Los diseños de investigación clínica, cuyo propósito es responder preguntas sobre causalidad, se pueden clasificar en relación con cuatro ejes: el número de grupos de estudio, la aplicación de una maniobra experimental, la direccionalidad causa-efecto y la fuente de la que se recaban los datos. Los diseños básicos más utilizados en epidemiología son el ensayo clínico, el estudio de cohortes, el estudio de casos y controles y la encuesta transversal. Este texto pretende facilitar la identificación y comprensión de cada uno de estos diseños mediante ejemplos relacionados con la asociación entre la vacunación con rotavirus y la invaginación intestinal.
Design of clinical research whose purpose is to answer questions about causality can be classified in relation to four axes: the number of study groups, the implementation of an experimental maneuver, cause-effect directionality and source from which the data are collected. The basic designs used in epidemiology are the clinical trial, the cohort study, the case-control study and the cross-sectional survey. This text aims to facilitate the identification and understanding of each of these designs through examples related to the association between rotavirus vaccination and intussusception.
1. Introduction
In October 1998, the first vaccine against rotavirus (Rotashield®) was used in the U.S. In July 1999 there were 15 cases reported of intussusception after application of the vaccine. Given the possible association between vaccination and this adverse event, in October 1999 the U.S. Advisory Commitee on Immunization Practices along with the Centers for Disease Control and Prevention withdrew its recommendation for the application of the vaccine against rotavirus and the pharmaceutical industry voluntarily ceased production. After 8 years, new vaccines against rotavirus1 became available.
The question “Does the oral vaccine against rotavirus cause intussusception?” has been attempted to be resolved in different ways. Because the findings of the various studies have been contradictory, in 2015 studies continue to be carried out.
Extensive literature reports are available on the theory of causation in general and on causation of health-related phenomena in particular. It is not the intention of this paper to delve deeply into the philosophy of causality; however, some of the more accepted criteria in epidemiology can be noted to assume that the association between two events is causal in nature, i.e., an independent variable has an effect on or can change the dependent variable.
The independent variable is the cause or risk factor under study, whereas the dependent variable is the effect or outcome under investigation. Some criteria of causality are the statistical association between the independent and dependent variables, the timing between the variables (the independent preceding the dependent), experimentation (the dependent variable is changed if the researcher manipulates the independent variable), and the dose-response relationship (the higher the magnitude of the independent variable, the greater magnitude in the change of the dependent variable).2. The first two criteria, statistical association and timing, are needed to establish causation. Without these two conditions it is assumed that the independent event is not the cause of the dependent event. Moreover, experimental evidence is, in itself, the best evidence of the causal relationship between two phenomena.
Either from the point of view of the clinician who critically reviews the scientific literature or the investigator who plans a project to answer a question, the first thing to do is identify the design of the research study. The study design is the logical structure used for construction of the project in order to provide elements that satisfy the casualty criteria mentioned above. There are various types of research designs, each with a different ability to provide evidence of a causal association between two phenomena. We continue with five clinical research studies whose objective was to provide evidence on the causal relationship between vaccination against rotavirus and intussusception.
2. Studies of clinical research to establish causality between rotavirus vaccination and intussusception
2.1. Study 1
All cases of intussusception reported to the System of Adverse Events Associated with Vaccine were identified. From 98 reported cases of intussusception, 87% received a previous dose of rotavirus vaccine and 56% were treated with surgery. There was a median of 4 days between vaccination and intussusception. Median age of cases was 4 months. Median hospital stay was 2 days and 2% of the patients died.3
Architecture of the study was descriptive, observational, no cause-effect directionality and retrolective.
Study design was a cross-sectional survey.a
2.2. Study 2
By reviewing medical and x-ray records, children between 1 and 12 months of age from 19 U.S. states who had been hospitalized for intussusception between 1998 and 1999 were identified. For each child with intussusception, four children of the same age and born in the same hospital were selected, and history of vaccination of all children was collected; 17% of 429 infants with intussusception and 13% of the 1,763 children without intussusception had previously received rotavirus vaccine.4
The study architecture was comparative, observational, cross-sectional, effect and cause directionality, and retrospective.
Study design was case-control.
2.3. Study 3
From 2006-2008, 207,621 doses of rotavirus vaccine (RotaTeq®, Merck) were applied to children between 4 and 48 weeks of age. Adverse events were recorded during the 30 days following application of the vaccine. Five cases of intussusception were identified. According to historical data, 6.75 cases were expected.5
Architecture of the study was comparative, observational, cause and effect directionality, and ambilective.
Study design was a randomized cohort.
2.4. Study 4
There were 68,038 children included. One group received three doses of rotavirus vaccine and a placebo. The maneuver was blinded. Active surveillance was conducted to identify adverse effects. One year after the first dose, intussusception occurred in 12 children treated with the vaccine and in 15 children given placebo.6
Architecture of the study was comparative, experimental, cause and effect directionality, and prolective.
Study design was randomized.
2.5. Study 5
Children 4-34 weeks of age who received at least one dose of rotavirus vaccine RotaTeq® (RV5) between 2006 and 2010 were included. Cases of intussusception were identified within 30 days after vaccination by reviewing the diagnoses recorded during hospitalization, emergency department or outpatient clinic visits. The incidence of intussusception in this group of children was compared against the incidence of intussusception in children who received other vaccines, but none against rotavirus during the same study period. Fourteen cases of intussusception were found within 30 days after application of 786,725 doses of RV5 and eight cases after 389,026 applications of other vaccines.7
Architecture of the study was comparative, observational, cause and effect directionality, and prolective.
Study design was cohort.
3. Axes to classify clinical research design
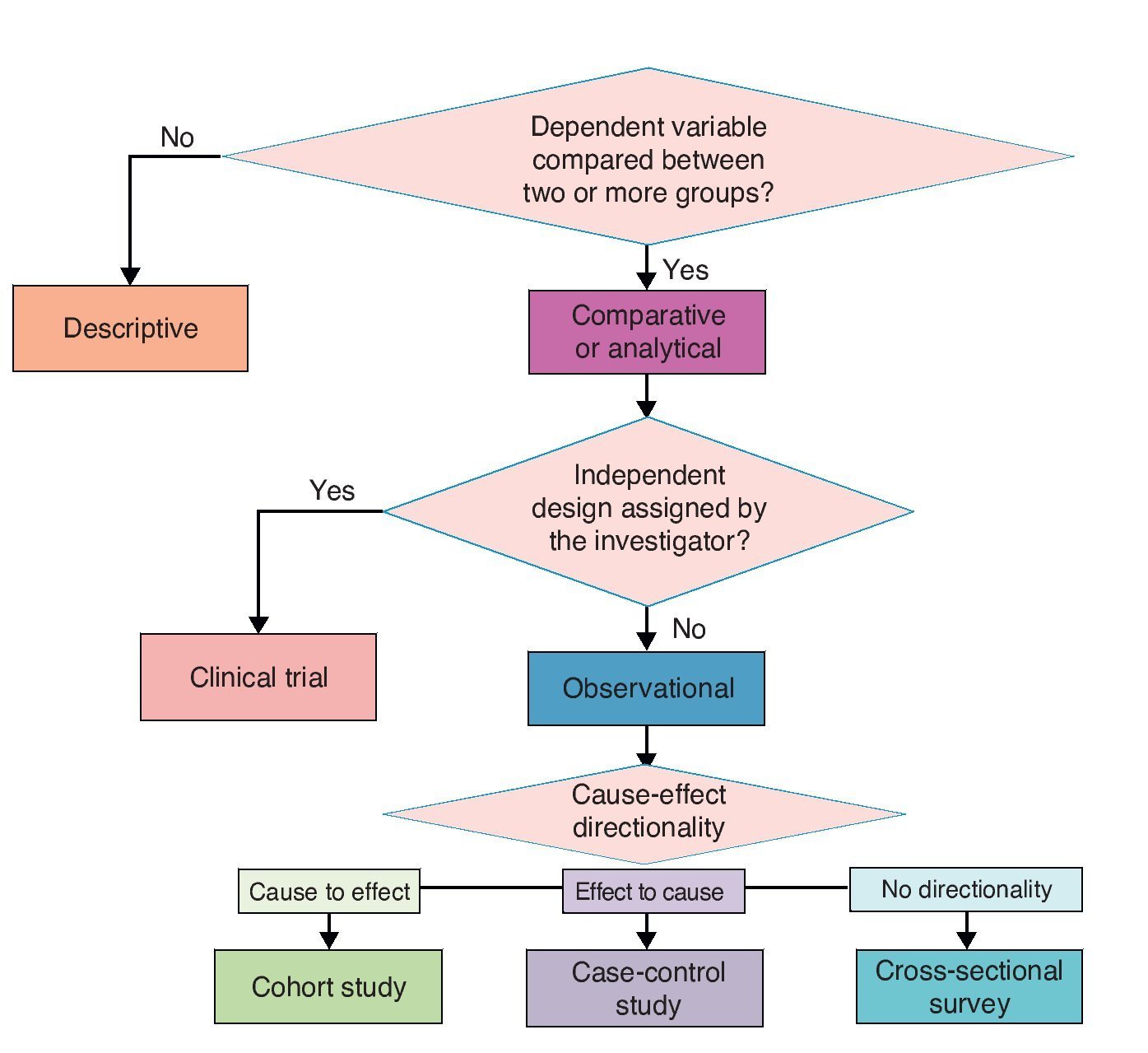
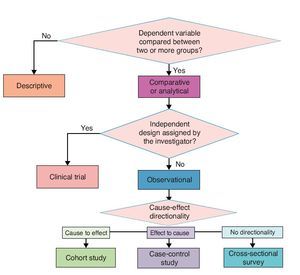
Investigation designs have been classified in different ways. A.R. Feinstein proposed an “architectural” classification in which each study is classified in accordance with five axes.8 The following is a modified version of this classification.
3.1. Axis 1. Number of study groups: descriptive vs. comparative
A study is descriptive if it comprises a single group; it is comparative if the dependent variable is contrasted between two or more groups. Some authors use the analytical description to refer to comparative studies because the purpose of these is to make inferences about the association between variables and test hypotheses.9 A descriptive study is impossible to estimate the degree of association between cause and effect; only the phenomenon can be characterized and, if it is longitudinal, the temporal sequence of events can be observed.
Study 1 is descriptive because it only reports data in the group of vaccinated children. The rest of the studies are comparative because they include data from at least two groups of children. In study 3, although the comparison group was not simultaneous, the dependent variable (intussusception) was compared with another group of similar children who had not been vaccinated and existed in the past (this group is referred to as a historical cohort). Comparative groups may be comprised of the same group of subjects at different points in time.
3.2. Axis 2. Assignment of an experimental maneuver: observational vs. experimental
This axis refers to the existence of an intervention designed by the investigator in order to evaluate its effect. When the investigator subjects one or more study groups to an intervention designed ex profeso for the study and the investigator decides which subjects would be submitted to this intervention, it is an experimental study. If the subjects are not involved in any proceedings assigned by the researcher, it is an observational study. Study subjects may have experienced interventions as part of their history or during the study, but these have not been decided by the investigator but by someone outside the project, e.g., the treating physician. In this case the study is not experimental but observational.
In the studies presented above, only study 4 is experimental and the rest are observational. Note that in studies 1, 2, 3 and 5, subjects also received the vaccine against rotavirus, but unlike study 4 it was not the investigator who decided to apply it or which study subjects received the vaccine as part of their health care.
3.3. Axis 3. Forming study groups according to the directionality between cause and effect: cohort, case and control or cross-sectional survey
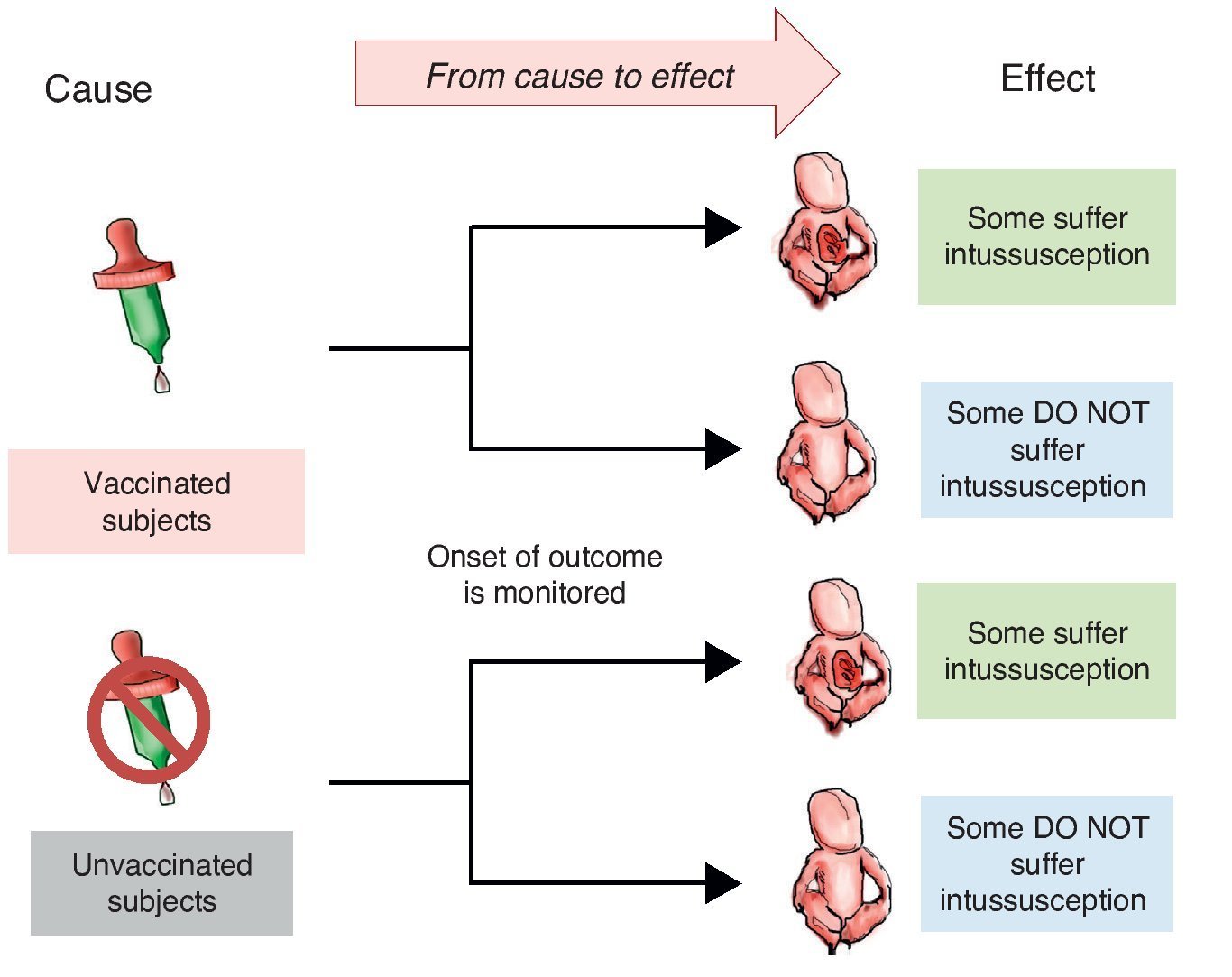
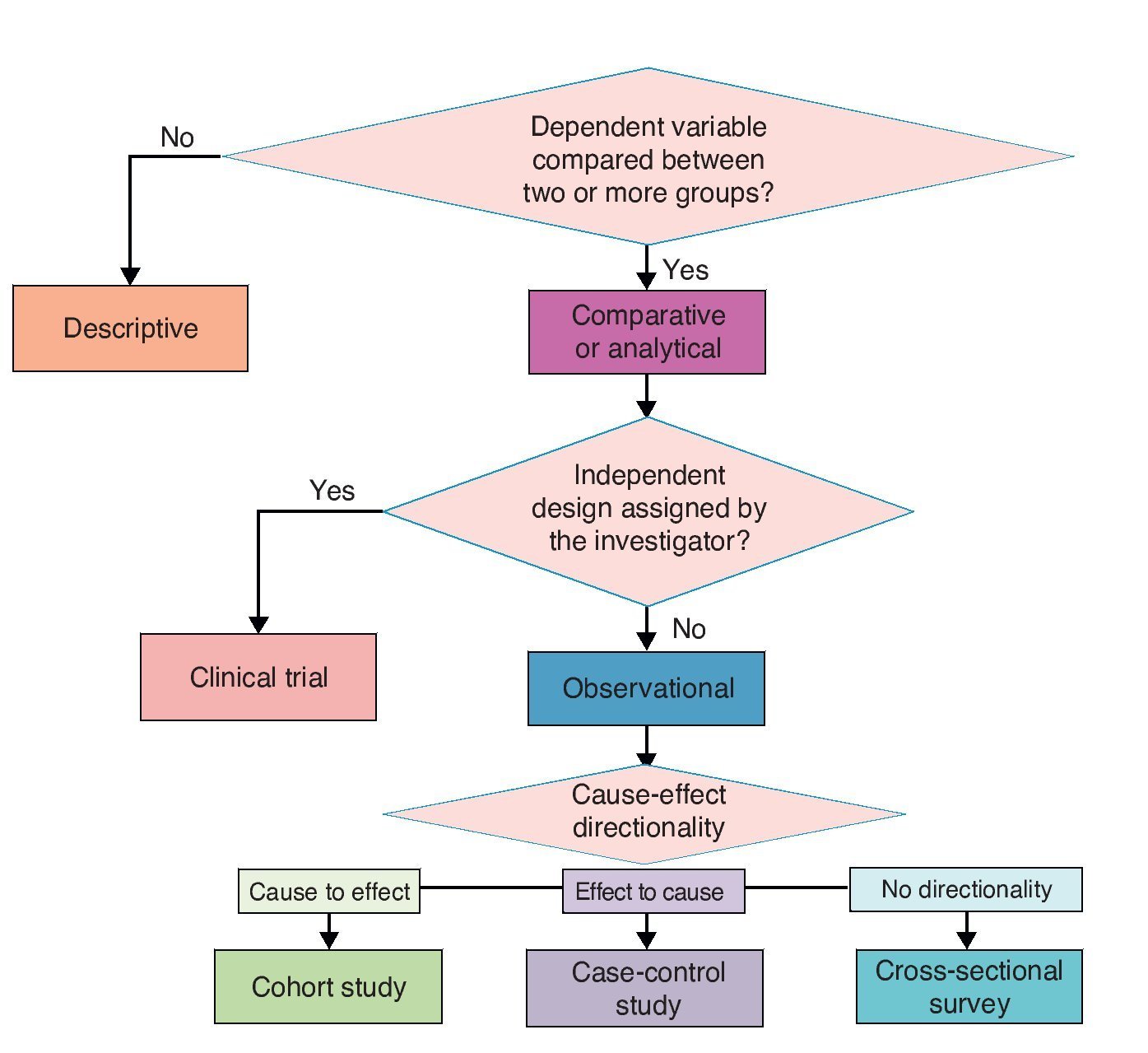
When designing a study to test a hypothesis about the causal association between two variables, you can choose among three ways to recruit participants.10 The first is to recruit two groups—one exposed to the suspected cause and one that is not. After a period of time we compare which group has a higher frequency and intensity of the effect. This is a study with directionality from cause to effect and each of the groups being compared is called cohort (Latin cohors: entourage, grouping). A cohort is a set of subjects who share a certain feature at one time and are observed over time. Therefore, studies that monitor two or more groups of subjects defined by their exposure and comparing the development of an event (effect) over time are called cohort (Figure 1).
Figure 1 Cohort design. Study groups are defined by the exposure to the cause (in this case vaccination) and the onset of action (intussusception) is monitored.
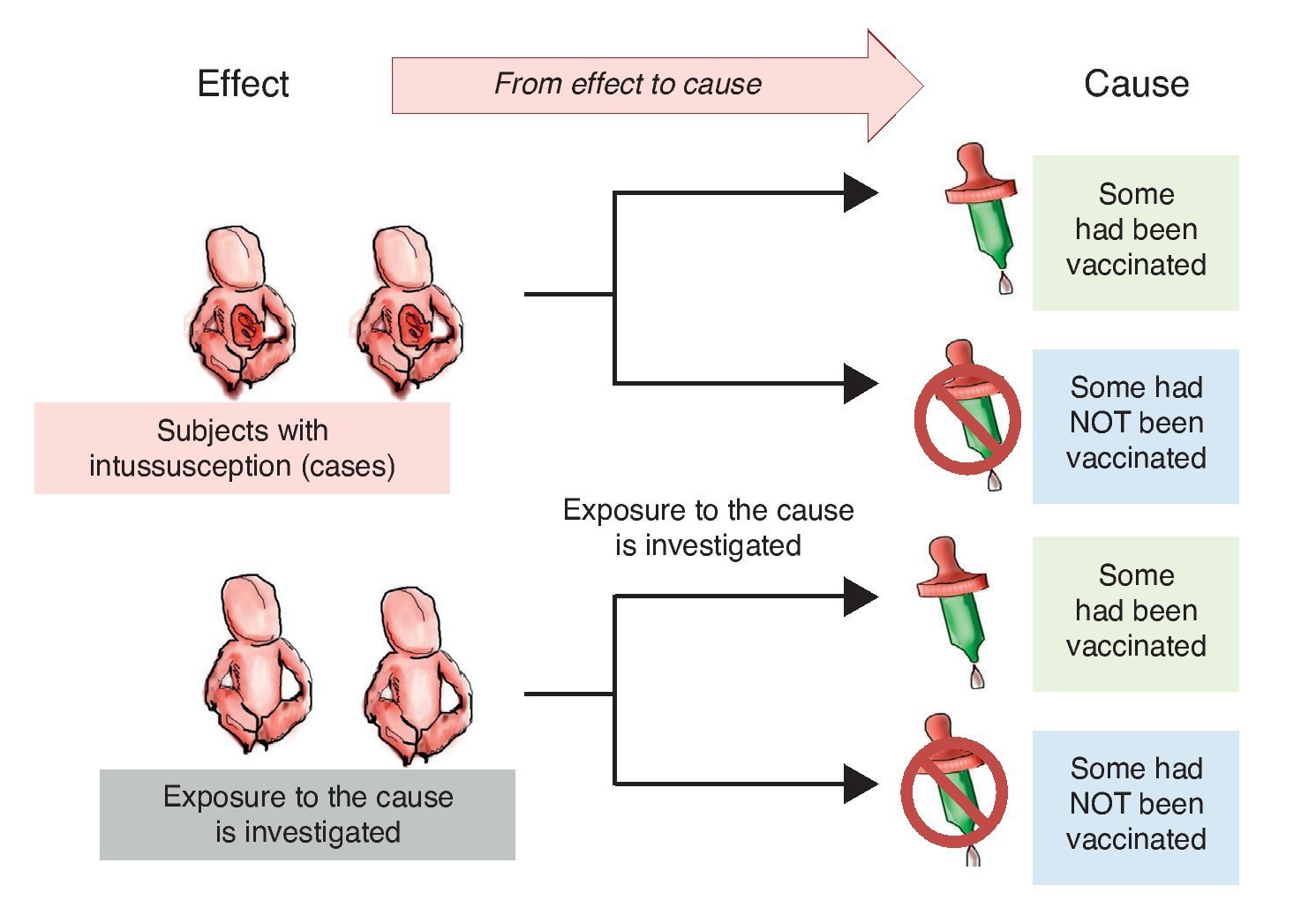
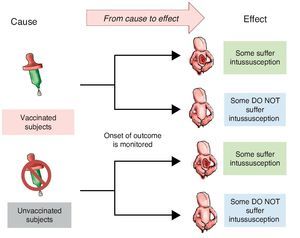
Investigators may also choose to use two study groups, not according to the exposure but in accordance with the effect; i.e., recruit a group that presents the effect or the disease and another group that does not in order to assess which of the two groups had greater exposure to the risk factor. This is a study with directionality from effect to cause. The group showing the effect is referred to as a group of cases and the group that does not have the intended effect is called the control group, the reason why such studies are referred to as case-control studies (Figure 2).
Figure 2 Case-control design. Study groups are defined by the presentation of the effect (intussusception) and whether or not they were exposed to the cause (vaccine) is investigated.
When there are no clues to what the possible cause and the possible effect are, a cause and effect directionality sometimes cannot be established. In these cases, only one study group is recruited: a population or a representative sample of this and several variables are measured in all subjects. This type of study is called a cross-sectional survey10 or cross-sectional analytical study.
Of the above five examples, study 2 has effect to cause directionality because it recruited participants at the time of the outcome—with intussusception or without—and vaccination history was subsequently collected as the alleged cause of intussusception. Studies 3-5 are cause and effect studies because all recruited patients at the time of exposure (vaccination) and from that time are followed until the occurrence of outcome (intussusception). In study 1 no cause and effect directionality is observed because it is a cross-sectional study.
3.4. Axis 4. Source from where data are collected: retrolective vs. prolective
There are two ways to collect data for a study. The first is after study planning directly from the primary source, e.g., by questioning subjects or using laboratory studies requested specifically for the study. In this case it is a prolective study. When it is from secondary sources existing before carrying out the project and usually using recorded data for purposes other than study, e.g., information files, records or databases, these studies are retrolective. Some projects may include data from both primary and secondary sources and in that case are ambilective.
It should be noted that, in general, each of the axis is independent of the other. Whereas a cohort study always has a cause and effect directionality and is often prolective, it can also be retrolective. Retrolectives cohorts are also called historical cohort. Case-control studies are generally retrolective but can also be prolective.
The proposed classification aims to provide a theoretical framework and a conceptual map for a better understanding of the logical structure of clinical research designs on causality. However, for practical purposes and specifically for purposes of publication, four basic types of clinical research on causality designs are recognized. In descending order in terms of their ability to provide evidence of causality, these four types are clinical trials, cohort, case-control and cross-sectional. These are basic models and each has multiple variants. Here are the essential characteristics of each one for easy identification (Figure 3).
Figure 3 Classification of basic clinical research designs.
4. Basic clinical research designs on causality
4.1. Clinical trial
The clinical trial is a comparative, experimental study with cause and effect directionality. In clinical trials of higher quality, the experimental maneuver is assigned randomly and blind to the study subjects. Clinical trials in which the maneuver is not randomly assigned are also known as quasi-experimental. The clinical trial is the design that allows providing the strongest evidence for a causal relationship between two phenomena, particularly if it is randomized and blinded. However, not all phenomena can be studied in a clinical trial because of the ethical, logistic and financial implications.
4.2. Observational studies
4.2.1. Cohort studies
Although there are descriptive cohort studies, for example, in order to estimate the incidence of an event or describe the natural history of disease, a cohort study generally refers to an observational study in which two or more cohorts defined by the degree of exposure to the risk factor under study are compared. In the English literature, the terms longitudinal and prospective study are sometimes used synonymously. We prefer to not use this terminology because it creates confusion with other meanings of these terms.
After the trial, the cohort study is the one in which further evidence produces the causal relationship between two phenomena. It has the advantages, compared to the case-control study, that exposure can be documented accurately as well as the temporal relationship between cause and effect. Moreover, various effects can be studied in the same exposure and it is useful for studying rare exposures. Compared with the case-control study, the cohort study has the disadvantage of requiring more time and usually more financial resources for its implementation. It also tends to be very efficient for rare effects.
4.2.2. Case-control studies
Case-control studies are observational studies where the frequency of exposure to a risk factor between a group of patients who have been exposed and a group of patients who have not are compared. In the English literature, the term retrospective study is sometimes used synonymously. Case-control studies have the advantage of requiring less time and budget than clinical trials and cohort studies. However, they have the disadvantage of being more susceptible to various biases. Therefore, the quality of evidence for a causal relationship is lower with respect to the two previously mentioned designs. They are particularly useful for rare diseases but are inefficient for rare exposures.
4.2.3. Cross-sectional studies
Cross-sectional studies are conducted at one point in time. Depending on whether one or more groups are formed, the study can be of two types:
a) Cross-sectional descriptive—generally used to estimate parameters (frequency, medium, etc.) of a variable in a population
b) Comparative cross-sectional (analytical)—in these studies the parameters are calculated in two or more groups; therefore, measures of association and differences among frequencies, mean or median of a variable among groups can be calculated.
As for the ability to demonstrate causation, cross-sectional studies are those that provide less evidence because, although comparative cross-sectional studies can calculate measures of association, they cannot establish the temporal relationship between dependent and independent variables.
For each particular problem in clinical research about causality, studies with different designs can be designed and carried out, but with the same objective. Sometimes the supporting evidence can be discordant. In others, certain types of designs may not be feasible for ethical, logistic or financial issues. In these cases, one should choose the evidence from studies with the strongest designs and best quality.
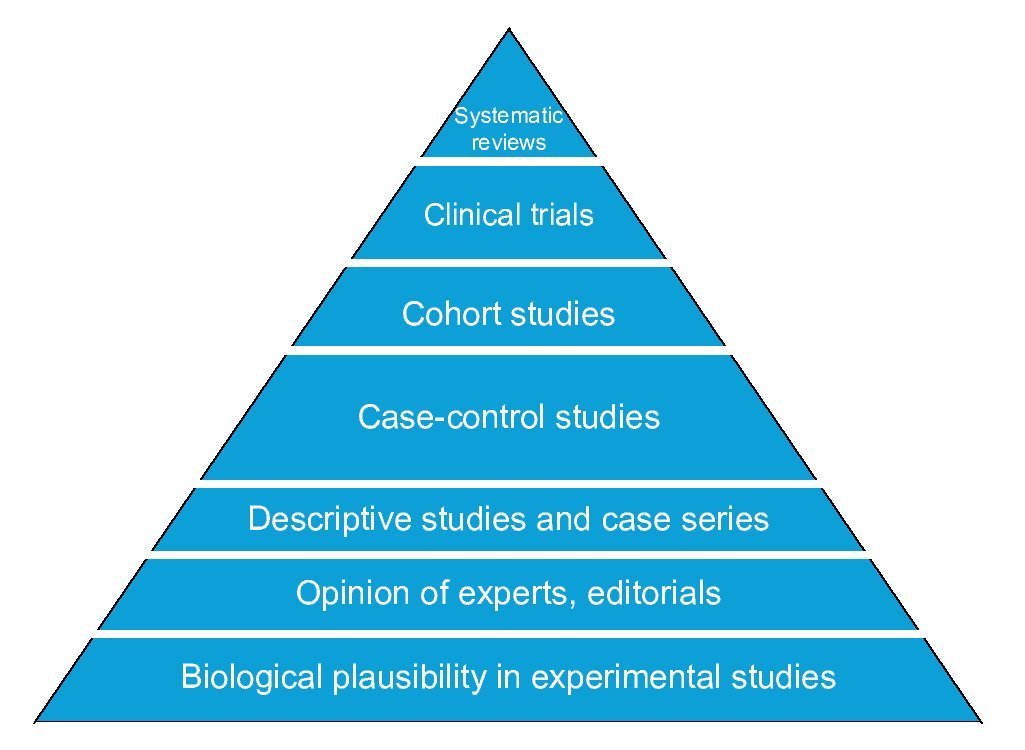
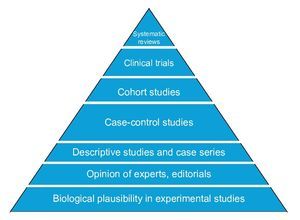
The basic designs to answer questions about causality in clinical research differ in four areas: number of study groups, implementation of an experimental maneuver, cause-effect directionality and the source from which the data are collected. The randomized clinical trial is the best evidence of causality because it satisfies the criterion of experimentation. Observational cohort and case-control studies also allow estimating the association between cause and effect as well as their timing; however, of these, the cohort design has greater strength than the case-control design that is less susceptible to bias. Comparative cross-sectional studies only make possible estimating the association between variables, but not the timing of events, so they have the weakest design to show causality. Descriptive studies do not provide information about causality because it is impossible to estimate the strength of association between cause and effect (Figure 4).
Figure 4 Pyramid of evidence of research designs. Designs higher up on the pyramid are those with the greatest strength to establish causality.
Of the examples discussed in this paper, study 1 does not allow making any inference about the relationship between rotavirus vaccine and intussusception because it is a descriptive study. In regard to study 2 with a case-control design and whose conclusion was a positive association between vaccination and intussusception, it can be said that it was an efficient way to provide evidence taking into account the speed with which conclusions were reached. In fact, in the absence of better evidence, this was sufficient to suspend the application of the vaccine against rotavirus. Studies with a cohort design and randomized clinical trials found no association between the vaccine and intussusception. Because these designs are stronger than cross-sectional and case-control studies, their findings are more reliable.
In science there is no definitive answer. For this reason, it is preferable to continue conducting studies on a question apparently already resolved. Concondances support a fifth criterion of casualty of those proposed by A.B. Hill: the consistency.2 Disagreements are a source of new research questions and new hypotheses. Moreover, in addition to the primary designs, there is a method to evaluate the combined results of several primary studies by systematic literature review and meta-analysis.
Conflict of interest
The authors declare no conflict of interest of any nature.
a The work cited included several approaches including a longitudinal and comparative (case series) part. For teaching purposes, it was simplified and presented only one of its parts to illustrate a descriptive cross-sectional design.
Received 14 September 2015;
accepted 15 September 2015
☆ Please cite this article as: Castilla-Peón MF, Ramírez-Sandoval JC, Reyes-Morales H, Reyes-López A. Diseño de estudios clínicos y causalidad: ¿la vacuna oral contra rotavirus causa invaginación intestinal? Bol Med Hosp Infant Mex. 2015. http://dx.doi.org/10.1016/j.bmhimx.2015.09.005
* Corresponding author.
E-mail:fernandacastillapeon@gmail.com (M.F. Castilla-Peón).