Central venous catheters (CVC) are needed for monitoring and treatment of critically ill patients; however, their use increases the risk of bacteremia. The aim of the study was to quantify the incidence of central venous catheter-related bacteremia (CVCRB) and to identify factors associated with this infection.
MethodsA prospective cohort study was conducted in a concentration hospital of western Mexico. The association of CVCRB and study variables was investigated using multivariate Cox regression analysis.
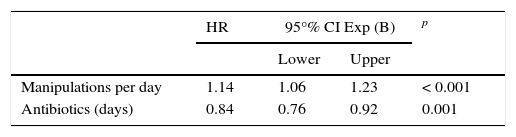
ResultsTwo hundred and four patients with CVC were studied. The mean age was 4.6 years; 66.2% were male. Insertion sites of the catheters were subclavian vein 72.5% (n = 148), jugular vein 20.1% (n = 41) and femoral vein 7.4% (n = 15). CVCRB incidence was 6.5 events/1,000 catheter-days; microorganisms identified were gram-positive cocci 37.5% (n = 6), gram-negative bacilli 37.5% (n = 6) and Candida albicans 25% (n = 4). It was observed that the increase in catheter manipulations per day was associated with bacteremia (HR 1.14, 95% CI 1.06–1.23), whereas the use of intravenous antibiotics showed a protective effect (HR 0.84, 95% CI 0.76–0.92).
ConclusionsIn addition to the strategies of maximum caution when placing or manipulating the catheter, we recommend decreasing, as much as possible, disconnects between the CVC and the infusion line. Antibiotics showed a protective effect, but the outcome is uncertain and the promotion of antimicrobial resistance should be considered.
El catéter venoso central (CVC) es necesario para la monitorización y tratamiento de pacientes en estado crítico; sin embargo, su uso incrementa el riesgo de bacteriemia. El objetivo del estudio fue cuantificar la incidencia de bacteriemia relacionada con catéter venoso central (BRCVC) e identificar los factores asociados con esta infección.
MétodosSe realizó un estudio de cohorte prospectivo en un hospital de concentración del occidente de México. Para conocer la asociación entre BRCVC y las variables en estudio, se realizó un análisis multivariado con regresión de Cox.
ResultadosSe estudiaron 204 pacientes con CVC. La edad promedio fue de 4.6 años; el 66.2% fue del sexo masculino. Los sitios de inserción del catéter fueron la vena subclavia (72.5%, n = 148), la vena yugular (20.1%, n = 41) o la vena femoral (7.4%, n = 15). La incidencia de BRCVC fue de 6.5 eventos por 1,000 días catéter. Los microorganismos identificados fueron cocos Gram positivos (37.5%, n = 6), bacilos Gram negativos (37.5%, n = 6) y Candida albicans (25%, n = 4). Se observó que la mayor manipulación del catéter por día se asoció con bacteriemia (RR 1.14, IC95% 1.06–1.23), mientras que el uso de antibióticos intravenosos mostró un efecto protector (RR 0.84, IC95% 0.76–0.92).
ConclusionesAdemás de las medidas máximas de precaución al momento de colocar o manipular el catéter, es conveniente disminuir lo más posible las desconexiones entre el equipo de venoclisis y el CVC. Los antibióticos mostraron un efecto protector; sin embargo, se debe considerar el riesgo de favorecer resistencias antimicrobianas.
Central venous catheters (CVC) are needed for monitoring and treatment of critically ill patients; however, their use increases the risk of bacteremia (OR 4.51 95% CI 2.49–8.18, p < 0.001).1–4 The presence of intravascular devices has been the main cause of hospital bloodstream infections, with a mortality rate of 25%.5–11
Microbial flora of the skin migrates through both outer and inner surfaces of the catheter or enters the bloodstream through contaminated solutions. In short-term intravascular devices, most central venous catheter-related bloodstream infections (CVCRB) are originated by the colonization of the outer surface, while in the long-term catheters infections occur mainly by contamination of the inner surface.9,12–14
An hour after the catheter is colonized, microorganisms can be identified 4cm away from the site of colonization.2,12 Subsequently, pathogens adhere to the catheter surface and form a protective film of extracellular polymers that surrounds bacteria and retains nutrients. Under these conditions, microorganisms may be resistant to antimicrobial treatment and phagocytic activity of the immune system.2,15
Clinical diagnosis of CVCRB is not specific. Microbiological analyses to confirm infections are performed in only 15 to 39% of patients with clinical manifestations. Differential time to positivity of blood cultures allow greater accuracy in diagnosis.6,16,17
Different conditions have been associated with the increased risk of CVCRB: age of the patient (< 10 years), catheter insertion without sterile barriers, difficulties during placement, bacterial colonization of the insertion site, placement in the femoral vein, total parenteral nutrition, blood transfusions and duration of catheter placement > 7 days.7,10,11,14,18–23 Since strategic planning for prevention requires understanding the epidemiology of the CVCRB, the objective of this study was to quantify the incidence of CVCRB and to identify the main risk factors in a concentration hospital in western Mexico.
2Patients and methodsA prospective cohort study was conducted in the Nuevo Hospital Civil de Guadalajara Dr. Juan I. Menchaca (HCGJIM) in Guadalajara, Jalisco, Mexico, from March 18, 2011 to June 24, 2012. The study was approved by the Ethics and Research Committees of the institution.
Patients in the pediatric Intensive Care Unit (ICU) and in the pediatric Emergency Room (ER) who had a CVC placed and had no bloodstream infection at the time of placing entered the cohort. Although patients were transferred to another department of the HCGJIM, their surveillance continued. Exclusion criteria were patients who died, who had catheter removal, those who presented bloodstream infection or were transferred to another hospital. Aseptic technique with povidone-iodine was performed prior the insertion of the intravascular catheter as well as a thorough washing of hands of the medical personnel; maximum sterile barriers (gloves, gown, cap and mask) were used during the procedure.24
2.1VariablesData from the cohort of patients, such as age, sex, diagnosis, anatomical region where the catheter was placed, type of catheter and complications during insertion were registered from the clinical files. The use of mechanical ventilation, total parenteral nutrition, antibiotics and transfusions were monitored daily. The number of manipulations of the catheter was quantified in nursing records. Any procedure requiring disconnection of both CVC and venoclysis equipment was defined as manipulation of the catheter.
2.2Sampling procedureAt least two samples for blood cultures were collected from patients who had clinical manifestations suggestive of infection related to the catheter (fever, hypothermia, tachycardia, bradycardia, leukocytosis, leukopenia, erythema or secretion from the catheter entry site): one through the CVC and the other from a peripheral vein. In each Pediatric FAN BacT/ALERT® PF (bioMérieux) bottle, ≥ 2ml of blood were inoculated. Before sampling, aseptic techniques were conducted with povidone-iodine at the puncture site and into the lumen of the catheter entry. Similar amounts of blood were inoculated in each blood culture bottle.25
Cultures were incubated in the automatic system of detection of microbial growth BacT/ALERT® 3D (bioMérieux). In case of bacterial growth, the system recorded the time of detection of positivity. Positive cultures were reseeded in blood and MacConkey’s agars. Identification of bacterial species was carried out in the MicroScan autoSCAN-4 System® (Beckman Coulter, Inc.). Cultures without growth were monitored for 7 days prior to classify them as negative.
CVCRB diagnosis was established when both blood cultures (catheter and peripheral) presented growth of the same microorganism, and if the blood culture of the catheter occurred before with a difference of time detection of positivity ≥ 2h. If both cultures were positive to the same germ but with a time of positivity < 2h, or if only the peripheral culture was positive, it was classified as non-catheter-related bloodstream infection. If only the culture collected from CVC presented growth, it was diagnosed as colonization of the catheter.
2.3Statistical analysisIncidence density of CVCRB was estimated with 95% confidence intervals (95% CI). For quantitative variables, means and standard deviations (SD) were calculated; qualitative variables were presented as frequencies and percentages. To evaluate the association between quantitative variables and CVCRB (bivariate analysis), Cox proportional risk model was used. For qualitative variables, hazard risk (HR) with 95% CI was estimated. Variables that showed a value of p ≤ 0.2 were included in multivariate Cox regression analysis. Information was analyzed using the statistical program IBM-SPSS Statistics v. 20.
3ResultsWe studied 204 patients with CVC. The mean age was 4.6 years (minimum 0.08, maximum 16, SD 5.17), and 66.2% (n = 135) were male. Most frequent diagnoses (ICD-10) were neoplasms (28.9%, n = 59), infectious diseases other than bloodstream infections (19.6%, n = 40), central nervous system diseases (15.2%, n = 31) and genitourinary system diseases (5.9%, n = 12).
Insertion of the catheter was performed in the subclavian vein (72.5%, n = 148), jugular vein (20.1%, n = 41) or femoral vein (7.4%, n = 15), from which 80.4% (n = 164) was with a multi-lumen catheter. Three patients presented immediate complications after placement. The average of catheter-days by patient was 11.2 (minimum 1, maximum 58, SD 8.9) and the average hospital stay at the moment of placing the catheter was 7.5 days (minimum 0, maximum143, SD 17.2).
The catheters studied (n = 204) accumulated 2,294 catheter-days. Fifteen CVCRB events, nine non-catheter-related bloodstream infections and 20 colonized catheters were identified. The incidence density of CVCRB was 6.5 events per 1,000 days (95% CI 3.8–10.5).
Patients without CVCRB (n = 189) discontinued the study for the following reasons: catheter removal (71.4%, n = 135), death (22.8%, n = 43), transfer to another hospital unit (1%, n = 2) or non-catheter-related bloodstream infections (4.8%, n = 9).
Microorganisms isolated (n = 16) were coagulase-negative staphylococci (n = 5), Candida albicans (n = 4), Enterobacter cloacae (n = 2), Staphylococcus aureus (n = 1) and other gram-negative bacilli with one isolation each (Escherichia coli, Acinetobacter baumannii, Serratia marcescens, Steno-trophomonas maltophilia). In one same event, two bacteria were isolated (Escherichia coli and Enterobacter cloacae).
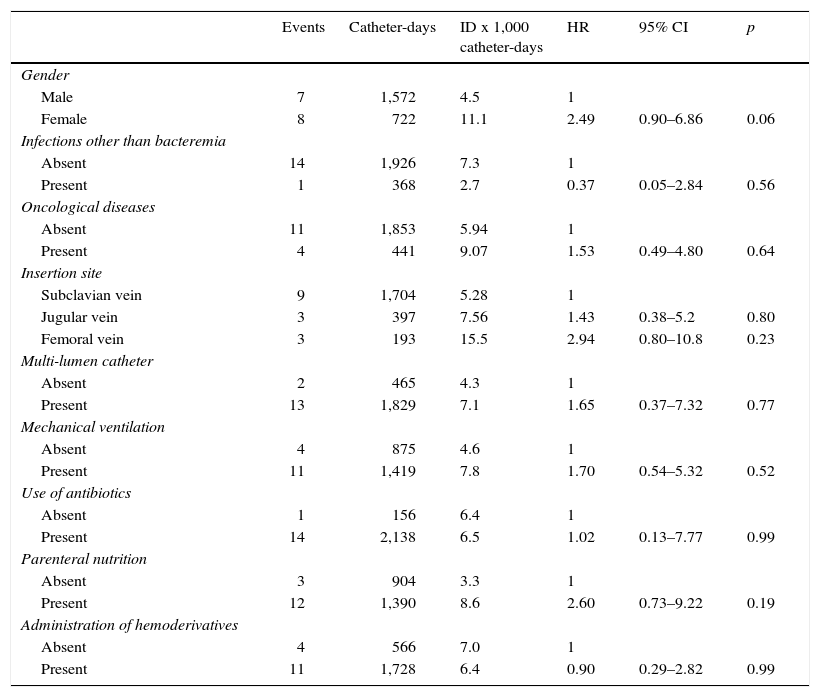
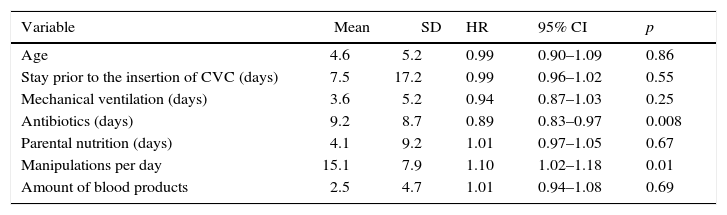
Incidence of CVCRB in function of the qualitative variables, HR and 95% CI, are listed in table 1. Mean and SD of quantitative variables and bivariate analysis with CVCRB are shown in table 2. There were no significant differences in the permanence of the catheter in patients with or without CVCRB (p < 0.56).
Incidence density of central venous catheter-related bloodstream infection based on qualitative variables
| Events | Catheter-days | ID x 1,000 catheter-days | HR | 95% CI | p | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 7 | 1,572 | 4.5 | 1 | ||
| Female | 8 | 722 | 11.1 | 2.49 | 0.90–6.86 | 0.06 |
| Infections other than bacteremia | ||||||
| Absent | 14 | 1,926 | 7.3 | 1 | ||
| Present | 1 | 368 | 2.7 | 0.37 | 0.05–2.84 | 0.56 |
| Oncological diseases | ||||||
| Absent | 11 | 1,853 | 5.94 | 1 | ||
| Present | 4 | 441 | 9.07 | 1.53 | 0.49–4.80 | 0.64 |
| Insertion site | ||||||
| Subclavian vein | 9 | 1,704 | 5.28 | 1 | ||
| Jugular vein | 3 | 397 | 7.56 | 1.43 | 0.38–5.2 | 0.80 |
| Femoral vein | 3 | 193 | 15.5 | 2.94 | 0.80–10.8 | 0.23 |
| Multi-lumen catheter | ||||||
| Absent | 2 | 465 | 4.3 | 1 | ||
| Present | 13 | 1,829 | 7.1 | 1.65 | 0.37–7.32 | 0.77 |
| Mechanical ventilation | ||||||
| Absent | 4 | 875 | 4.6 | 1 | ||
| Present | 11 | 1,419 | 7.8 | 1.70 | 0.54–5.32 | 0.52 |
| Use of antibiotics | ||||||
| Absent | 1 | 156 | 6.4 | 1 | ||
| Present | 14 | 2,138 | 6.5 | 1.02 | 0.13–7.77 | 0.99 |
| Parenteral nutrition | ||||||
| Absent | 3 | 904 | 3.3 | 1 | ||
| Present | 12 | 1,390 | 8.6 | 2.60 | 0.73–9.22 | 0.19 |
| Administration of hemoderivatives | ||||||
| Absent | 4 | 566 | 7.0 | 1 | ||
| Present | 11 | 1,728 | 6.4 | 0.90 | 0.29–2.82 | 0.99 |
ID: Incidence density; HR: hazard risk; 95% CI: 95% confidence interval.
Bivariate analysis of quantitative variables and CVCRB
| Variable | Mean | SD | HR | 95% CI | p |
|---|---|---|---|---|---|
| Age | 4.6 | 5.2 | 0.99 | 0.90–1.09 | 0.86 |
| Stay prior to the insertion of CVC (days) | 7.5 | 17.2 | 0.99 | 0.96–1.02 | 0.55 |
| Mechanical ventilation (days) | 3.6 | 5.2 | 0.94 | 0.87–1.03 | 0.25 |
| Antibiotics (days) | 9.2 | 8.7 | 0.89 | 0.83–0.97 | 0.008 |
| Parental nutrition (days) | 4.1 | 9.2 | 1.01 | 0.97–1.05 | 0.67 |
| Manipulations per day | 15.1 | 7.9 | 1.10 | 1.02–1.18 | 0.01 |
| Amount of blood products | 2.5 | 4.7 | 1.01 | 0.94–1.08 | 0.69 |
SD: standard deviation; HR: hazard risk; 95% CI: 95% confidence interval; CVC: central venous catheter.
Variables with a value of p ≤ 0.2 were included in the multivariate analysis, and non-significant variables were excluded step by step. The final model was integrated with the following variables: days of administration of antibiotics and average manipulations per day (Table 3).
Variables associated with central venous catheter-related bloodstream infection identified with multivariate analysis using Cox regression
| HR | 95°% CI Exp (B) | p | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Manipulations per day | 1.14 | 1.06 | 1.23 | < 0.001 |
| Antibiotics (days) | 0.84 | 0.76 | 0.92 | 0.001 |
HR: hazard risk; 95% CI: 95% confidence interval.
In 2012, information from the National Healthcare Safety Network (NHSN) indicated that, in not cancer pediatric care departments, CVCRB rate was quantified from 0.5 to 1.4 events per 1,000 catheter-days. The highest frequency was observed in the areas of surgery and surgical cardiology. Patients of cancer services have reported a frequency of 2.3 CVCRB events per 1,000 patient-days.26 In this study, the incidence of CVCRB was 6.5 events by 1,000 catheter-days, and in patients with oncological diseases, 9.07 events for 1,000 days. Both frequencies were higher than those reported by the NHSN. It has been identified that intravascular devices placed in ICUs are more likely to be associated with bloodstream infections.18 Abramczyk et al., in São Paulo Hospital, University of São Paulo, quantified an incidence of 10.2 CVCRB events for 1,000 catheter in children in an ICU.27
With regard to the etiologic agents of CVCRB in patients of the HCGJIM, a high frequency of isolates of gram-negative bacilli was observed. This condition is probably associated with external contamination and not with commensal skin bacteria, which are the most frequently reported in various studies.9,12 Safdar and Maki described that 45% of CVCRB are caused by external colonization of the catheter,9 while Garland et al. identified that 67% (n = 10) of CVCRB events were secondary to an inner lumen colonization in newborn patients.12 In both studies, the most frequently isolated bacteria were coagulase-negative staphylococci (77.1% and 93.3%, respectively). Hammarskjöld et al. quantified an incidence of catheter colonization of 7.6 events for 1,000 days. The predominant bacteria were coagulase-negative staphylococci (60%), and the duration of catheterization showed a tendency to increase the probability of colonization (HR 1.009, 95% CI 1.003–1.015).3 In the present cohort, the estimated incidence of colonization was 8.7 events per 1,000 catheter-days, and 75% of bacteria were Staphylococcus sp. Although it has been proposed that catheter colonization may precede infections, this condition is not a specific predictor of bloodstream infections. Koh et al. noted that only 5.8% of arterial lines and 7.5% of colonized venous lines developed CVCRB. No differences in the frequency of colonization between both types of vascular lines were identified (HR 1.17, 95% CI 0.41–3.36), and catheters placed in an ER showed an increased risk (HR 4.45, 95% CI 1.42–13.9).8
In this study, it was noted that the increase of catheter manipulations per day relates to bloodstream infections (HR 1.14, 95% CI 1.06–1.23) while the use of intravenous antibiotics showed a protective effect (HR 0.84, 95% CI 0.92–0.76). Some previously described risk factors for CVCRB (< 10 years of age, difficulties during insertion, parenteral nutrition, mechanical ventilation, transfusions, multiple lumen catheters or femoral vein placement)7,19,21 showed no significant association. However, this result may be due to the sample size. Mahieu et al. studied the influence of different forms of catheter manipulation on the frequency of CVCRB. Disconnection of the catheter for disinfection of the hub (OR 1.2, 95CI 1.1–1.3%) and blood sampling (OR 1.4, 95% CI 1.1–1.8) increased the risk of bloodstream infections, while heparinization (OR 0.9, 95% CI 0.8–1.0) and exit site antisepsis (OR 0.9, 95CI% 0.8–1.0) showed a protective effect.13
In addition to hand washing and the use of maximum sterile barriers when inserting the catheter,12,24 it is suggested to reduce as much as possible disconnects between the venoclysis equipment and CVC. The lowest number of medication doses should be administered per day; intravenous solutions should be prepared every 24h instead of every 8h, and the use of infusion pumps28 is recommended, especially for drugs that require frequent dose changes.
In a similar study of cases and controls, Vilela et al. observed that the concomitant use of antibiotics in patients with CVC decreased the risk of infection (OR 0.06, 95% CI 0.016–0.29).20 However, the protective effect of antimicrobials is uncertain due to the possibility of false-negative results of blood cultures if the samples were obtained when antibiotics were being administered. The risk of antimicrobial resistance of microorganisms attached to catheters and protected by extracellular polysaccharides should also be considered.
According to the NHSN, an increased frequency of infections related to CVC is present in school hospitals.26 Therefore, in these hospitals, preventive measures should be strengthen, including the appropriate training for the correct insertion and maintenance of the catheter, the monitoring of infection rates, hand hygiene, the use of infusion solutions equipment, the use of sterile gloves for manipulation of the intravenous lines, and the withdrawal of the catheter as soon as possible.6,29
A limitation of this study was the sample size, which may explain the absence of the association between the variables described as risk factors and the CVCRB. The protective effect of antibiotics for CVCRB should be assessed through diagnostic tests other than blood cultures due to the possibility of false-negative results.
Finally, it is suggested to decrease as much as possible disconnects between the line of infusion and CVC. Strategies to achieve this include administering medications with the lowest number of possible doses, indicate 24h solution infusions and the use of continuous infusion pumps. Although antibiotics have a protective effect for CVCRB, its indication is not recommended until the impact on antimicrobial resistance is assessed.
Ethical disclosureRight to privacy and informed consentThe authors declare that no patient data appear in this article.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
FundingThere was no external funding. Resources for the realization of tests and procedures were financed by the institution, and the rest of expenses arising from the research were funded by the authors.
Conflict of interestThe authors declare no conflicts of interest of any nature.
Please cite this article as: Lona-Reyes JC, et al. Bacteriemia relacionada con catéter venoso central: incidencia y factores de riesgo en un hospital del occidente de México. Bol Med Hosp Infant Mex. 2016;73:105-10.