Chemical pesticides, widely used in agriculture and vector-borne disease control, have shown toxic effects on the environment and the people in contact with them. Bacillus thuringiensis is a widely used bacterium for alternative and safer control of insect pests. Its toxins are specific for insects but innocuous for mammals and may be used as powerful adjuvants when applied with vaccines. The objective of this work was to characterize some autochthonous B. thuringiensis strains, which could be used for the control of a local pest (Diatraea considerata Heinrich) that affects sugar cane crops in Sinaloa, Mexico. Also, to evaluate these strains as a source of Cry toxins, which may be used in the future as adjuvants for some vaccines.
MethodsEight strains from field-collected dead insects were isolated. These were microbiologically identified as B. thuringiensis and confirmed by amplification and sequencing of 16S rDNA. Bioassays were performed to evaluate their pathogenicity against D. considerata, and Cry toxins were identified by proteomic analyses.
ResultsAn increased mortality among larvae infected with strain Bt-D was observed, and its toxin was identified as Cry1Ac.
ConclusionsThe observed data showed that the selected strain was pathogenic to D. considerata and seemed to produce Cry1Ac protein, which has been reported as an adjuvant in different types of immunization.
Los pesticidas químicos, ampliamente usados en agricultura y en el control de vectores transmisores de enfermedades, han mostrado efectos tóxicos sobre el medio ambiente y las personas expuestas a ellos. Bacillus thuringiensis es una bacteria ampliamente utilizada como una alternativa segura y eficaz en el control biológico de plagas agrícolas. Sus toxinas son específicas de insectos, pero inocuas para mamíferos, e incluso poseen gran potencial para ser usadas como adyuvantes en vacunas. El objetivo de este trabajo fue caracterizar cepas autóctonas de B. thuringiensis con efectividad contra el gusano barrenador (Diatraea considerata Heinrich) de la caña de azúcar en cultivos del estado de Sinaloa, México, y como fuente de proteínas Cry, con potencial de utilizarse como adyuvantes en vacunas.
MétodosSe lograron aislar ocho cepas a partir de insectos muertos en campos agrícolas, las cuales fueron identificadas microbiológicamente como B. thuringiensis, lo que se confirmó por amplificación y secuenciación del 16S rDNA. La efectividad de los aislados para el control del gusano barrenador fue evaluada mediante bioensayos y las toxinas Cry fueron identificadas por análisis proteómico.
ResultadosSe observó una mortalidad elevada en las larvas infectadas con las cepas de estudio. Particularmente, la cepa Bt-D, de la cual el análisis molecular mostró que posee una toxina tipo Cry1Ac.
ConclusionesLos resultados mostraron que la cepa Bt-D posee un elevado potencial patogénico hacia D. considerata y produce la proteína Cry1Ac, de la cual existen reportes de su aplicación como adyuvante en diferentes formas de inmunización.
Chemical pesticides are commonly used in large-scale commercial crops and for vector-borne disease control worldwide, including Mexico, particularly in agricultural zones such as Sinaloa. Unfortunately, the use of these chemicals has been associated with several diseases, such as leukemia, diabetes and other degenerative disorders.1 To achieve an equilibrium between social, environmental and economic interests, Integrated Pest Management indicates that one reliable option is the use biological control agents,2,3 such as Bacillus thuringiensis (Bt).
Bt is a Gram-positive bacterium that develops very resistant spores during the stationary phase and simultaneously produces crystalline inclusions during sporulation. These inclusions are composed of proteins with highly specific insecticidal activity, which is why Bt has been widely used in products for the control of pests, mainly Lepidoptera, Diptera, and Coleoptera.4 These proteins are commonly known as Cry proteins or δ-endotoxins and are classified into different families depending on their amino acid sequence.5 There are reports that indicate that Lepidopteran insects are usually susceptible to the toxins of the Cry1 family. For example, Diatraea grandiosella, which affects sugar cane crops in Sinaloa, Mexico, is known to be susceptible to Cry1Ab, Cry1Ba and Cry9Ca.6 Surprisingly, there are no reports on the use of Bt for the control of D. considerata.
Bt is not only a safe pest control agent, but also a source of other useful proteins and toxins (such as parasporins) that have shown cytotoxic effects on human cancer cells.7,8 Moreover, some studies have reported that Cry toxins could be used as adjuvants in vaccines, since they enhance the immune response.9–11 Therefore, in this work, eight regional Bt strains were isolated and characterized to evaluate their entomopathogenic effect on D. considerata and to identify Cry toxin subtypes.
2Methods2.1Source of the strainsReference strains (Bt kurstaki HD73 and HD1) used for this study were kindly provided by the Sciences Center of Sinaloa. Native strains were isolated from dead insects (Spodoptera frugiperda) collected from the fields and labeled as Bt-A, B, C, D, 5, 15, 22 and 64. Insects were macerated in 0.85% sterile saline solution and subjected to thermic shock (85°C for 10min and 4°C for 2min). Subsequently, a loopful of the solution was streaked in nutrient agar and incubated at 30°C for 24h. The colonies that showed typical Bacillus morphology (Gram-positive, opaque, whitish and irregular) were re-cultured in nutrient agar at 30°C for 48h. Then, bacteria were stained with Scheaffer-Fulton endospore stain and visualized in a light microscope at 100X. Cultures that showed spore-forming bacilli and parasporal crystals production were selected.
2.2Molecular analysisFor DNA extraction, vegetative cells were obtained by culturing the strains in nutrient broth at 150rpm and 37°C for 24h. DNA was extracted using heat and LiCl-precipitation.12 Briefly, cells were lysed with extraction buffer (100mM Tris-HCl, pH 8; 10mM EDTA, pH 8; 1% SDS; 100mM LiCl) and chloroform at 80°C. Samples were centrifuged at 13,000rpm for 10min, and the upper aqueous phase was recovered, mixed with an equal volume of 4M LiCl and incubated at 4°C overnight. Samples were centrifuged, and the supernatant was mixed with isopropanol and centrifuged again. DNA pellet was recovered, washed with 70% ethanol and resuspended in sterile distilled water. DNA integrity was visualized on a 1.5% agarose gel and quantified by spectrophotometry.
Amplification of the 16S rDNA fragment was carried out in a total volume of 50μl containing PCR buffer [50mM KCl, 20mM Tris-HCl (pH 8.3), 2.5mM MgCl2], 0.2μM each primer, 0.2mM dNTPs, 1.25 U AmpliTaq DNA polymerase (Applied Biosystems) and 10 ng of DNA. The reactions were cycled 35 times under the following parameters: 95°C for 40 s, 55°C for 40 s and 72°C for 90 s with 5′-AGAGTTTGATCMTGGCTCAG-3′ and 5′-TACGGYTACCTTGTTACGACTT-3′ oligonucleotides as primers.
Sequencing was performed by Macrogen Inc (using an ABI3730XL DNA analyzer). The sequences obtained were analyzed using Chromas Lite software, and alignment and neighbor-joining tree method were performed using CLC Sequence Viewer software. The sequences were compared with the ones in GeneBank (https://blast.ncbi.nlm.nih.gov/Blast.cgi) using the algorithm BLAST to confirm they belonged to Bacillus thuringiensis.
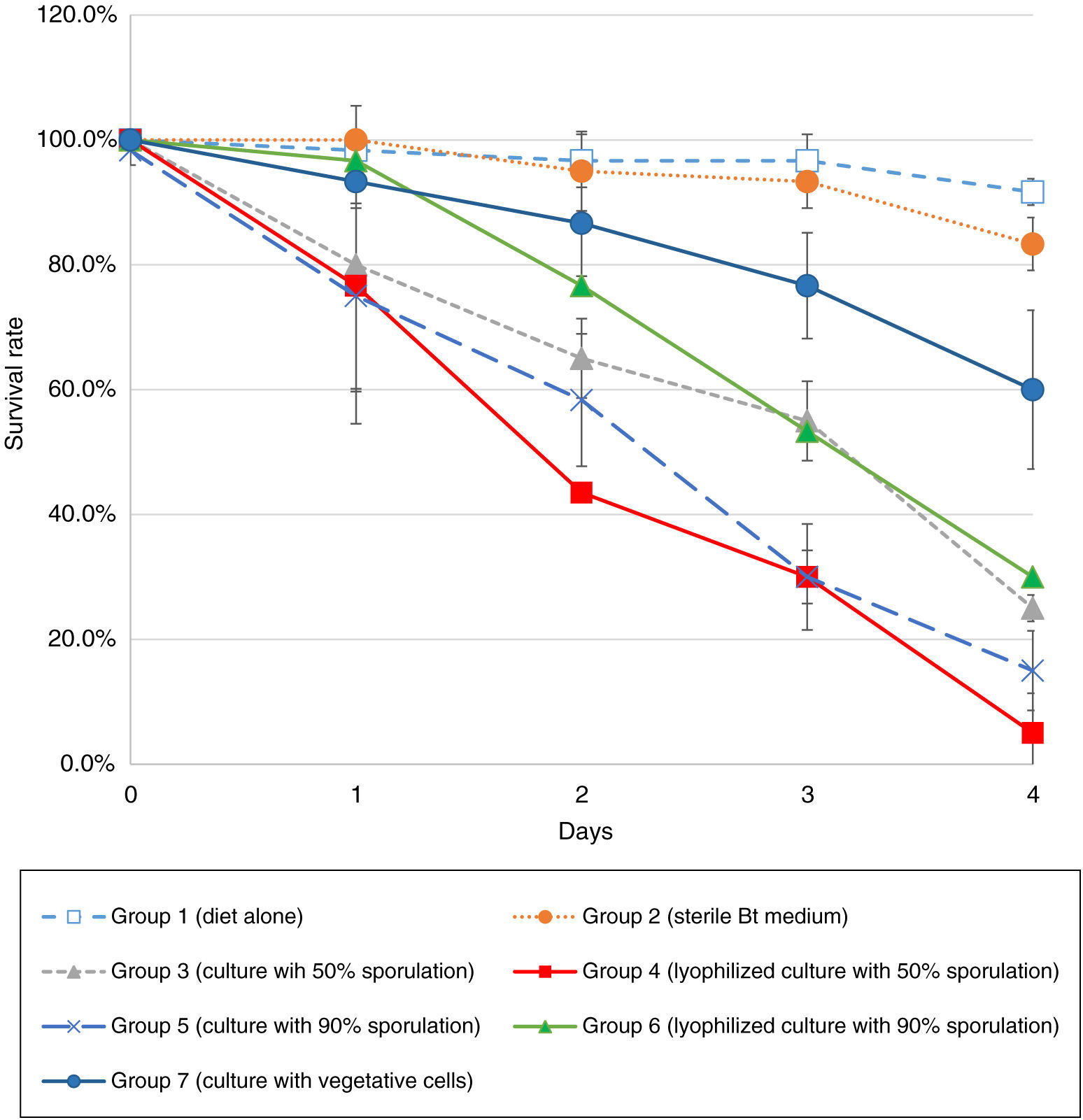
2.3BioassaysWe performed preliminary bioassays with Galleria mellonella larvae to confirm that the strains were pathogenic for Lepidopteran insects. Based on these results, we selected the most pathogenic strain (Bt-D). For the bioassays with D. considerata Heinrich, we used first-instar larvae. Larvae were fed with an artificial diet13 inoculated with the selected Bt strain since proteins have to be ingested to achieve a toxic effect. Experimental groups were fed with the following diets: alone (group 1), diet with sterile Bt medium (group 2), culture with 5.7 x 109 CFU/g (50% sporulation, group 3), lyophilized culture with 5.7 x 109 CFU/g (50% sporulation, group 4), culture with 3.51 x 109 CFU/g (> 90% sporulation, group 5), lyophilized culture with 3.51 x 109 CFU/g (>90% sporulation, group 6), and culture with 5.37 x 106 CFU/g (only vegetative cells, group 7). Each group contained 30 larvae, and the survival rates were monitored every 24h for four days.
Bacteria were cultured in soybean medium14 using a bioreactor with constant conditions (30°C, 600-800rpm, > 20% dissolved oxygen, pH 7.4). Fresh as well as lyophilized cultures were used to assess the importance of the formulation in the efficacy of the biological agent.
2.4Proteomic analysisThe strains were grown in nutrient broth at 37°C and 150rpm until they reached Abs600=0.2-0.3. Subsequently, bacteria were inoculated into soybean medium in a 1:20 volume ratio in shaken flasks with 1:4 liquid-air ratio. Samples were then incubated at 30°C and 150rpm for 96h or until they reached > 90% sporulation. Cultures were centrifuged at 5,000rpm for 15min; the supernatant was discarded, and the pellet containing the spores and proteins was resuspended and solubilized in an alkaline buffer15 (50mM NaHCO3, 50mM Na2CO3, 50mM EDTA, pH10) at 37°C and 150rpm for 2h. Samples were centrifuged again at 5,000rpm for 15min, and supernatants were recovered as well. The total amount of protein was quantified by the Bradford protein assay.16 The proteomic profile was obtained by SDS-PAGE.
Once one of the local strains (Bt-D) was selected, Western blot analysis was performed to select the protein bands that would be processed for mass spectrometry analysis. For this purpose, a commercial rabbit polyclonal antibody against Cry1Ab toxin (Abcam, ab51586) was used. An HRP-conjugated goat anti-rabbit IgG was used as a secondary antibody.
The spots cut from the gels (∼1mm2) were processed following Bruker's standard protocol for in-gel protein digestion. Briefly, gel particles were washed four times with 50mM NH4HCO3 and acetonitrile. The proteins were then reduced and alkylated with 10mM DTT (56°C for 45min) and 55mM iodoacetamide (20min at room temperature in the dark). Samples were washed again with acetonitrile and NH4HCO3. The supernatant was removed, and gel particles were air-dried. For the digestion of the proteins, trypsin (25 ng/μl in 25mM NH4HCO3) was used. The samples were incubated at 37°C overnight. The supernatant was recovered and stored at -20°C. The particles were then incubated with 50mM NH4HCO3 at 37°C overnight, and the supernatant was stored at -20°C. Peptides were extracted with trifluoroacetic acid 0.1%/acetonitrile (1:1) for 30min at room temperature. Finally, all supernatants were mixed and dried in a vacuum centrifuge.
Peptides were resuspended in trifluoroacetic acid and MALDI-TOF (MS/MS). For their identification, an Ultraflextreme mass spectrometer (Bruker, USA) with LIFT fragmentation was performed. An AnchorChip target and α-Cyano-4-hydroxycinnamic acid (HCCA) matrix were used, following the dried droplet protocol suggested by the manufacturer. The laser intensity was adjusted to 50% for the acquisition of parent masses and 60-70% for fragment masses, with three or four repetitions. For the analysis, spectra with 1x103–1x104 intensity peaks were considered. The identity of the protein was matched using the eubacteria Swiss-Prot database and the Mascot search engine with the following parameters: enzyme, trypsin; missed cleavages, 1; fixed modification, carbamidomethyl C; variable modification, oxidation M; parent tolerance, 0.2Da; fragment tolerance, 0.5Da. Positive protein identifications were those rendering a MASCOT score higher than 30. The toxins of both Bt kurstaki HD73 and HD1 reference strains were used as positive controls.
2.5StatisticsFor the bioassays with first-instar larvae, data were analyzed by one-way ANOVA with IBM SPSS v.20 software. p values < 0.05 were considered as statistically significant.
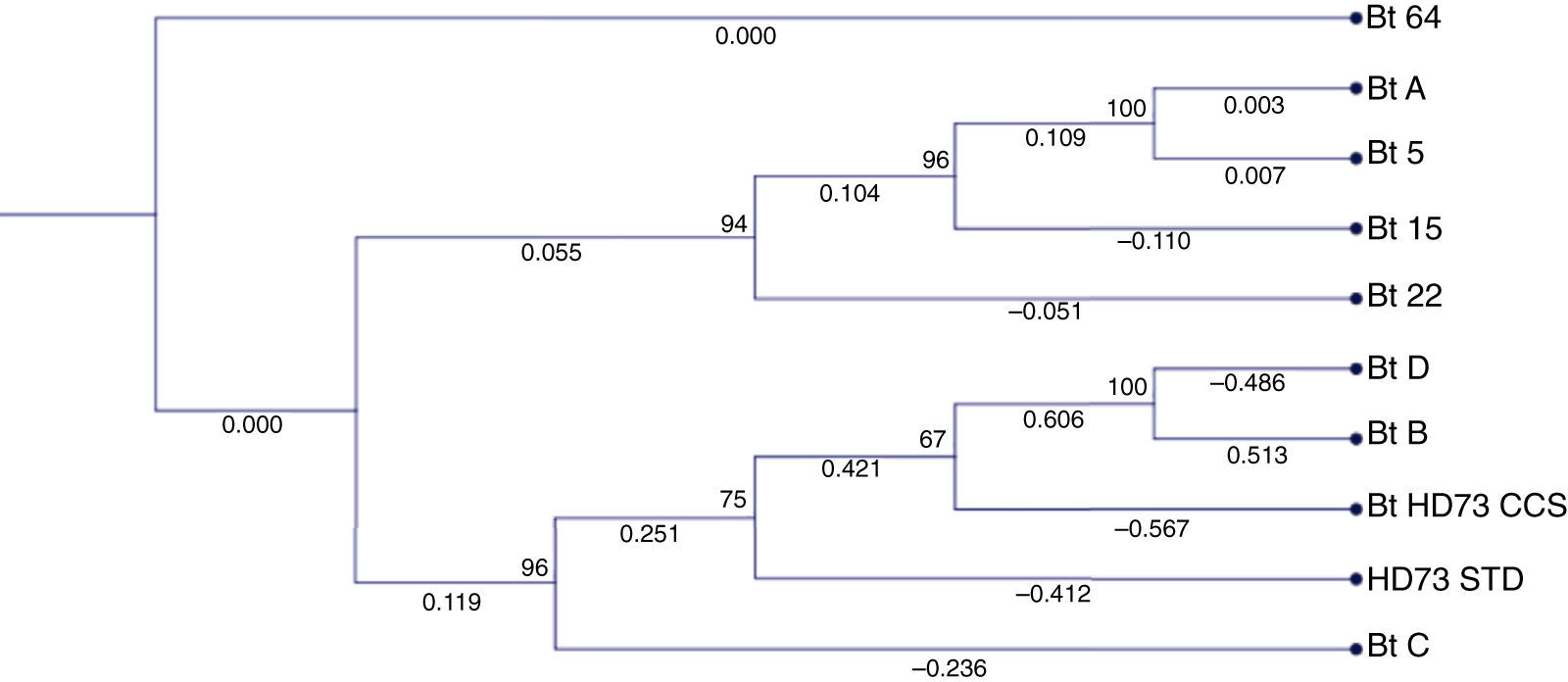
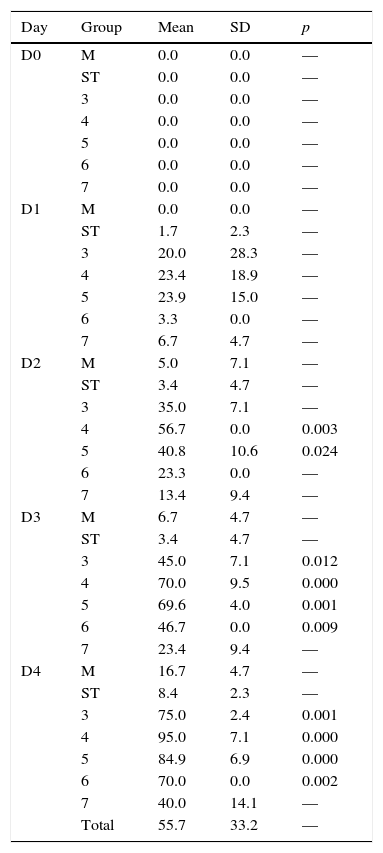
3Results3.1Molecular analysisThe sequences of the amplified segments (16S rDNA) were compared with the ones available in the NCBI database, which demonstrated that the strains were all Bacillus thuringiensis (Figure 1). The phylogenetic tree showed an aggrupation between the strains Bt-A, 5, 15 and 22, and another branch for the strains Bt-D, B, HD73, and C (Figure 1). The strain Bt-64 showed a major divergence from the others, which matches with its macroscopic and microscopic morphological characteristics (data not shown).
3.2BioassaysThe survival rates of first-instar larvae were drastically decreased in the groups that contained a significant sporulation percentage, therefore, high levels of parasporal crystals (Figure 2). Since day 2, first-instar larvae groups 4 and 5 had a significant decrease in survival rates (Table 1). The following days all groups had statistically significant differences versus the control group, except from group 7. Since group 7 treatment consisted of vegetative cells (no sporulation and crystal production), those results emphasize the role that parasporal crystals have over the entomopathogenic effect. Although there are strains that produce VIP toxins during the vegetative growth,5 it does not seem to be the case for this strain in particular.
Survival percentage for bioassays with first-instar larvae.
| Day | Group | Mean | SD | p |
|---|---|---|---|---|
| D0 | M | 0.0 | 0.0 | — |
| ST | 0.0 | 0.0 | — | |
| 3 | 0.0 | 0.0 | — | |
| 4 | 0.0 | 0.0 | — | |
| 5 | 0.0 | 0.0 | — | |
| 6 | 0.0 | 0.0 | — | |
| 7 | 0.0 | 0.0 | — | |
| D1 | M | 0.0 | 0.0 | — |
| ST | 1.7 | 2.3 | — | |
| 3 | 20.0 | 28.3 | — | |
| 4 | 23.4 | 18.9 | — | |
| 5 | 23.9 | 15.0 | — | |
| 6 | 3.3 | 0.0 | — | |
| 7 | 6.7 | 4.7 | — | |
| D2 | M | 5.0 | 7.1 | — |
| ST | 3.4 | 4.7 | — | |
| 3 | 35.0 | 7.1 | — | |
| 4 | 56.7 | 0.0 | 0.003 | |
| 5 | 40.8 | 10.6 | 0.024 | |
| 6 | 23.3 | 0.0 | — | |
| 7 | 13.4 | 9.4 | — | |
| D3 | M | 6.7 | 4.7 | — |
| ST | 3.4 | 4.7 | — | |
| 3 | 45.0 | 7.1 | 0.012 | |
| 4 | 70.0 | 9.5 | 0.000 | |
| 5 | 69.6 | 4.0 | 0.001 | |
| 6 | 46.7 | 0.0 | 0.009 | |
| 7 | 23.4 | 9.4 | — | |
| D4 | M | 16.7 | 4.7 | — |
| ST | 8.4 | 2.3 | — | |
| 3 | 75.0 | 2.4 | 0.001 | |
| 4 | 95.0 | 7.1 | 0.000 | |
| 5 | 84.9 | 6.9 | 0.000 | |
| 6 | 70.0 | 0.0 | 0.002 | |
| 7 | 40.0 | 14.1 | — | |
| Total | 55.7 | 33.2 | — |
All groups were compared to group 2 (ST), which was considered as the negative control.
SD, standard deviation; M, diet alone; ST, sterile medium.
For cultures with 50% sporulation, treatments with lyophilized spore-crystal mixture were more efficient than fresh cultures (group 3 versus 4, Figure 2), which underlines the importance of formulation when an agrobiological product is applied. However, in the 90% sporulation cultures, lyophilization did not improve insecticidal activity (group 5 versus 6, Figure 2). The high standard deviation (SD) values obtained in some days (especially day 1) could be due to the inner variation among individuals. Although all larvae belong to the same population, there is a natural diversity among them, which causes a higher susceptibility in some individuals. Moreover, the controlled variable in the experiment was the concentration of Bt inoculated to the diet; however, larvae fed at libitum, so the amount of spore-crystal complex ingested by each larva was not controlled.
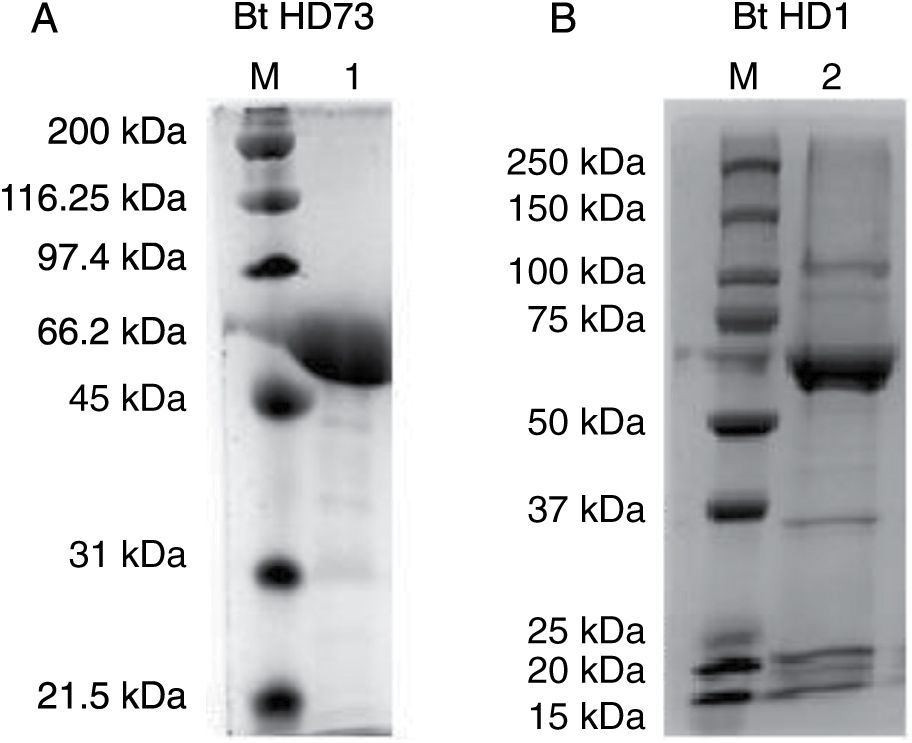
3.3Proteomics analysisFor the Bt kurstaki HD73 strain, a protein band with a molecular weight of ∼65 kDa was identified as Crystal protein Cry1Ac. In the case of the Bt kurstaki HD1 strain, a protein band with a molecular weight of ∼65 kDa was identified as Crystal protein Cry1Aa (Figure 3), according to reported data.17,18 These results were confirmed by MALDI-TOF analysis, where the protein rendered a > 40 score.
The proteomic analysis of our local strain Bt-D showed that it produces a protein with a calculated molecular weight of 67 kDa (Figure 4), identified as a Cry1Ac toxin by Western blot analysis, which may be the responsible for the insecticidal activity observed in the bioassays. It is known that Cry proteins are originally produced as protoxins, which are then solubilized and cleaved in the midgut of the Lepidopteran insects when they are ingested, becoming active toxins.19 Since an alkaline buffer solution was used to solubilize the proteins in the samples,20 the bands observed as ∼67 kDa proteins are the active forms of the toxin. Two bands detected by Western blot were observed on lanes 3 and 4 maybe because the native strain produced more than one toxin, whose epitope is similar or equal to Cry1Ac, and causes the protein to bind to the primary antibody.
Bacillus thuringiensis strain Bt-D protein profile. A) 12% SDS-PAGE. M, molecular weight marker; lane 1, 12h culture (vegetative cells only); lane 2, 24h culture; lane 3, 48h culture (50% sporulation); lane 4, 96h culture (> 90% sporulation). B) Western blot membrane for Bt-D strain. M, molecular weight marker; lane 1, 12h culture (vegetative cells only); lane 2, 24h culture; lane 3, 48 h culture (50% sporulation); lane 4, 96h culture (> 90% sporulation). Each lane was loaded with 30μg of protein.
Although Bacillus thuringiensis has been used as a biopesticide for a long time, most of the studies with the genus Diatraea have been done only with D. grandiosella, D. saccharalis and D. magnifactella, as reviewed by Hernández-Velázquez et al.21 Regarding these species, Sikorowski et al. reported that the Bt product they used also caused mortality on first- and second-instar larvae in days 1 to 3.22 In another study with D. grandiosella, the efficacy of Cry1Ab over field collected (transgenic sugar cane crops) and laboratory-raised larvae was evaluated, and it was shown that field insects were more susceptible to the toxin.23 In this investigation, insects were kept under laboratory conditions for a more homogeneous population.
Some evidence suggests that an autochthonous strain is more efficient than a foreign one to control a local pest, according to the study published by Fonseca et al.,24 where they evaluated local strains against Diatraea magnifactella, a sugarcane borer closely related to Diatraea considerata.
In the bioassays, it was observed that treatments with a lyophilized culture of Bt-D were more efficient than the fresh cultures since they increased the mortality rates. The additives in the formulation of the lyophilized powder can influence the entomopathogenic effect,25 act as cryoprotectants and stimulate the need of feeding of the insects and, therefore, toxin ingestion.24,26,27 Since we only performed identification of the Bt-D strain toxin, further analysis should be carried out to obtain the complete sequence of the protein and confirm if it is Cry1Ac.
Some toxins such as Cry1Ac and Cry1A, which are conventionally known to be toxic to Lepidopteran insects, have shown promissory activities when applied as adjuvants. There are several studies focused on viral and bacterial diseases, such as hepatitis B, meningoencephalitis, and pneumococcal infections, suggesting that these Cry1A adjuvants can achieve their purpose when applied either intraperitoneally, intranasally, or even intragastrically. However, during oral exposure, some authors comment that the protein should be protected from degradation with a vehicle.28–30 Further research is needed to evaluate the effect of repeated inhalation of Cry1A protein on immune protection against other pathogens: for example, influenza virus in a murine model.
In addition to the benefits of a Cry1Ac toxin producing strain, it would be interesting to investigate if Bt-D (as well as the other autochthonous isolates) produces a kind of parasporin, another type of Bt proteins that are known to be toxic to human cancer cells, as stated before. Although the proteomic analysis is usually more accurate to determine if a strain is producing these toxins, some studies8 have used PCR molecular analysis to find if these strains harbor the genes necessary for the production of toxins such as parasporins.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare no conflicts of interest of any nature.
This work was supported by the 106152 National Council of Science and Technology (CONACYT) and PROFAPI 2014/168 (UAS) grants.