucrados en este proceso es la sobreexpresión de proteínas antiapoptóticas como Bcl-2 y Bcl-XL. El bloquear la actividad de estas proteínas puede contribuir a la sensibilización de las células tumorales, permitiendo que los fármacos quimioterapeúticos funcionen de forma adecuada.
Métodos y resultadosEn este trabajo se llevó a cabo la transfección de células de cáncer de próstata (PC3) por un plásmido que codifica para una proteína recombinante que contiene un péptido antagónico perteneciente a la región BH3 de la proteína Bax fusionada a la proteína reportera GFP (BaxGFP).
Esta proteína fue capaz de inducir apoptosis en las células PC3. El transporte selectivo de este plásmido hacia la célula tumoral empleando Salmonella enterica serovar Typhimurium cepa SL3261, un vector bacteriano vivo atenuado, permitió la sensibilización de la célula tumoral a la acción de fármacos como el cisplatino mediante un proceso denominado bactofección.
ConclusionesEstos resultados sugieren que Salmonella enterica puede emplearse como un vector acarreador de secuencias nucleotídicas que codifican para moléculas heterólogas empleadas en la terapia antitumoral.
In the past few years, chemotherapy has become the preferred method for the treatment of different types of cancer. However, the development of drug resistance mechanisms by tumor cells has become one of the key obstacles to their elimination.
Among chemotherapy resistance mechanisms is the dysregulation of encoding proteins controlling apoptosis, including proteins from the Bcl-2 family.1 Overexpression of antiapoptotic proteins, such as Bcl-2 and Bcl-XL, is associated with chemotherapy resistance in non-Hodgkin lymphoma,2 nephroblastomas,3 ovarian cancer,4 monocytic leukemias,5 squamous cell carcinoma6 and acute T-cell leukemia.7 Therefore, blocking the activity of these proteins in tumors may result in the sensitization and death of tumor cells.
One of the strategies to block Bcl-2 and Bcl-XL is the use of synthetic peptides derived from the BH3 region of the Bax, Bak and Bad proteins that have the ability to bind to Bcl-2 and Bcl-XL. Thus, these induce the release of pro-apoptotic factors such as cytochrome C.8,9 Studies on the usefulness of these peptides in antitumor therapy have been conducted by coupling them to fusogenic peptides of the Antennapedia protein that can destabilize the cell membrane and facilitate the passage of Bax peptides to the tumor cell cytosol and induce cell death.10,11 Although the entry of Bax peptides into the tumor cell is solved by coupling to the fusogenic peptides, it is still necessary to overcome other inconveniences relating to the use of peptides in antitumor therapy, such as peptide stability after administration, and their effective and selective direction toward the tumor cell.
The use of live-attenuated bacterial vectors has been proposed to solve these problems since they can migrate into the tumor microenvironment and become true protein, peptide and nucleotide sequence factories once localized within the tumor.12 One of the live-attenuated bacterial vectors used for this purpose is Salmonella enterica since it is highly selective for the nutrient-rich and microaerophilic tumor microenvironment.13,14 This study evaluated the ability of the live-attenuated bacterial vector Salmonella enterica to transfer plasmids into prostate cancer tumor cells (PC3) through a process known as bactofection. Transferred plasmids contained a sequence encoding a peptide from the BH3 region of the Bax protein fused to the green fluorescent reporter protein(GFP). Once the Bax peptide that antagonizes the antiapoptotic activity of the Bcl-XL and Bcl-2 proteins has been translated in the cell, it will sensitize the tumor cells to die by apoptosis as a result of the activity of the chemotherapy agent cisplatin.
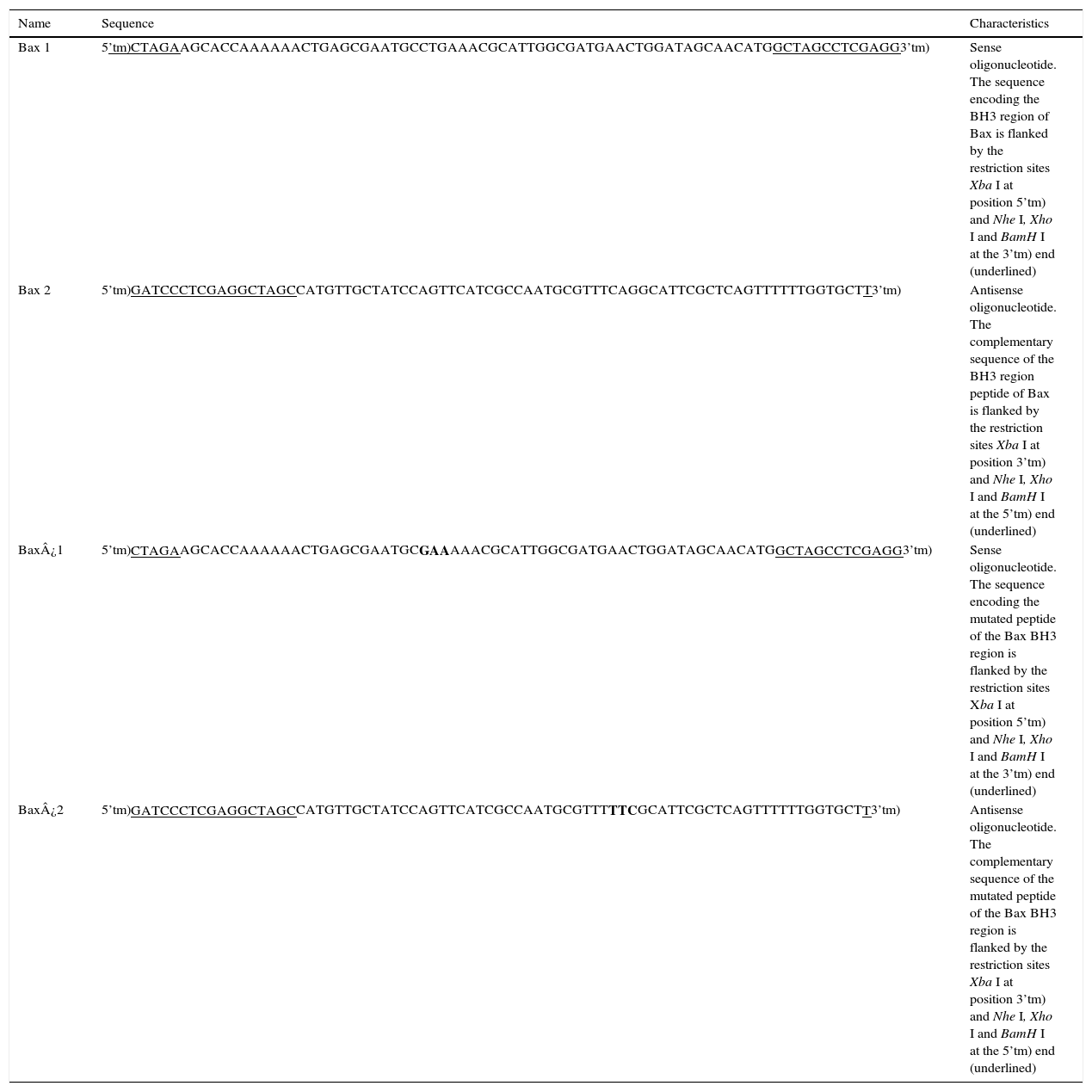
2Methods2.1Bacterial strains, oligonucleotides, and plasmidsThe following bacterial strains were used: Escherichia coli DH5α (Gibco, BRL. Gaithersburg MD, USA) and Salmonella enterica serovar Typhimurium SL3261 mutated in Aro A (Salmonella enterica SL3261).15 The oligonucleotide sequences encoding the BH3 region peptide of the pro-apoptotic protein Bax and the mutant peptide Bax¿, as well as the plasmids obtained in this study, are listed in Tables 1 and 2, respectively.
Oligonucleotides.
| Name | Sequence | Characteristics |
|---|---|---|
| Bax 1 | 5’tm)CTAGAAGCACCAAAAAACTGAGCGAATGCCTGAAACGCATTGGCGATGAACTGGATAGCAACATGGCTAGCCTCGAGG3’tm) | Sense oligonucleotide. The sequence encoding the BH3 region of Bax is flanked by the restriction sites Xba I at position 5’tm) and Nhe I, Xho I and BamH I at the 3’tm) end (underlined) |
| Bax 2 | 5’tm)GATCCCTCGAGGCTAGCCATGTTGCTATCCAGTTCATCGCCAATGCGTTTCAGGCATTCGCTCAGTTTTTTGGTGCTT3’tm) | Antisense oligonucleotide. The complementary sequence of the BH3 region peptide of Bax is flanked by the restriction sites Xba I at position 3’tm) and Nhe I, Xho I and BamH I at the 5’tm) end (underlined) |
| Bax¿1 | 5’tm)CTAGAAGCACCAAAAAACTGAGCGAATGCGAAAAACGCATTGGCGATGAACTGGATAGCAACATGGCTAGCCTCGAGG3’tm) | Sense oligonucleotide. The sequence encoding the mutated peptide of the Bax BH3 region is flanked by the restriction sites Xba I at position 5’tm) and Nhe I, Xho I and BamH I at the 3’tm) end (underlined) |
| Bax¿2 | 5’tm)GATCCCTCGAGGCTAGCCATGTTGCTATCCAGTTCATCGCCAATGCGTTTTTCGCATTCGCTCAGTTTTTTGGTGCTT3’tm) | Antisense oligonucleotide. The complementary sequence of the mutated peptide of the Bax BH3 region is flanked by the restriction sites Xba I at position 3’tm) and Nhe I, Xho I and BamH I at the 5’tm) end (underlined) |
Mutations in Bax¿ sequences are shown in bold.
Plasmids.
| Name | Size | Characteristics |
|---|---|---|
| pEGFP-N1 | 4.7 kb | Reporter plasmid expressing the green fluorescent protein (GFP)* |
| pBaxGFP | 4.7 kb | Plasmid carrying the sequence for expression of the BH3 region peptide of Bax coupled to GFP |
| pBax¿GFP | 4.7 kb | Plasmid carrying the sequence for the expression of the BH3 region mutated peptide of Bax coupled to GFP |
The sequences encoding the Bax and Bax¿ peptides were obtained by the sequences coupling technique. Briefly, Bax and Bax¿ sense and antisense oligonucleotides were hybridized using a 5nM concentration of each one in a mixture incubated at 90°C for 15min and gradually cooled. The hybridization product was analyzed by 3% agarose gel electrophoresis in TAE 1X buffer (Tris-potassium acetate 0.04M, EDTA 0.001M) and purified with an agarose gel extraction kit (Qiagen). Xba I and BamH I restriction sites flanked the coupling products obtained for the sequences encoding the Bax and Bax¿ peptides. This restriction allows their ligation with the T4 DNA ligase enzyme (Invitrogen) to the pEGFP-N1 vector (Clontech) after previous purification and digestion with the Nhe I and BamH I enzymes, thus yielding the pBaxGFP and pBax¿GFP plasmids.
2.3Bacterial culturesEscherichia coli DH5α and Salmonella enterica SL3261 were transformed with the generated plasmids and cultured in brain heart infusion medium (BHI) (Difco) at 37°C and 50μ/4g/ml kanamycin. Salmonella strains were cultured in medium supplemented with 0.01% 2,3-dihydroxybenzoic acid (Sigma–Aldrich). Salmonella enterica SL3261 strain was transformed by electroporation with the MicroPulser Electroporator equipment (BioRad), using 2mm cuvettes. Briefly, electrocompetent bacteria were mixed with 0.5μ/4g of plasmid DNA (pEGFP-N1, pBaxGFP or pBax¿GFP), and placed in the electroporation cuvette where a 2.5kV pulse was applied; selection of positive clones was performed with kanamycin at a concentration of 50μ/4g/ml.
2.4Cell lines and cell culturesProstate cancer cell line (PC3) was cultured in Advanced RPMI medium (GIBCO), 3% fetal bovine serum (FBS) and 1% antibiotics-antimycotics containing 10,000 U/ml penicillin G, 10mg/ml streptomycin, and 25μ/4g/ml amphotericin B.
2.5Transfection assaysProstate cancer cell line (PC3) was transfected with Lipofectamine®) 2000 (Invitrogen) according to the manufacturer's specifications. Briefly, 10x105 cells were placed in each well of a 24-well plate and transfected with a mixture of 0.5μ/4g plasmid DNA (pEGFP-N1, pBaxGFP or pBax¿GFP) and 1μ/4l of Lipofectamine®) 2000 in unsupplemented Advanced RPMI medium. The mixture was incubated for 4h; cells were subsequently washed with unsupplemented medium and incubated for 24h in supplemented medium to conduct the corresponding analyses.
2.6Expression of the recombinant proteins BaxGFP and Bax¿GFPThe expression assays of the recombinant proteins BaxGFP and Bax¿GFP were conducted 24h after PC3 cellular transfection and analyzed by confocal microscopy. Briefly, transfected PC3 cells were treated with the mitochondrial marker MitoTracker (Invitrogen) at a concentration of 1μ/4M for 30minutes. The cells were immediately washed in unsupplemented Advanced RPMI medium. PC3 cells were subsequently trypsinized and collected. Finally, 1x105 cells were placed on a 20 x 20mm coverslip and treated with Poly-L-lysine (Invitrogen). Cells were fixed with 4% paraformaldehyde and mounted on a slide with 15μ/4l Vectashield®) (Vector) mounting medium. Preparations were analyzed by confocal microscopy (Leica TCS Sp8X).
2.7TUNEL apoptosis assaysTUNEL assay was conducted in PC3 cells transfected with the pEGFP-N1, pBaxGFP or pBax¿GFP plasmids, using the In Situ Cell Death Detection TMR red kit (Roche) following the manufacturer's instructions. Cell analysis was conducted in a FACSCALIBUR (Becton Dickinson) cytometer. For the immunocytochemical TUNEL assay, the In Situ Cell Death Detection POD kit (Roche) was used, and analysis was performed by bright field microscopy with an Olympus inverted microscope (model IX73).
2.8PC3 cell bactofection with Salmonella enterica SL3261The PC3 cell line was infected for 30minutes with Salmonella enterica SL3261 containing the pEGFP-N1, pBaxGFP or pBax¿GFP plasmids, in 24-well plates at a multiplicity of infection (MOI) of 100. Cultures were subsequently treated with Advanced RPMI medium supplemented with gentamicin 200μ/4g/ml for 1h to eliminate remaining bacteria. Finally, supplemented Advanced RPMI medium was added, and cultures were incubated for 72h at the end of which the expression of the recombinant proteins BaxGFP, Bax¿GFP and GFP was evaluated by fluorescence microscopy with an Olympus inverted microscope (model IX73).
2.9Chemotherapy sensitization assaysAfter PC3 cell line was infected with Salmonella enterica SL3261 containing the pEGFP-N1, pBaxGFP or pBax¿GFP plasmids, and cultures were subsequently treated with Advanced RPMI medium and incubated, cisplatin, the chemotherapy agent, was added at a concentration of 20μ/4M, 12h before concluding the incubation period.
2.10Statistical analysisDifferences between groups of cells treated with the different recombinant Salmonellas and conditions were determined by analysis of variance (ANOVA) and Student's t-test (in the case of independent samples), with a 95% confidence interval. The average of three or more independent experiments±standard deviation (SD) is shown in all cases.
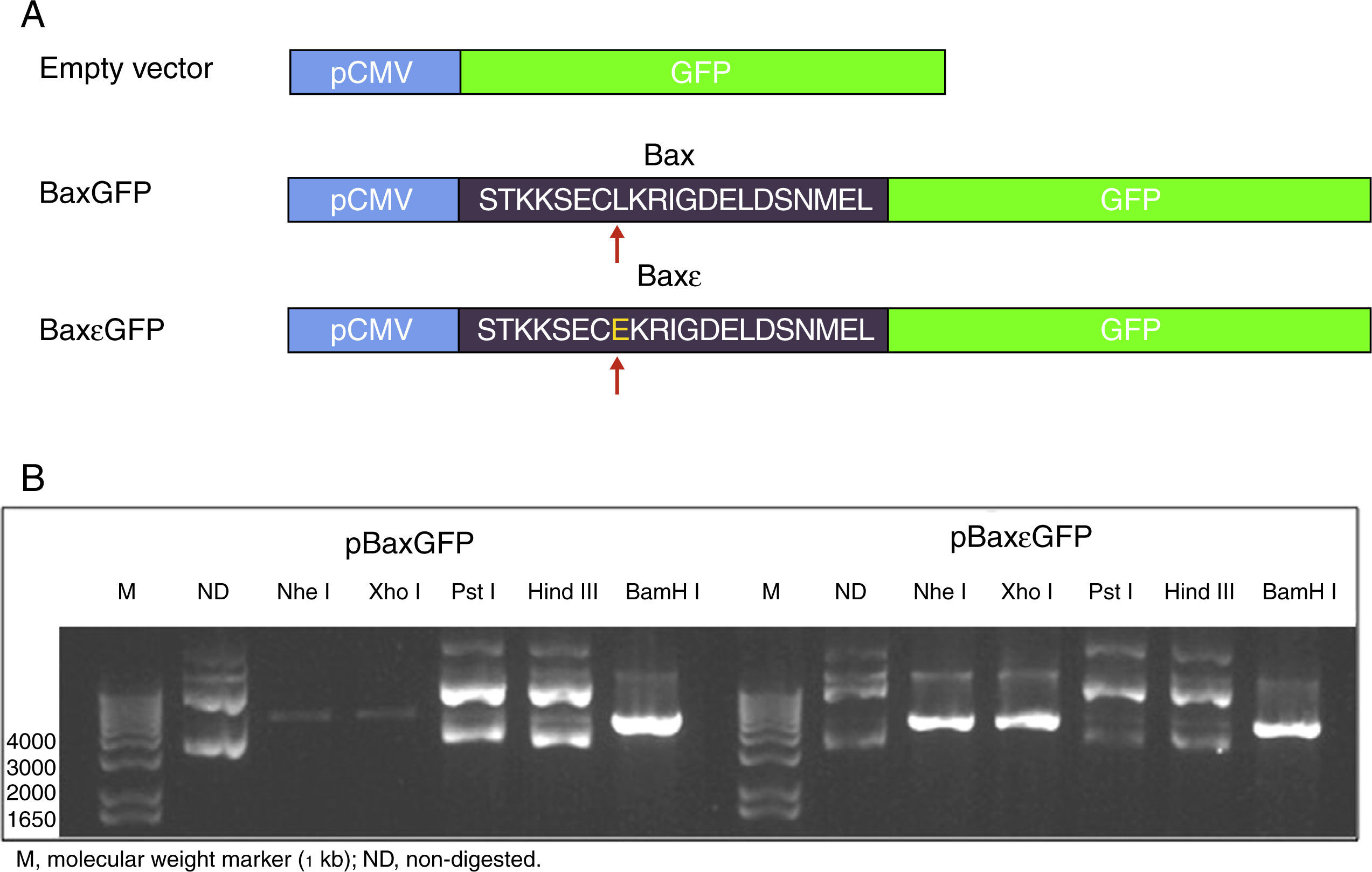
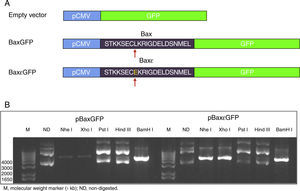
3Results3.1Construction of the pBaxGFP and pBax¿GFP plasmidsIn this study, we report the construction of an expression vector for eukaryotic cells in which the BH3 region peptide of the Bax protein was fused to the reporter protein GFP. Similarly, the sequence encoding the antagonist Bax peptide in which leucine was substituted by glutamic acid at position 8 (L8E) was used as a control to test the specificity of the system. This substitution allows Bax peptide to lose affinity for the pro-apoptotic proteins of the Bcl-2 family such as Bcl-XL. Figure 1 shows the representative maps of the constructions as well as the restriction map of plasmid digestion with the restriction enzymes Nhe I, Xho I and BamH I, and indicates the presence of the encoding sequences. The insertion of fragments encoding the Bax and Bax¿ peptides led to the loss of the Nhe I site of the pEGFP-N1 product as a result of ligation, with the Xba I site at position 5’tm) of the hybridization product (Table 1). Digestion of the pEGFP-N1 plasmid (encode GFP protein) with the Nhe I and BamH I enzymes leads to the loss of the Xho I site, located in the multicloning site. However, insertion of the Bax and Bax¿ fragments containing the restriction sites for the Nhe I, Xho I and BamH I enzymes at the 3’tm) end (Table 1), allows recuperation of the sites for Nhe I and Xho I, and verification that the fragments were adequately inserted. The obtained plasmids were pBaxGFP and pBax¿GFP (Table 2); that encoding proteins BaxGFP and Bax¿GFP, respectively.
Construction of the pBaxGFP and pBax¿GFP plasmids. (A) Representative map of the construction of the BaxGFP and Bax¿GFP recombinant proteins, in which the replacement of leucine by glutamic acid at position 8 (L8E) is shown. (B) Restriction analysis of the inserted fragments in which the Nhe I, Xho I and BamH I sites that were incorporated due to the cloning of fragments encoding the Bax and Bax¿ peptides are shown.
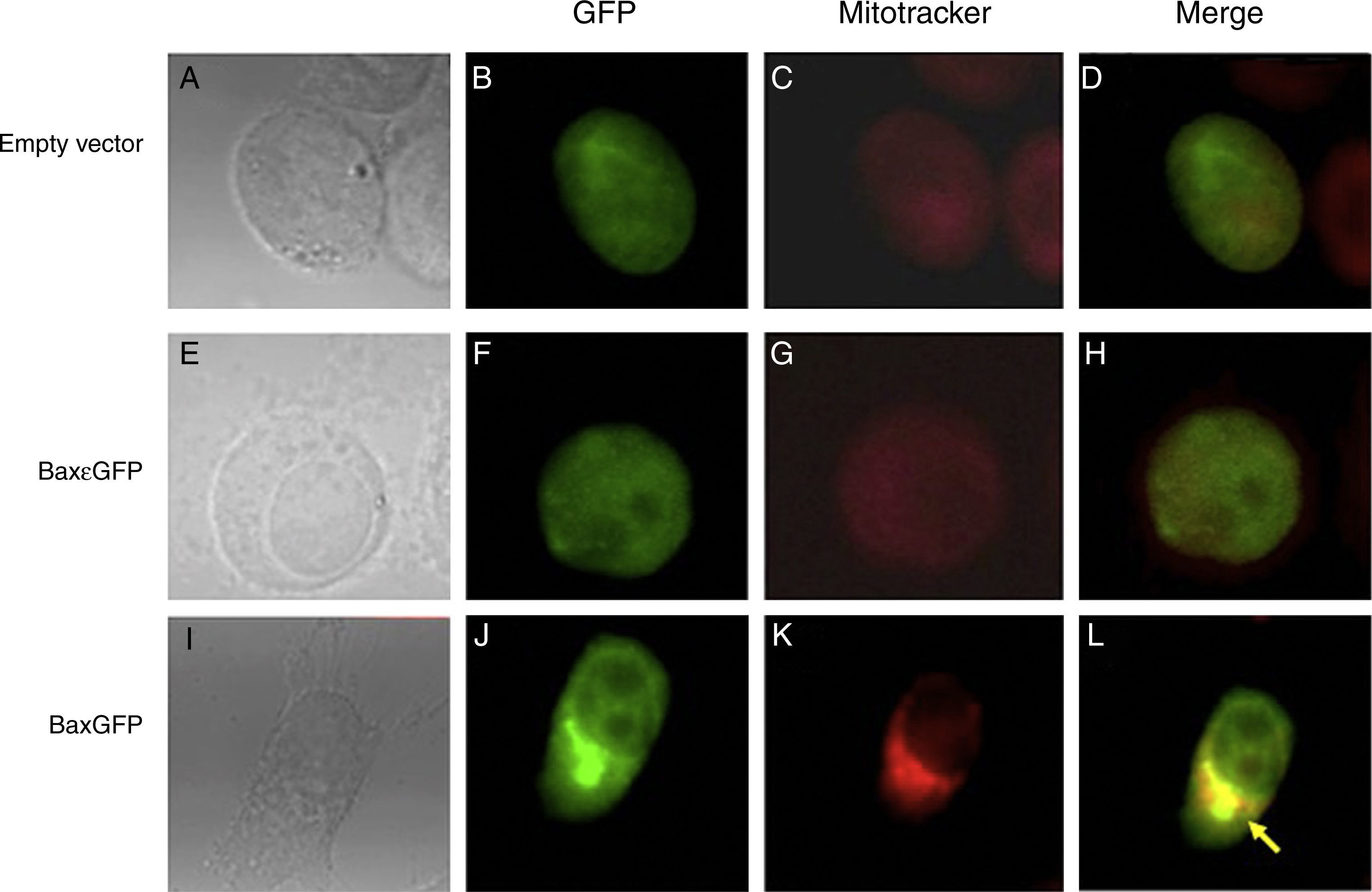
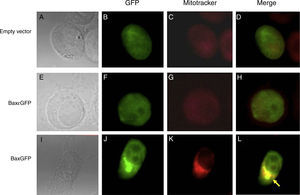
PC3 prostate cancer cell line was used to evaluate the expression and localization of the recombinant proteins BaxGFP and Bax¿GFP. The cells were transfected with the empty vector (pEGFP-N1), pBaxGFP or pBax¿GFP plasmids, and 24h posttransfection, mitochondria were stained and analyzed by confocal microscopy. Figure 2 shows the cells in a bright field (2A, 2E, and 2I), cells expressing GFP (2B, 2F, and 2J), cellular mitochondrial staining with MitoTracker (2C, 2G, and 2K) and the overlap of GFP and MitoTracker (2D, 2H, and 2L). As can be seen in the overlapping images, only the recombinant protein BaxGFP (Figure 2L) co-located with the mitochondrial marker, suggesting a possible interaction with the anti-apoptotic proteins anchored to the mitochondrial membrane. While Figures 2D and 2H correspond to the empty vector and the recombinant Bax¿GFP protein, respectively, the expression of GFP remains dispersed in the cytosol and does not colocalize with the mitochondrial marker.
Co-localization of the recombinant protein BaxGFP with the mitochondrion. PC3 cells transfected with the pBaxGFP, pBax¿GFP, and empty vector (pEGFP-N1) were treated with the mitochondrial marker MitoTracker for 30minutes and analyzed by confocal microscopy. (A, E, I) PC3 cells in a bright field. (B, F, J) Expression of GFP protein. (C, G, K) Mitochondria stained with MitoTracker. (D, H, L) Overlapping images of GFP and MitoTracker (representative image of three independent experiments).
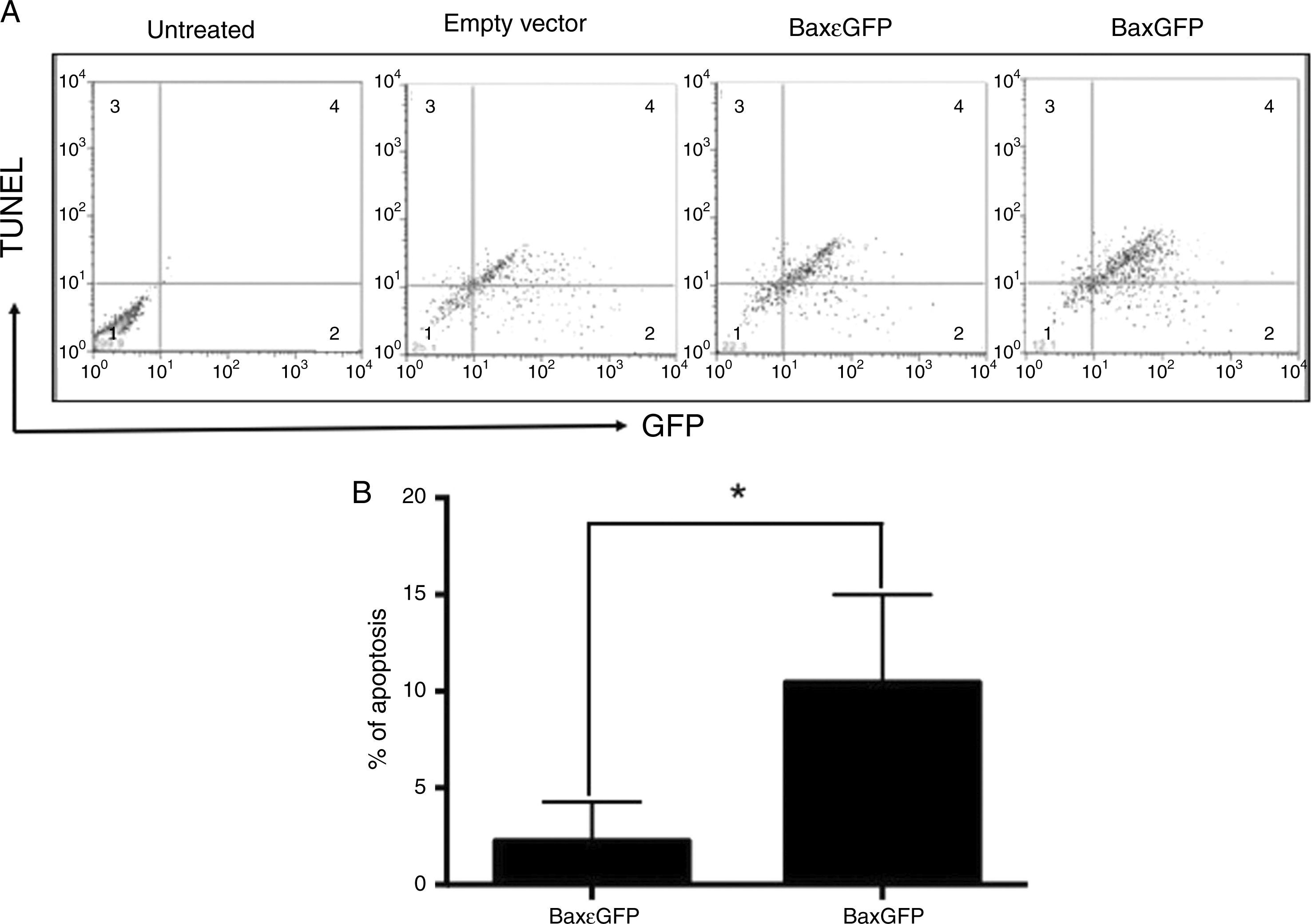
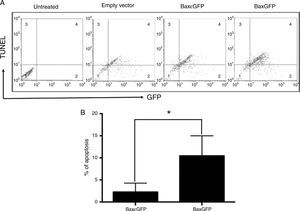
Once the interaction of the BaxGFP protein with the mitochondria was analyzed, we examined whether these proteins were able to induce apoptosis in PC3 cells. Therefore, we conducted a TUNEL assay of PC3 cells after their transfection with the empty vector, pBaxGFP or pBax¿GFP plasmids, and determined the percentage of apoptotic cells by flow cytometry. Results showed that transfection of PC3 cells with the pBaxGFP plasmid generated the highest number of double positive cells (GFP and TUNEL), compared with the cells transfected with pBax¿GFP and the empty plasmids (Figure 3A).
The recombinant protein BaxGFP induces apoptosis in PC3 cells. (A) Representative dot plot of three independent experiments in which the distribution of non-transfected or transfected PC3 cells with the empty vector (pEGFP-N1), pBaxGFP or pBax¿GFP plasmids is shown. Quadrant 1 shows the GFP (-) and TUNEL (-) cells; Quadrant 2 shows the GFP (+) cells; Quadrant 3 shows the TUNEL (+) cells; and Quadrant 4 shows the GFP (+) and TUNEL (+) cells. (B) PC3 cellular apoptosis quantification induced by the recombinant proteins BaxGFP and Bax¿GFP. Each column represents the mean and standard deviation of three independent experiments (*p<0.05).
In these assays, we found that the empty plasmid also induced apoptosis, probably due to the transfection method. Apoptosis induced by transfection of the empty vector was subtracted from the samples transfected with the pBaxGFP and pBax¿GFP plasmids to understand the real effect of the antagonist Bax peptide on apoptosis induction in PC3 cells. As shown in figure 3B, the expression of the recombinant protein BaxGFP induced more apoptosis in PC3 cells compared to the expression of the Bax¿GFP protein; this difference was statistically significant (p<0.05).
These results demonstrated that the transfection of a plasmid encoding the recombinant protein BaxGFP in tumor cells was capable of colocalizing with mitochondria and also induced apoptosis.
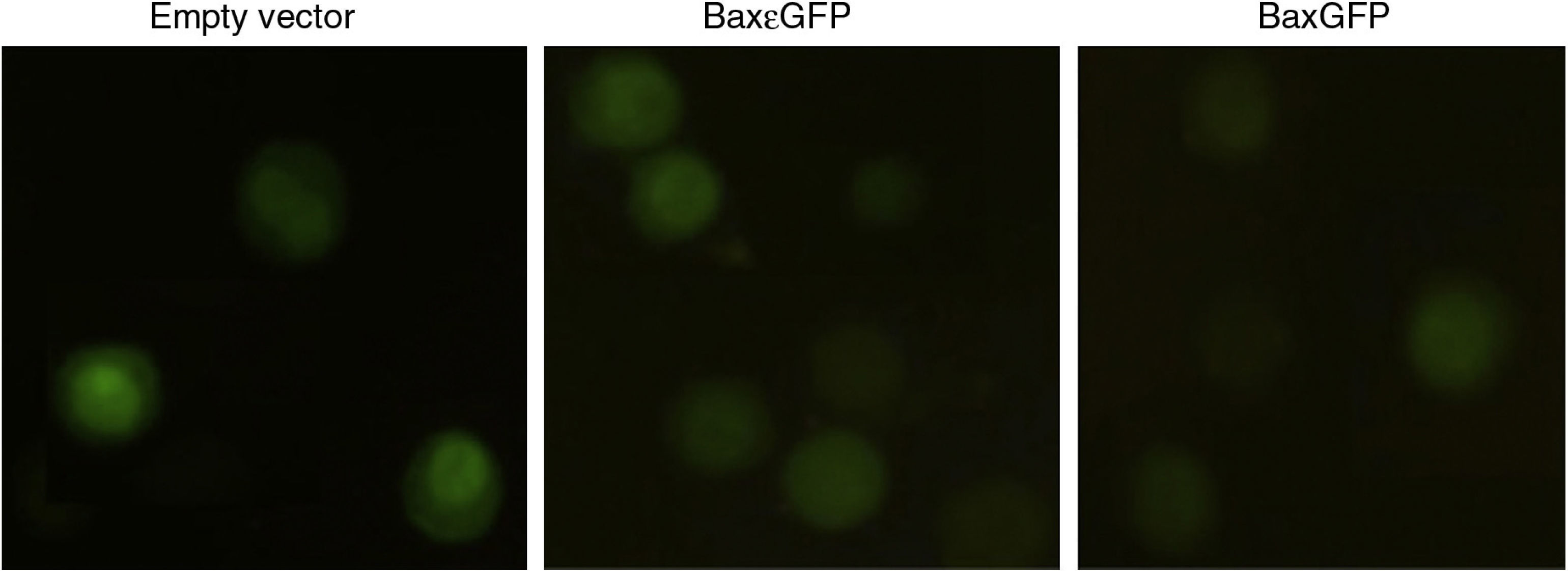
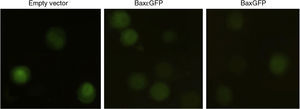
3.4Bactofection of the pBaxGFP and pBax¿GFP plasmids in tumor cells mediated by Salmonella entericaTo analyze the usefulness of a live-attenuated bacterial vector in the transportation of pBaxGFP and pBax¿GFP plasmids as well as their release into a eukaryotic cell (bactofection), Salmonella enterica SL3261 was transformed by electroporation with pEGFP-N1(empty vector), pBaxGFP and pBax¿GFP plasmids, and used in the bactofection assays of PC3 cells. Figure 4 shows that Salmonella enterica SL3261 is capable of releasing the plasmids encoding GFP, Bax¿GFP and BaxGFP in the PC3 cell line. This expression was observed 72h after the infection.
Bactofection of the plasmid BaxGFP in PC3 cells. PC3 cells of epithelial lineage were infected with transformed Salmonella enterica SL3261 for 1h and subsequently treated with gentamicin (200μ/4g/ml) for 1h to eliminate non-infecting bacteria. After cell infection (72h), they were fixed and analyzed by fluorescence microscopy to determine the expression of the GFP proteins and the recombinant proteins Bax¿GFP and BaxGFP.
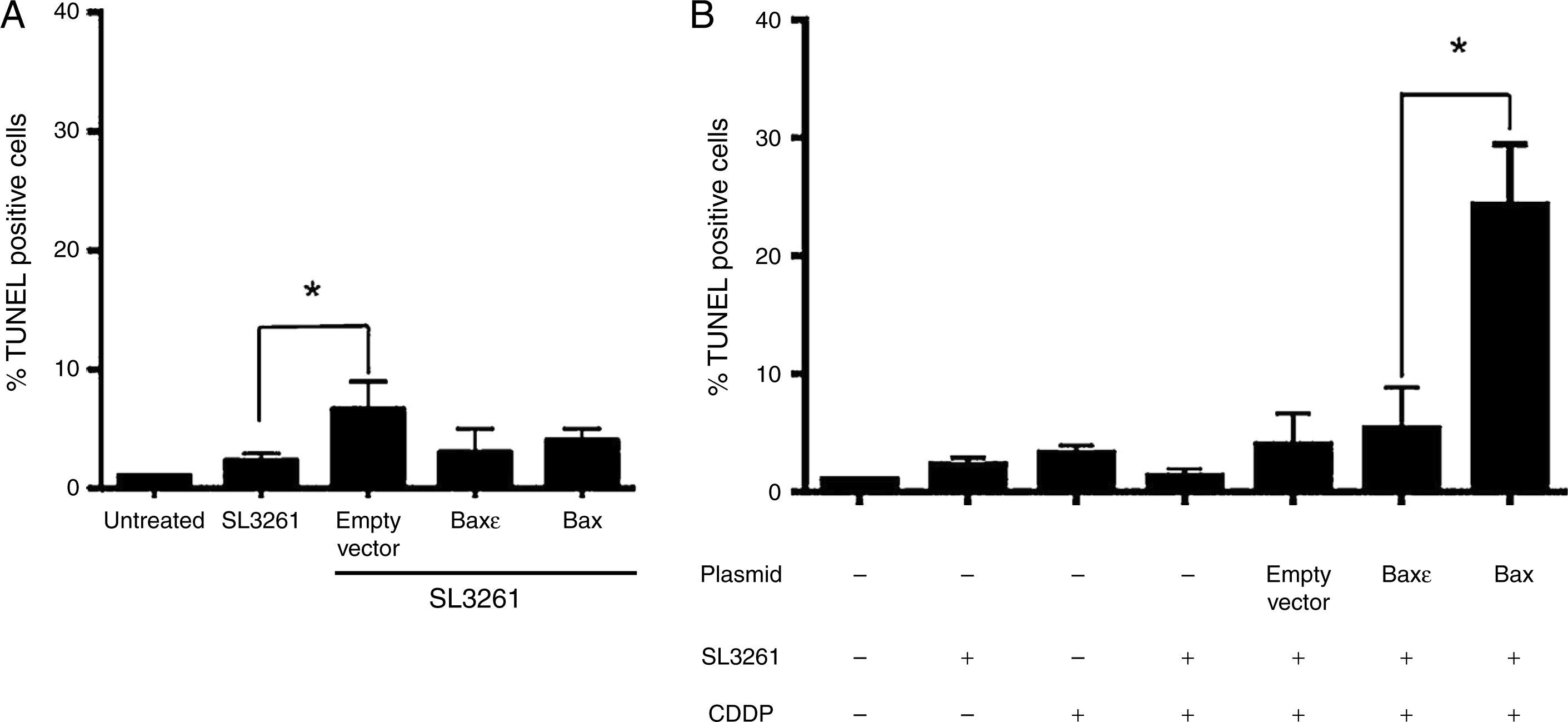
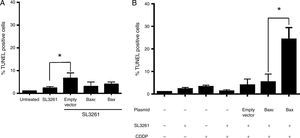
After confirming that Salmonella enterica was capable of transferring genetic material in the form of plasmids into tumor cells and that these could produce the recombinant protein BaxGFP, the next step was to evaluate whether they could induce apoptosis in PC3 cells. Therefore, we conducted the bactofection of PC3 cells using the Salmonella enterica strains transformed by pBaxGFP, pBax¿GFP or pEGFP-N1 (empty vector) as mentioned before; 72h after bactofection, cell death was determined by TUNEL assays. Bactofection of PC3 cells with pBaxGFP, pBax¿GFP, and pEGFP-N1 plasmids did not induce significant cell death, and no statistically significant differences were observed (Figure 5A).
Bactofection of pBaxGFP plasmid sensitizes PC3 cells to the effect of cisplatin. (A) PC3 cells were infected with Salmonella enterica or transformed Salmonella enterica with the pBaxGFP (labeled as Bax) or pBax¿GFP (labeled as Bax¿) plasmids or the empty vector. TUNEL assay by immunocytochemistry was conducted 72h after infection. (B) PC3 cells were treated with cisplatin (at a concentration of 20μ/4M), 12h before completing the 72h after bactofection; subsequently, the TUNEL assay by immunocytochemistry was conducted. Representative data from three independent experiments (*p<0.05).
Since bactofection with BaxGFP plasmid was not sufficient to induce apoptosis in PC3 cells, we decided to determine if BaxGFP expression was capable of sensitizing tumor cells to the effect of chemotherapy agents such as cisplatin, a drug used in the treatment of prostate cancer. Thus, 12h before completing the 72h of PC3 cell bactofection with the Salmonella enterica BaxGFP and Bax¿GFP strains and the empty vector, cells were treated with cisplatin at a concentration [20μ/4M] that induces sub-optimal apoptosis percentages when evaluated by TUNEL assay with immunocytochemistry. The treatment of PC3 cells with Salmonella enterica SL3261 transformed with the pEGFP-N1 and pBax¿GFP plasmids in the presence of 20μ/4M cisplatin, induced apoptosis in less than 10% of cells (Figure 5B). Background levels of apoptosis were observed in PC3 cells treated with dimethyl sulfoxide, the vehicle used to dissolve the cisplatin (data not shown). On the other hand, treatment of PC3 cells with Salmonella enterica SL3261 carrying the pBaxGFP plasmid in the presence of 20μ/4M cisplatin generated approximately 30% of cell death. This data suggest that the expression of the BaxGFP recombinant protein sensitized PC3 cells to the effect of cisplatin.
4DiscussionCurrently, drug resistance is a key problem in cancer chemotherapy. One mechanism involved is the overexpression of proteins that control cell death, such as the anti-apoptotic proteins Bcl-xL and Bcl-2 from the Bcl-2 family, which have been associated with chemotherapy resistance in different types of cancer, such as nephroblastoma, ovarian cancer, monocytic leukemia, squamous cell carcinoma and acute T cell leukemia.3–7 Recently, a new strategy using synthetic peptides from the BH3 region of proapoptotic proteins such as Bax and Bak as antagonists of antiapoptotic proteins has been described. Peptides derived from Bax and Bak have been reported to block the activity of the different antiapoptotic proteins such as Bcl-2 or Bcl-XL,16,17 and even MCL-1,18 and to induce apoptosis in different cell lines.8,10,11 However, the therapeutic use of these peptides in vivo models entails the problem of degradation as well as low specificity against the tumor cell, prompting the need to evaluate several peptide-release systems or sequences encoding these peptides.
In the present study, the capacity of the live-attenuated bacterial vector Salmonella enterica serovar Typhimurium (strain SL3261) to mediate bactofection of prostate cancer cells with sequences encoding an antagonist peptide of the Bax protein BH3 region was evaluated. Moreover, we assessed the induction of apoptosis in this cell line to reverse drug chemoresistance associated with overexpression of proteins such as Bcl-XL.19
As stated before, two plasmids were constructed: one, carrying the sequence of the BH3 region peptide from the Bax protein fused to the GFP reporter protein (pBaxGFP). The other, carrying the sequence of the BH3 region of the Bax protein with a substitution of leucine by glutamic acid at position 8 that inhibits its specificity for Bcl-2 family antiapoptotic proteins (pBax¿GFP).20 These plasmids were used to transfect PC3 cells and showed that BaxGFP and Bax¿GFP proteins were adequately expressed and had the capacity to interact with mitochondria. Our results showed that the recombinant protein BaxGFP co-localized with PC3 cell mitochondria, an area in which antiapoptotic proteins such as Bcl-XL are distributed.21 This colocalization was not observed where the empty vector or the recombinant protein Bax¿GFP were present due to the leucine replacement with glutamic acid, as previously mentioned.
The results also revealed that PC3 cells transfected with the pBaxGFP plasmid induced more apoptosis compared to cells transfected with the empty vector or the Bax¿GFP plasmid; the difference with the latter was statistically significant (p<0.05), suggesting that the BaxGFP recombinant protein is capable of inducing apoptosis of tumor cells. These results are consistent with previously reported findings by Li R et al., in which the use of antagonist peptides from the BH3 region of Bax and Bak efficiently mediated the release of cytochrome C, using synthetic peptides in a head and neck squamous cell carcinoma model.10 Although these results are encouraging in terms of promoting apoptosis in tumor cells, a transport mechanism is required to carry and selectively release plasmids toward and into the tumor cells.
For this reason, several research groups have begun to evaluate the usefulness of Salmonella enterica as a bacterial vector with great selectivity for the tumor microenvironment,13 and with the ability to transport plasmids toward tumor cells.14,22 Although the mechanism through which Salmonella enterica releases genetic material in mammal cells is not exactly known this bacterium also possesses the intrinsic ability to induce an immune response capable of slowing tumor growth or eliminating it.23,24 Our study evaluated the ability of Salmonella enterica SL3261 to mediate bactofection of the plasmid encoding the recombinant protein BaxGFP, so that tumor cells per se can produce the antagonist peptides against Bcl-XL and, hence, become apoptotic.
The results obtained in this study confirmed that Salmonella enterica was able to transfer the plasmid pBaxGFP that encodes the antagonist peptide (Figure 4). However, when apoptosis of these cells was analyzed no significant cell death activity was observed, unlike that observed in the transfection assays with lipofectamine and when using the pBax¿GFP plasmid as a control. Perhaps, the amount of transferred plasmid by bactofection was lower than the plasmid quantity used in the transfection assays with lipofectamine (Figure 5A).
On the other hand, when analyzing whether bactofection of the pBaxGFP plasmid into PC3 cells with Salmonella enterica would foster sensitization to apoptosis after treatment with cisplatin, we detected that only PC3 cells treated with Salmonella carrying the pBaxGFP plasmid induced increased apoptosis compared to controls (Figure 5B). These findings further support those studies suggesting that Salmonella enterica is an efficient carrier of sequences encoding inhibitory25,26 or immune modulating molecules.27–29 In this case, we propose that Salmonella enterica is an efficient live-attenuated bacterial vector for the transport of heterologous molecules including genetic material (plasmid) toward tumor tissue and that tumor cells per se produce the peptides that will sensitize them to chemotherapeutic drugs and promote their death by apoptosis.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingR.L.P. thanks the financial support from Fondos Federales (HIM-2013-028 SSA. 1068, HIM-2014-032 SSA.1137).
Conflict of interestTha authors declare no conflicts of interest of any nature.
The authors thank the Directorate for Research Support and Postgraduate Programs at the University of Guanajuato for their support in the translation and editing of the English version of this article.