The relevance of the microenvironment in the initiation, promotion, and progression of cancer has been postulated. Mesenchymal stem cells (MSCs) have been identified as important components of the tumor stroma, which are capable of affecting the development of cancer through various mechanisms. In particular, MSCs immunosuppressive properties play an important role. It has been shown that bone marrow-derived and other healthy tissues-derived MSCs are capable of regulating the immune response by affecting the activation, maturation, proliferation, differentiation, and effector function of cells of the immune system, such as neutrophils, macrophages, dendritic cells, natural killer cells (NK) and T-lymphocytes. Similar mechanisms have been identified in MSCs associated with different types of tumors, where they generate an immunosuppressive microenvironment by decreasing the cytotoxic activity of T-lymphocytes and NK cells, skew macrophage differentiation towards an M2 phenotype, and decrease the secretion of Th1-type cytokines. Also, the cytokines, chemokines, and factors secreted by the transformed cells or other cells from the tumor stroma are capable of modulating the functions of MSCs.
Se ha postulado la relevancia del microambiente en la iniciación, promoción y progresión del cáncer. Las células troncales mesenquimales (MSC, del inglés mesenchymal stem cells) se han identificado como un componente fundamental del estroma tumoral. Estas son capaces de favorecer el desarrollo del cáncer mediante varios mecanismos. En particular, sus propiedades inmunosupresoras juegan un papel importante. Se ha demostrado que las MSC de médula ósea y otros tejidos sanos son capaces de regular la respuesta inmune al afectar la activación, maduración, proliferación, diferenciación y función efectora de las células del sistema inmune, como neutrófilos, macrófagos, células dendríticas, células NK y linfocitos T. Mecanismos similares se han identificado en las MSC asociadas con diferentes tipos de tumores, donde estas se encargan de generar un microambiente inmunosupresor al disminuir la actividad citotóxica de linfocitos T y células NK, polarizar a los macrófagos hacia un fenotipo M2, y disminuir el patrón de secreción de citocinas tipo Th1. Asimismo, las citocinas, quimiocinas y factores secretados por las células transformadas u otras células del estroma tumoral son capaces de modular las funciones de las MSC.
Despite the efforts in the development of new strategies against cancer, many cases remain unresponsive to chemotherapy, radiotherapy and immunotherapy treatments, whose main target are the neoplastic cells; however, several studies have evidenced the importance of the tumor microenvironment in the initiation, promotion, and progression of cancer. The tumor microenvironment has several components, such as transformed cells, leukocytes, fibroblasts, endothelial cells, pericytes and mesenchymal stem cells (MSCs). These cells are responsible for the secretion of cytokines, chemokines, peptides, metalloproteases and components of the extracellular matrix, which altogether contribute to the generation of a permissive microenvironment for the development of neoplasia.1
In this context, the interaction between transformed cells and tumor stromal cells occurs through cell-cell contact and secreted factors, which act in an autocrine and a paracrine form. It has been shown that in response to signals generated by transformed cells, cellular components of the tumor stroma can proliferate, differentiate, migrate, secrete cytokines, chemokines and growth factors, remodel the extracellular matrix, induce angiogenesis, and recruit cells of the immune system. These are important processes that favor the progression of cancer.
Inflammation plays a key role in all stages of cancer development. Due to their persistence, the areas where a tumor develops are considered as wounds that do not heal. They are characterized by the presence of immune cells, such as dendritic cells (DC), macrophages, neutrophils, eosinophils, T-lymphocytes, and natural killer cells (NK); as well as cytokines, such as tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-1 (IL-1), and transforming growth factor-β (TGF-β). It has been shown that the inflammatory tumor microenvironment favors the migration of MSCs to these sites,2-5 where they stimulate the development of the tumor through the following mechanisms:
- 1.
MSCs are precursors of approximately 20% of the tumor-associated fibroblasts (TAFs), which express α-smooth muscle actin (αSMA).2 The exposure of bone marrow or adipose tissue-derived MSCs to tumor cells produced factors such as TNFα and IL-1β, induce their differentiation towards TAFs,3,6 capable of secreting pro-inflammatory chemokines (CCL2/MCP-1, CXCL8/IL-8, and CCL5/RANTES) that stimulate the development of the tumor.7
- 2.
MSCs promote angiogenesis: pro-inflammatory cytokines like IFNγ and TNFα, normally expressed in the tumor microenvironment, activate MSCs to express the hypoxia-inducible factor 1-alpha (HIF1α), and secrete high amounts of vascular endothelial growth factor (VEGF).8,9
- 3.
MSCs secrete trophic factors promote chemoresistance10 and the maintenance of tumor-initiated cells.11,12
- 4.
MSCs stimulate the epithelium-mesenchyme transition, the invasion and metastasis of tumor cells through the secretion of CXCL12/SDF-1, CCL1, CCL8/IL-8, IL-6 and prostaglandin E2 (PGE2).3,13 In carcinoma cells, MSCs decrease the expression of the epithelial marker E-cadherin and induce the expression of mesenchymal markers such as fibronectin and vimentin, as well as the transcription factor snail.13 In addition, it has been shown that MSCs-secreted TGF-β participates in the migration of breast cancer cells.14
- 5.
MSCs are capable of regulating the immune response.
In this review, we focused on the analysis of the immunosuppressive capacity of MSCs and the effect of this feature in the development of a tumor.
2Mesenchymal stem cellsMSCs are adult pluripotent stem cells originally isolated from bone marrow (BM).15 Currently, their isolation from different tissues have been achieved, but until now an MSCs marker has not been defined. Therefore, the International Society of Cell Therapy has established a series of guidelines to define these cells. It is necessary that MSCs show the following characteristics: positive expression of CD105, CD73, CD90; low levels of HLA-I and negative for HLA-II, CD11b, CD14, CD34, CD45, and CD31; likewise, these cells must possess adipogenic, osteogenic and chondrogenic differentiation capacity.16,17
3Immunosuppressive properties of MSCsImmunosuppressive properties of MSCs have been demonstrated using different in vitro and in vivo study models. MSCs can regulate the function of innate immune system cells (neutrophils, macrophages, and NK cells) and adaptive immune system cells (DCs, B lymphocytes, and T-lymphocytes). During the regulation of the immune response, MSCs use mechanisms that involve cell contact and secretion of different molecules.18-24 Early studies have focused on the study of the immunosuppressive mechanisms of the bone marrow-derived MSCs (BM-MSCs). However, several laboratories are currently investigating this property in other healthy tissues and tumor-derived MSCs. First, we will describe the mechanisms of immunosuppression exerted by MSCs from healthy tissues; then we will focus on the mechanisms found in tumor-derived MSCs.
3.1Mechanisms of immunosuppression exerted by MSCsThe information generated so far has made it possible to propose a model in which MSCs present in tissues appear to be in a steady state, with minimal or absent immunoregulatory molecules expression; however, upon an inflammatory environment, MSCs become activated, and the expression of immunosuppressive molecules is induced or increased. It has been shown that IFNγ alone or in combination with TNF-α, IL-1α, IL-1β or IL-17 is the main cytokine involved in this process.20,22,25 Moreover, IFNγ induces the expression of molecules on the membrane of MSCs, such as the intercellular adhesion molecule 1 (ICMA-1), vascular cell adhesion molecule 1 (VCAM-I), programmed death-ligand 1 (PD-L1), Jagged-1 and 2, and human leukocyte antigen G1 (HLA-G1). These molecules are involved in the regulation of the immune response.
IFNγ induces increased expression of ICAM-1/CD54 and VCAM-I/CD106 in MSCs.26 Both molecules are important in the recruitment of leukocytes to sites of inflammation and allow high-affinity interaction between MSCs and immune cells. When VCAM-I and ICAM-I are blocked with antibodies, the negative effect of MSCs on the proliferation of T-lymphocytes decreases, which is partially re-established.27 A mechanism by which the interaction between these adhesion molecules with their respective ligands [lymphocyte function-associated antigen 1 (LFA-1) and very late antigen-4 (VLA-4)] in T-lymphocytes can inhibit their proliferation, is likely to occur because this event induces the expression of the cytotoxic T-lymphocyte antigen 4 (CTLA-4).28,29 CTLA-4 is a negative regulator of the immune response, and T regulatory lymphocytes (Treg) express it constitutively. Recently, our group demonstrated that the interaction between BM or umbilical cord blood (UCB) derived MSCs and the activated T-lymphocytes increases the expression of CTLA-4 in CD4+ T-lymphocytes. This interaction was associated with a lesser proliferation of this cell type, increased secretion of IL-10 and high concentrations of IFNγ, suggesting the differentiation of Treg lymphocytes, although the regulatory function of the cell populations generated in these co-cultures was not assessed. The results indicate the importance of cell-cell contact in the capacity of the MSCs to promote the differentiation of these populations.24
Another evidence that highlights the importance of cell contact in the immunosuppression exerted by MSCs is the induction of the expression of PD-L1 by IFNγ. The activation of the PD-1/PD-L1 pathway is important for the termination of the immune response, tolerance to self-antigens and foreign antigens, and it contributes to the generation of the immunosuppressant environment found in tumors in an important way. Using BM, UCB or placenta-derived (PL) MSCs, it has been shown that PD-L1 participates in the immunosuppression of MSCs on activated T-lymphocytes by decreasing their proliferation and affecting their differentiation. Through this molecule, MSCs decrease the differentiation of Th17 cells and the secretion of IL-17.30-34 A recently proposed mechanism indicates that MSCs secrete IL-25, which acts in an autocrine way by stimulating the expression of PD-L1, which in turn induces a decrease in the secretion of IL-17.35 In addition, PD-L1 promotes the differentiation of Th1 lymphocytes to Treg Foxp3+ lymphocytes36; also, an increase in the expression of PD-1 in CD4+CD25+ activated lymphocytes in the presence of MSCs has been observed.31,32
Notch/Jagged is another important pathway in the immunosuppression exerted by MSCs, which contributes to a decreased proliferation of T-lymphocytes when they are activated in the presence of MSCs.37 Furthermore, it leads to the expansion of Treg lymphocytes.38 Also, the interaction between MSCs and hematopoietic stem cells CD34+ generates a population of regulatory DCs through the activation of the Notch pathway. These populations are characterized by high secretion of IL-10 and low secretion of IL-12, low capacity to activate T-lymphocytes and the capability to stimulate the differentiation of Treg cells specific to alloantigens.39
On the other hand, HLA-G1 is a molecule involved in the early interaction between MSCs and activated T-lymphocytes; apparently, the initial contact between both cell types is crucial to triggering the immunosuppression and essential to induce the secretion of IL-10 in T-lymphocytes. It has been shown that the elimination of the cell contact using transwell chambers,24 or the use of antibodies directed to HLA-G1 reduces the concentration of IL-10 in the supernatants of T-lymphocytes-MSCs cocultures.40 Similarly, IL-10 stimulates the membrane expression of HLA-G1 in MSCs and the secretion of HLA-G5 through a positive feedback mechanism, which in turn induces an increased expression of IL-10 in T-lymphocytes.40,41 This positive feedback mechanism promotes an anti-inflammatory environment characterized by the generation of T CD4+ lymphocytes with high expression of CD10 (a subunit of the IL-10 receptor), IL-10, and Treg populations CD4+ CD25+ Foxp3+.24,40,42,43
One of the main molecules involved in the immunosuppressive activity exerted by MSCs is the intracellular enzyme indoleamine-2, 3-dioxygenase (IDO), which is responsible for the catabolism of the amino acid tryptophan, depleting it from the environment and generating metabolites as kynurenine. IFNγ induces the expression of IDO in MSCs; the effects of its enzymatic activity are reflected in the modulation of the function of various components of the immune system. For example, it affects the proliferation and cytotoxic activity of NK cells and inhibits the maturation of DCs. It is known that depending on the cytokines to which macrophages are exposed they can be polarized towards a pro-inflammatory phenotype (M1) or an anti-inflammatory (M2) phenotype. It has also been shown that the activity of IDO induces the skew of monocytes towards an M2 phenotype, while it decreases the proliferation of T-lymphocytes, inhibits the differentiation of Th17 populations and favors the differentiation of T regulatory lymphocytes Foxp3+ and IL-10+ IFNγ+ CD4+ or Tr1.18,21,44
Another mediator involved in the immunosuppressive mechanism of MSCs is PGE2, which is constitutively expressed by MSCs although its secretion is increased in an inflammatory environment. PGE2 decreases the proliferation of T-lymphocytes and promotes the differentiation of T regulatory lymphocytes CD4+ CD25+ Foxp3+ and Tr1.33,45 Also, PGE2 decreases the differentiation of monocytes to CDs, skews macrophages to an M2 phenotype and decreases the proliferation and cytotoxic activity of NK cells.46,47
As it can be noticed, several mechanisms employed by MSCs in the regulation of the immune response entail an increase in the secretion of the anti-inflammatory cytokine IL-10. It has been shown that high concentrations of this cytokine generated in cocultures of MSCs with activated T-lymphocytes induce the expression of PD-1 in populations of Treg lymphocytes CD4+ CD25+, increasing its immunosuppressive capacity.31 In addition, IL-10 decreases the maturation of DCs and their ability to produce IL-12.48 MSCs secrete high amounts of TGF-β, which decreases the proliferation of NK cells49 and favors the differentiation of Treg lymphocytes CD4+ CD25+ Foxp3+.50 All the previous findings mean that the mechanisms of immunosuppression exerted by MSCs, when occurring in a tumor context, interfere with the immune response against cancer.
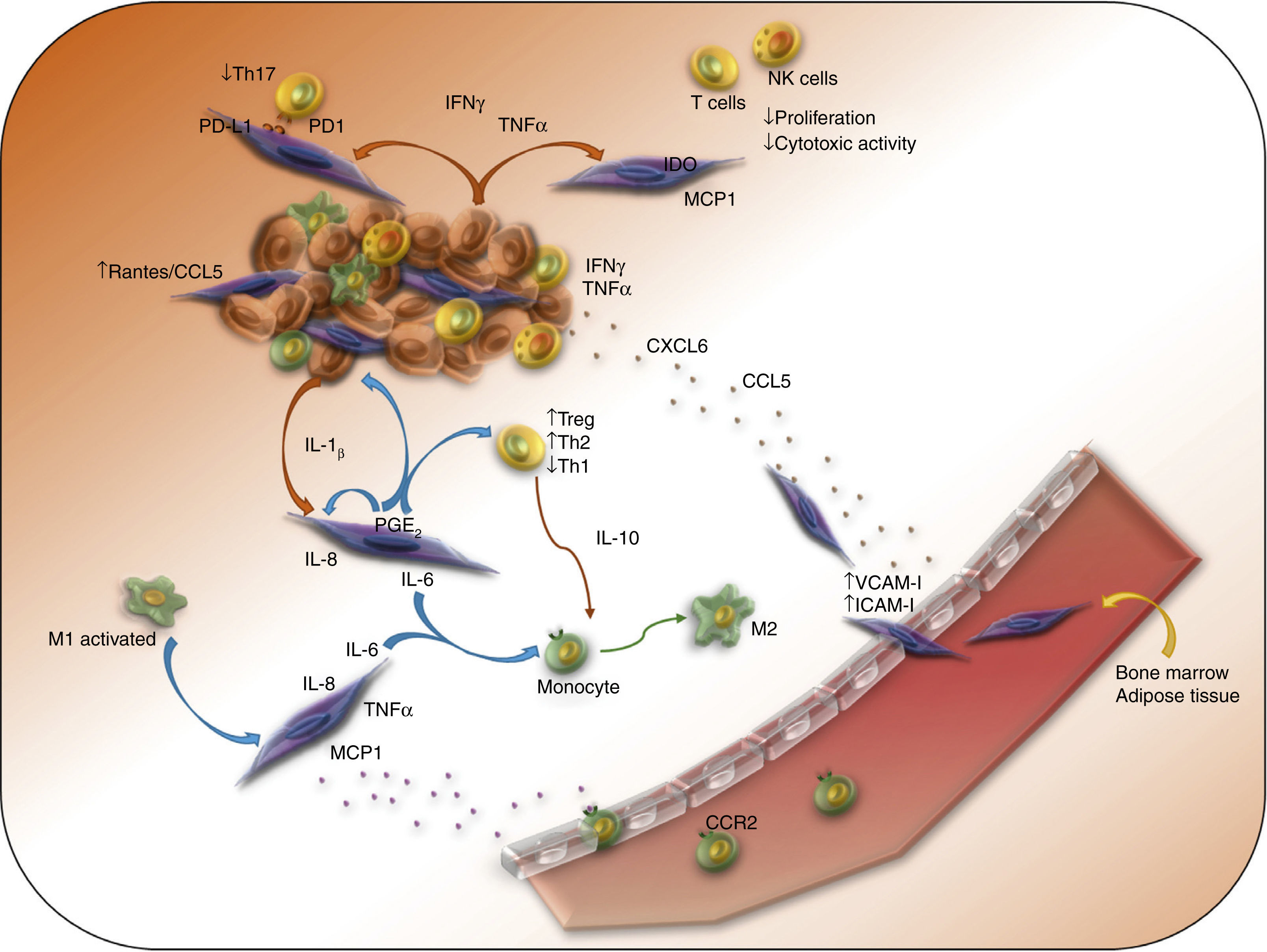
3.2Mechanisms of immune suppression observed in MSCs derived from tumorsSome evidence suggests that BM or adipose tissue-derived MSCs can migrate to sites of tumor development, characterized by persistent inflammation and secretion of different chemokines, whose receptors are mainly expressed by MSCs (CCR1, 4, 5, 7, 9 and 10, as well as CXCR4, 5 and 6).2-4 High concentrations of pro-inflammatory cytokines present in the tumor environment promote the expression of ICAM-I and VCAM-I in MSCs, which induces its adhesion to the endothelium and its subsequent extravasation (Figure 1). Once located in the tumor stroma, MSCs favor the evasion of the immune response by transformed cells. In a murine melanoma model, it was observed that MSCs promote tumor development mainly in an allogenic context, which highlights the importance of an inflammatory environment in the immunosuppressive activity of these cells.51 Further studies showed the importance of IFNγ and TNF-α in this process because they induce an increase in the expression of enzymes, cytokines, chemokines and growth factors by the MSCs, whose immunosuppressive activity favor the promotion and progression of the tumor.52,53
MSCs form part of the tumor stroma, and they promote tumor development through different immunosuppressive mechanisms. The presence of pro-inflammatory cytokines such as IFNγ, TNF-α and IL-1β activate MSCs, which express different immunosuppressive factors and chemokines that attract immune cells to the sites of tumor development, where T-lymphocytes and macrophages and NK cells are polarized towards an anti-inflammatory phenotype.
The role of the IFNγ and TNF-α in the immunosuppressive function of the MSCs on the immune cells at the tumor stroma has been analyzed using murine models. The effect of these cytokines is relevant in the interaction of MSCs with macrophages on tumor development sites. It has been shown that TNF-α-activated MSCs, or lymphoma-derived MSCs recruit monocytes and macrophages more efficiently than BM-derived MSCs through the secretion of ligands of CCR2.54 In particular, M1 macrophages, which have antitumor activity, are capable of activating MSCs to an immunosuppressive phenotype with high expression of mRNA inducible nitric oxide synthase (iNOS), IL-6, COX-2, and MCP1/CCL2. The latter mediates its recruitment to the site of development of the tumor through its interaction with CCR2 expressed in macrophages, where MSCs-secreted IL-6 can polarize macrophages towards an M2 phenotype (Figure 1).53,55 Similar results have been found in pancreas cancer, where tumor tissue-derived MSCs have greater expression of COX-2, TGF-β, IL-10 and IL-6 mRNA than those derived from a healthy pancreas. In this model, IL-10 and IL-6 promote the polarization of macrophages to an M2 phenotype, with reduced expression of iNOS and increased expression of arginase 1 and CD206.55 In addition, it has been noted that TNF-α-activated MSCs induce an immunosuppressive phenotype in bone marrow-derived neutrophils of normal mice or from the spleen of mice carrying tumor, characterized by an increase in the activity of arginase and iNOS.56 Altogether, these results indicate that TNF-α-activated MSCs favor the development of tumors through the polarization of macrophages and neutrophils towards an anti-inflammatory phenotype.
As previously mentioned, the enzymatic activity of IDO is very important in the mechanisms of immunosuppression exerted by MSCs. Regarding this subject, Ling et al. developed a model of murine humanized MSCs capable of expressing IDO (MSCs-IDO). It was determined that through the regulation of the immune response, MSCs-IDO favors the development of melanoma and lymphoma in vivo. In melanoma tumors, there is a reduction in the infiltration of CD8+ T-lymphocytes, although no differences are observed in T CD4+ lymphocytes, NK or B lymphocytes.57 The role of IDO has also been shown in the development of head and neck squamous cell carcinoma. The presence of cells with features of MSCs have been identified, and these cells are capable of reducing the proliferation of CD4+ and CD8+ T-lymphocytes, through the activity of IDO (Figure 1).58
Also, the immunosuppressive effect of MSCs on T-lymphocytes and NK cells through other mechanisms has been demonstrated. In an in vitro model of co-culture of BM-MSCs, peripheral blood mononuclear cells, and breast cancer cells, it has been observed that BM-MSCs protect tumor cells from the immune system elimination by inhibiting the function of NK cells and T cytotoxic lymphocytes (Figure 1). In addition, they favor the differentiation of regulatory T cells. This effect is accompanied by the increase of type Th2 cytokines and TGF-β, as well as the decrease of Th1 type cytokines.59 Similar results have been obtained using in vivo models, in which MSCs also decrease the strength of the cytotoxic capacity of NK cells and CD8+ T-lymphocytes, induce the differentiation of Treg lymphocytes, increase serum levels of IL-4, IL-10, and TGF-β, and diminish the levels of IFNγ.60 This evidence indicates that MSCs abolish the cellular immune response against transformed cells resulting in increased tumor development.
In addition to the effect of MSCs on the immune cells present in the tumor, it has been demonstrated that cytokines, chemokines, and factors secreted by transformed cells and immune cells also influence the immunosuppressive ability of MSCs. As an example, we can cite the effect of IL-1β and TNF-α secreted by breast cancer cells. TNF-α acts on BM-MSCs through TNF-RI and TNF-RII by the activation of the NF-κB pathway, resulting in the secretion of pro-inflammatory chemokines, such as CCL2 and CXCL8/IL-8, which contribute to the inflammatory tumor environment since they promote the migration of monocytes.7 Similarly, it has been observed that the supernatant of metastatic breast cancer cell lines, partly through the production of IL-1β and the activation of the NFκB pathway in BM-MSCs, induces increased expression of CXCL1, CXCL6, and CXCL8, which favors, even more, the tumor development in a mouse xenotransplant in vivo model.61,62 In addition, colon and breast carcinoma cell lines induce an increased expression of PGE2 in MSCs mainly through IL-1α and IL-1β. PGE2, which acts in an autocrine way, together with IL-1, induces the expression of IL-6, IL-8, and CXCL1.13 These results indicate that the exposure of MSCs to TNF-α and IL-1β secreted by transformed cells, induces the activation of the NFκB pathway in MSCs and the secretion of pro-inflammatory chemokines.
The effect of transformed cells on the function of MSCs is not exerted only through secreted factors; it has been demonstrated that the cell-cell contact is very important. The interaction of MSCs and breast cancer cells induces an increase in the expression of RANTES/CCL5 in MSCs; this event requires close contact between both cell types, and occurs more rapidly than the increase in the secretion of other cytokines (Figure 1).13,63 It has been observed that the treatment of BM-MSCs with conditioned medium from head and neck squamous carcinoma cell lines increases the expression of CD54/ICAM-I in MSCs.61 This event is important for the interaction of immune cells and tumor cells with MSCs. Regarding this matter, it has been observed that the contact between BM-MSCs and leukemic cells through VLA-4 and VCAM-I induces an increase in the expression of IL-8, IL-6, CCL2, and VCAM-1 in MSCs, which contributes to the survival of transformed cells.64 These results highlight the importance of the cell-cell interaction between tumor cells and MSCs.
As mentioned before, immune cells are a key component of the tumor stroma, and they also have influence in the biology of MSCs. LPS-activated macrophages induce the secretion of pro-inflammatory cytokines as IL-6, IL-8 and TNF-α in MSCs through the activation of NFκB and STAT3. Also, these MSCs are capable of favoring the proliferation of transformed cells and increasing the development of tumor in a mouse model of gastric cancer.65
In addition, the analysis of the effect of tumor-infiltrating lymphocytes activated with melanoma cells on BM-MSCs determined that lymphocyte secreted factors can polarize MSCs to a Th1 phenotype, characterized by the transcription of pro-inflammatory genes and immunoregulatory genes, such as IDO.66 Several authors have proposed the ability of MSCs to acquire a pro-inflammatory or an anti-inflammatory type, depending on the context, and the presence of TLR-3 or TLR-4 ligands. Until now, these results are contradictory.37,67-69 Jin et al. (2016) conducted a single-cell transcriptomic analysis.70 They determined that IFNγ and TNF-α are the main cytokines involved in the activation of MSCs by the induction of the expression of pro-inflammatory cytokines and chemokines as IL-12, IL-15, CXCL9, CXCL10, CXCL11, as well as immunosuppressant factors such as IDO, HLA-G, and PD-L1. The authors determined both patterns of expression in the same cell, suggesting that the presence of pro-inflammatory or anti-inflammatory phenotypes observed in MSCs is not due to the existence of two populations.70 It is important to determine which tumor microenvironment stimuli is able to polarize MSCs to an anti-inflammatory phenotype, as it has been observed in different in vitro and in vivo models, where the immunosuppressive phenotype predominates. This information would allow the possibility of generating pro-inflammatory cell populations capable of inducing an immune response against the tumor, or anti-inflammatory cell populations aimed to promote the acceptance of a transplanted organ or the healing of an injury.
4Conclusions and prospectsMSCs form part of the tumor stroma, and they maintain constant communication with transformed cells and immune cells (which are part of the tumor microenvironment) through the expression of membrane molecules and secreted factors. These cells and factors regulate the function of MSCs through the secretion of IFNγ, TNF-α, and IL-1β, and activate MSCs to express and secrete different immunosuppressive molecules as IDO, PD-L1, VCAM-I, ICAM-I, COX-2, PGE2, TGF-β, and HLA-G, as well as pro-inflammatory chemokines as CXCL1, CXCL6, CXCL8, CXCL9, CXCL10, and CXCL11. These last chemokines favor the migration of immune cells to sites of tumor development; once located, are regulated by MSCs regarding function and their skew towards an immunosuppressive phenotype. The tropism of MSCs to inflammatory environments makes them suitable for their use as therapeutic weapons, by designing MSCs able to express molecules directed against neoplastic cells or molecules capable of inducing an effective immune response.
Conflict of interestThe authors declare no conflicts of interest of any nature.
To M.Sc. Ignacio Martínez Martínez for the revision of the manuscript and his helpful comments.