1. Summary of the clinical history (A-12-05)
1.1. History
Preterm newborn with abdominal wall defect admitted to the HIMFG at 24 h of life. The 16-year-old mother received prenatal care, folic acid and multivitamins from the first trimester of pregnancy. Three obstetric ultrasounds were performed. The latter showed severe oligohydramnios and an abdominal wall defect.
The baby was born in a general hospital via cesarean section at 36 weeks gestation (WG). Weight at birth was 2200 g and length of 42 cm; Apgar score was 7/9. There was an abdominal wall defect with exposure of the bowel loops and stomach, which were wrapped in gauze and covered with a sterile bag. Mechanical ventilation via an endotracheal tube was started. The baby was maintained NPO with intravenous solutions and antibiotic coverage with cefotaxime and amikacin. Under this management she was transferred to the Hospital Infantil de México Federico Gómez (HIMFG).
On admission to the HIMFG the following data were recorded: weight 2620 g, heart rate 160/min, respiratory rate 50/min, blood pressure 53/37 mmHg, temperature 36ºC, capillary refill 2 sec. Physical examination showed the newborn to be well-hydrated with pale skin, unconscious due to sedation, continuous murmur on the second left intercostal space, and abdomen with a 4-cm diameter wall defect to the right of the umbilical cord with exposed swollen bowel loops, pink/reddish in color, covered with a bag and fixed with bandages. The remainder of the examination was without alterations.
In the neonatal intensive care unit she remained under fasting conditions, intravenous fluids and antibiotic coverage with amikacin, ampicillin and metronidazole. Laboratory studies on admission showed changes in liver function tests: total bilirubin (TB) 2.66 mg/dl, direct bilirubin (DB) 2.56 mg/dl, aspartate aminotransferase (AST) 269 U/l, ala-nine aminotransferase (ALT) 82 U/l, albumin 0.8 g/dl, globulins 1.6 g/dl, total proteins 2.4 g/dl.
Patent ductus arteriosus of 2 mm diameter with 21 mmHg pressure gradient was identified on echocardiogram. Right ventricular systolic pressure (RVSP) was 48 mmHg.
On surgical examination there were no perforations or intestinal ischemia. A sutured silo was placed to the aponeurosis such that the bowel loops were retracted towards the abdominal cavity by gravity. The patient was maintained on parenteral nutrition postoperatively. Ventilatory management was complicated and required increase in parameters until the end of the first postoperative week when high frequency ventilation and administration of nitrous oxide was initiated. Due to the presence of right basilar infiltrate on plain chest x-ray and an increase in leukocytes and bands, the antibiotic scheme was changed to cefepime and amikacin.
The patient was hemodynamically instable during the second postoperative week and aminergic and milrinone support was initiated. Strategies of alveolar recruitment, nitrous oxide and high frequency ventilation with high oxygen concentration were continued. The antibiotic scheme was changed to meropenem and vancomycin.
Hemodynamic state improved during the third postoperative week, which allowed for the aminergic and ventilatory support to be decreased. Bowel loops appeared pale pink with cetrine fluid. Transfontanellar ultrasound identified a grade II intraventricular bleed. Echocardiogram carried out that week demonstrated PSVD 24 mmHg, ejection fraction of 79%, shortening fraction of 45% and ductus arteriosus almost closed.
During the fourth week of admission, albumin infusion was begun at 25% at 0.5 mg/kg because of albumin persisting <1.5 g/dl. Bowel loops continued to show good color and gradual descent into the abdominal cavity. However, the patient presented with ventilatory and hemodynamic deterioration and mannans 190 pg/ml. Administration of amphotericin B was begun and aminergic and ventilatory support was increased until cardiorespiratory arrest occurred at 24 days of life, which did not respond to resuscitation maneuvers.
2. Imaging (Dra. Maria Teresa Valadez)
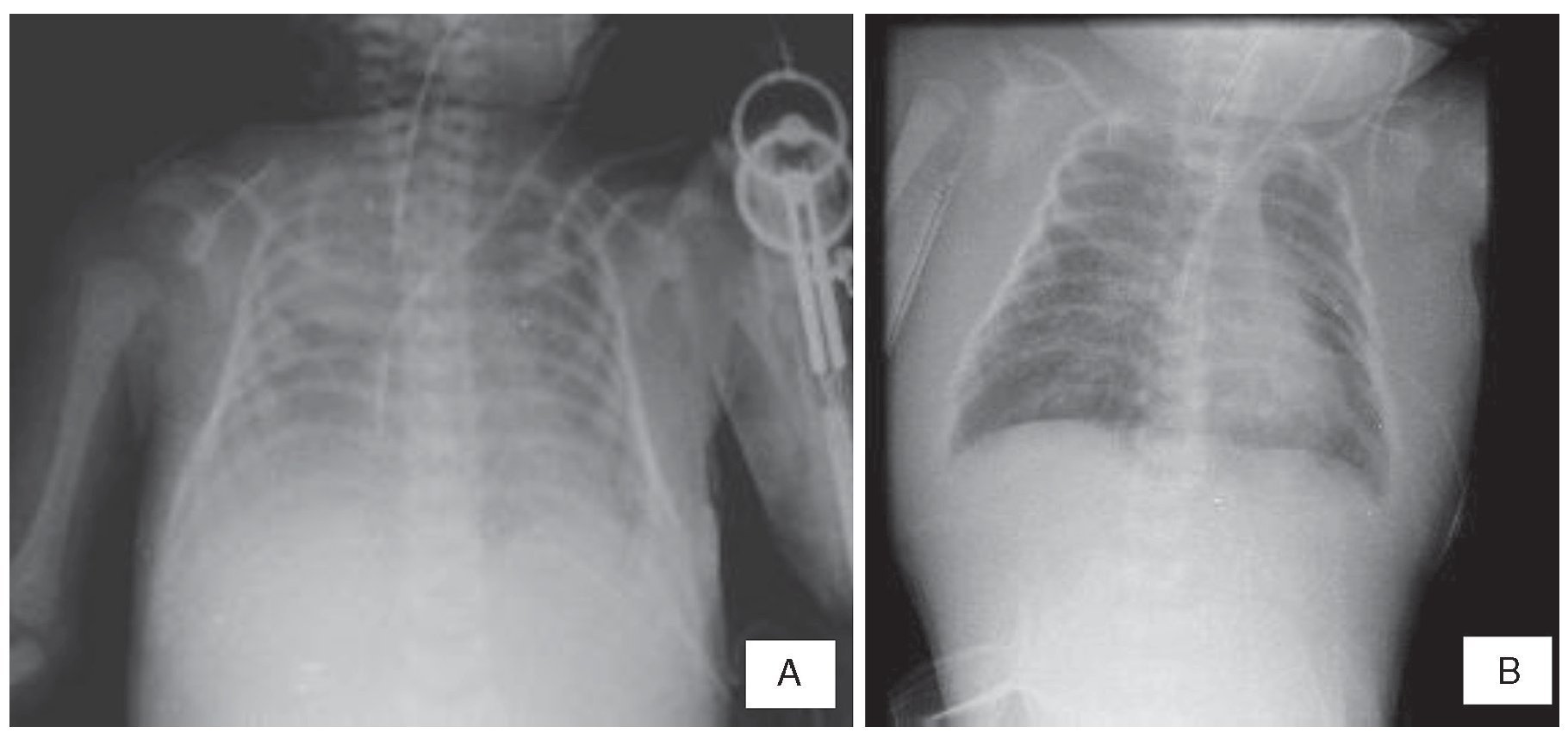
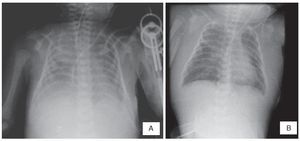
The last x-rays showed effacement of the costodiaphragmatic angles, opacity in the left lung and thickening of the soft tissues (Figure 1A). An area of consolidation cannot be ruled out. Soft tissue edema can be seen diffusely up to the neck and a pulmonary infiltrate suggestive of acute pulmonary edema (Figure 1B).
Figure 1 (A) Thickening of the soft tissues and effacement of the costophrenic angles is seen. (B) Persistence of the edema in the soft tissues of the chest and neck and data of pulmonary edema.
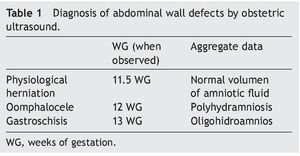
In our environment, diagnosis of gastroschisis is not established as frequently as it should be. One possible explanation is that the physicians performing the obstetric ultrasounds are not radiologists but OB/GYN and neonatologists who are not always experienced in imaging methodology. For this reason, a summary of the useful ultrasound findings are presented in Table 1. Other findings associated with the severity of damage in gastroschisis have been described, such the presence of a dilated fixed loop >2.5 cm.
3. Case presentation (Dra. Ma. Guadalupe Vega González)
Treatment of a newborn with abdominal wall defect should be initiated from the prenatal period. Physiological closure of the abdominal wall takes placed between the 10th and 13th WG. Diagnosis of gastroschisis is possible at the end of the first trimester with an ultrasound with sensitivity from 60-75% and specificity from 95%. A differential diagnosis should be done with oomphalocele in which in contrast to gastroschisis there is a membrane covering the loops. Thickness and dilation of the intraabdominal loops can be measured and with this estimate the risk of intestinal atresia and poor prognosis.1
Being the child of an adolescent mother and product of a first gestation are risk factors for congenital malformations such as gastroschisis. Intrauterine growth restriction is an associated factor as it is found in 20−60% of the cases. History of severe oligohydramnios should have alerted the health personnel because this finding is associated with high infant mortality and indicates the need for referral to a specialized center that has a perinatologist, obstetrician, pediatric surgeon and neonatologist.2
Management should begin with airway protection and fluid resuscitation according to the requirements of each patient. Subsequently, an orogastric tube should be placed to decrease the pressure in the mesenteric vessels. In order to protect the eviscerated structures it is recommended that they be covered with a sterile bag, preferably Silastic, to avoid loss of proteins, fluids and heat through the bowel loops.3 It is important that personnel who receive the newborn with gastroschisis are aware that they should cover the bowel loops with sterile bags immediately after the resuscitation to avoid contamination and damage to the intestinal serosa. If the pediatric surgeon is present to quickly perform reduction of the bowel loops, the intestine can be kept covered with moist sponges that allow for continuous hydration.4
From January 2014 in the neonatal intensive care unit of the HIMFG, a management protocol of patients with gastroschisis consisting of the following was carried out:
1. Safe referral and transfer of the patient with direct assessment, bowel loops covered with plastic bags and without use of moist gauze.
2. Surgical management that includes use of pre-armed silo or closure of the wall without sutures, depending on the clinical conditions of the patient.
3. Use of gentle anesthesia to avoid the need for intubation
4. Medical management with early initiation of parenteral and enteral feeding and antibiotic coverage. Using this management protocol, mortality decreased to 2.5% (E. Bracho-Blanchet, personal communication).
Due to the ease of placement of the pre-armed silos at the patient’s bedside without the need for general anesthesia, reintroduction of the bowel loops to the abdominal cavity has been facilitated in patients with gastroschisis abdominal wall defects. These silo bags are economical and efficient pre-armed devices for the progressive reduction of severe gastroschisis, with a ring whose internal diameter adapts itself to the defect of the peritoneal cavity. Placement of the silo decreases the risk of intraabdominal hypertension and compartmental syndrome associated with primary closure of the defect.
Patients with severe hypoalbuminemia, i.e., <1.5 g/dl, have high mortality and high frequency of failure to tolerate enteral feeding and require prolonged parenteral nutrition with a mean of 28 days after abdominal wall closure. Low albumin levels are due to the loss of proteins during the fetal period through the exposed intestine. Albumin infusion could be considered although its use is controversial in neonatology due to the described adverse effects.5
From the infectious diseases point of view, in this case the presumptive diagnosis of invasive candidiasis was established, which is a nosocomial infection present in 0.5-1.2% and with mortality up to 75% according to the patient’s weight and Candida species involved.6 Among the risk factors for nosocomial invasive candidiasis are prolonged hospitalization, prolonged administration of wide spectrum antibiotics and placement of tubes and catheters. Confirmatory diagnosis is carried out with cultures; however, sensitivity is very low. Treatment is frequently initiated empirically. Final diagnoses are mentioned below:
1. Late preterm newborn of 36 WG
2. Restriction of symmetrical intrauterine growth
3. Gastroschisis type abdominal wall defect
4. Secondary peritonitis
5. Hypoalbuminemia
6. Persistence of ductus arteriosus in closure routes
7. Persistent pulmonary arterial hypertension
8. Nosocomial pneumonia
9. Hypoxic respiratory failure
10. Grade II intraventricular hemorrhage
4. Pathology (Dra. Ma. de Lourdes Cabrera Muñoz)
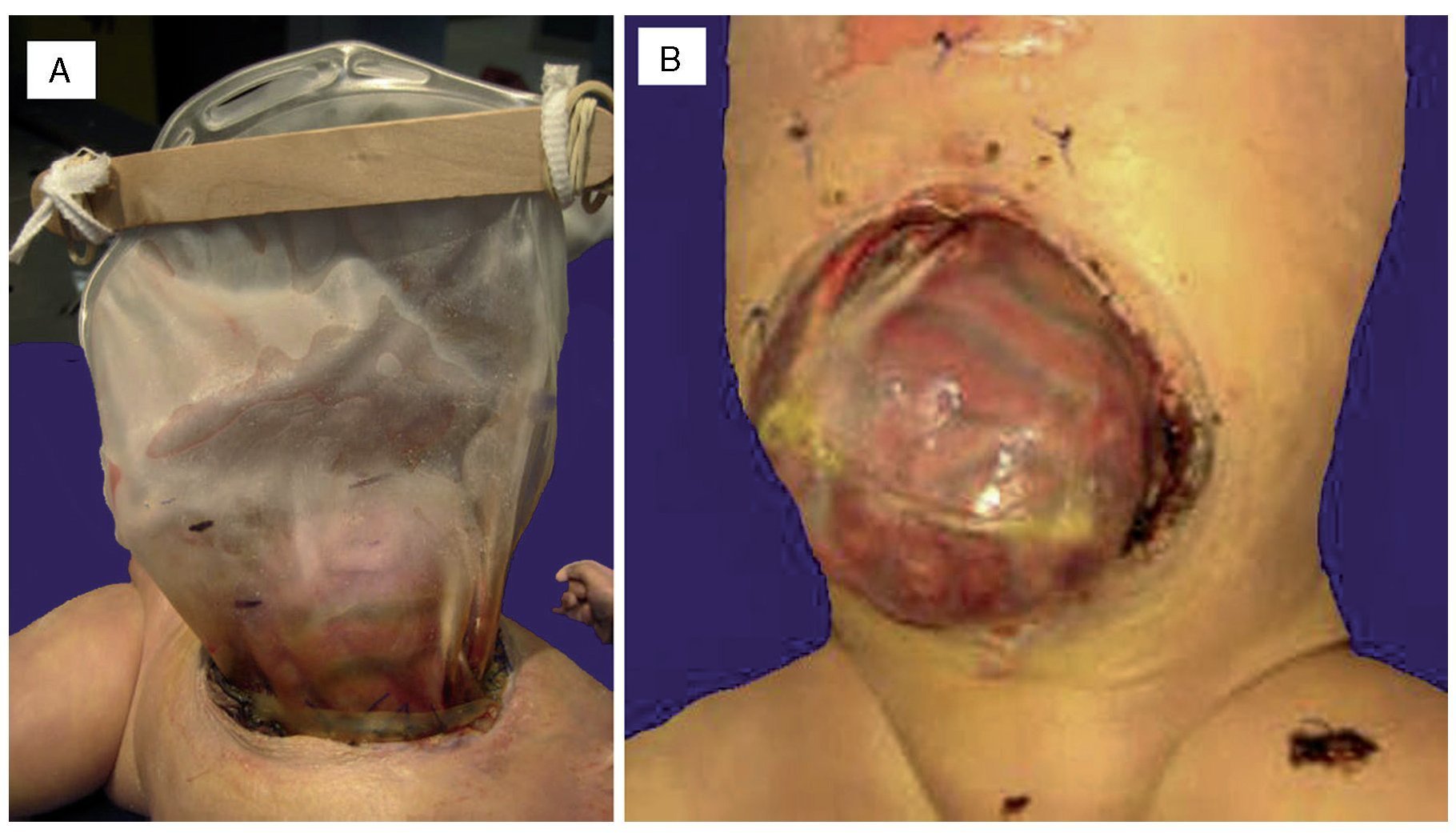
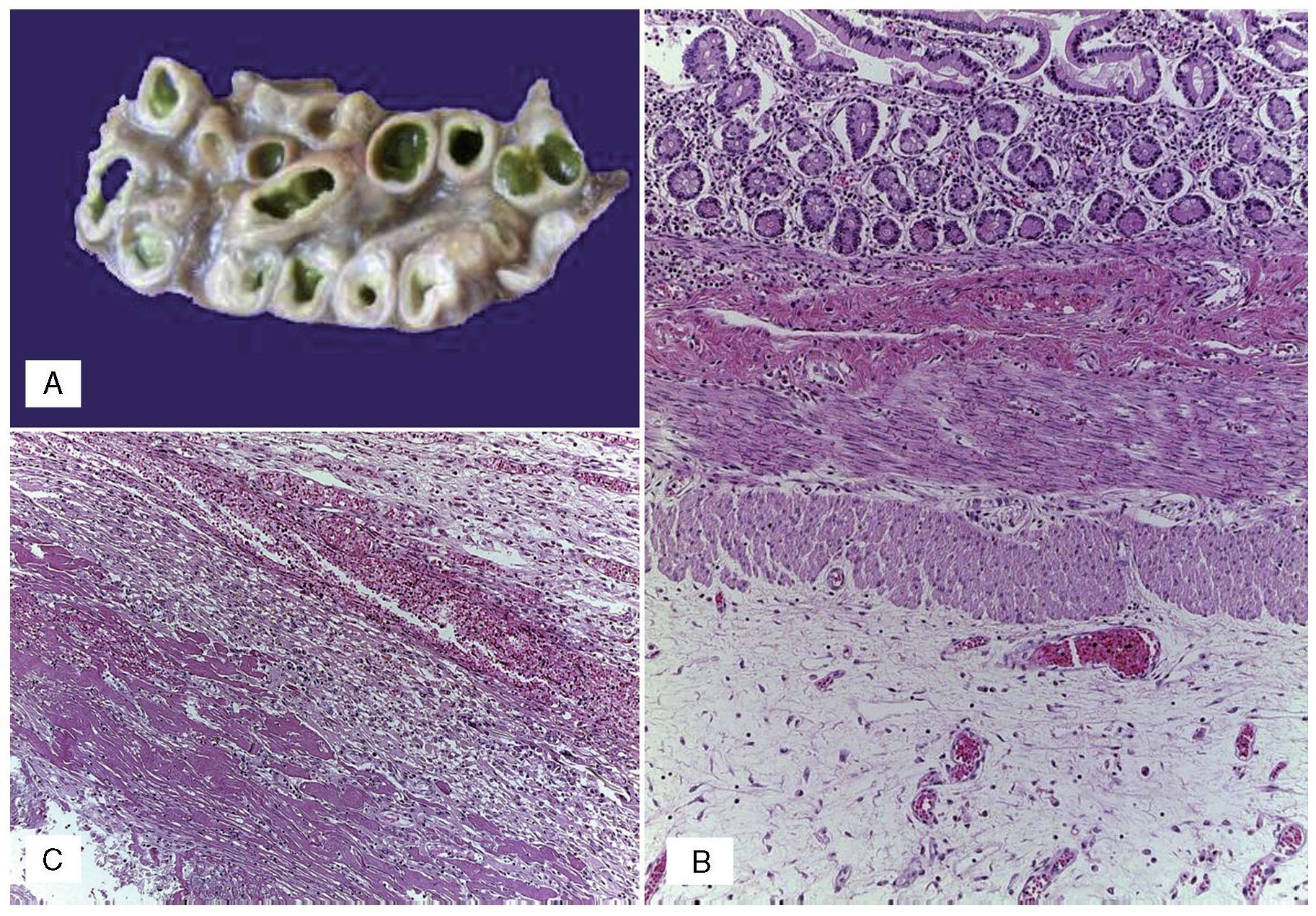
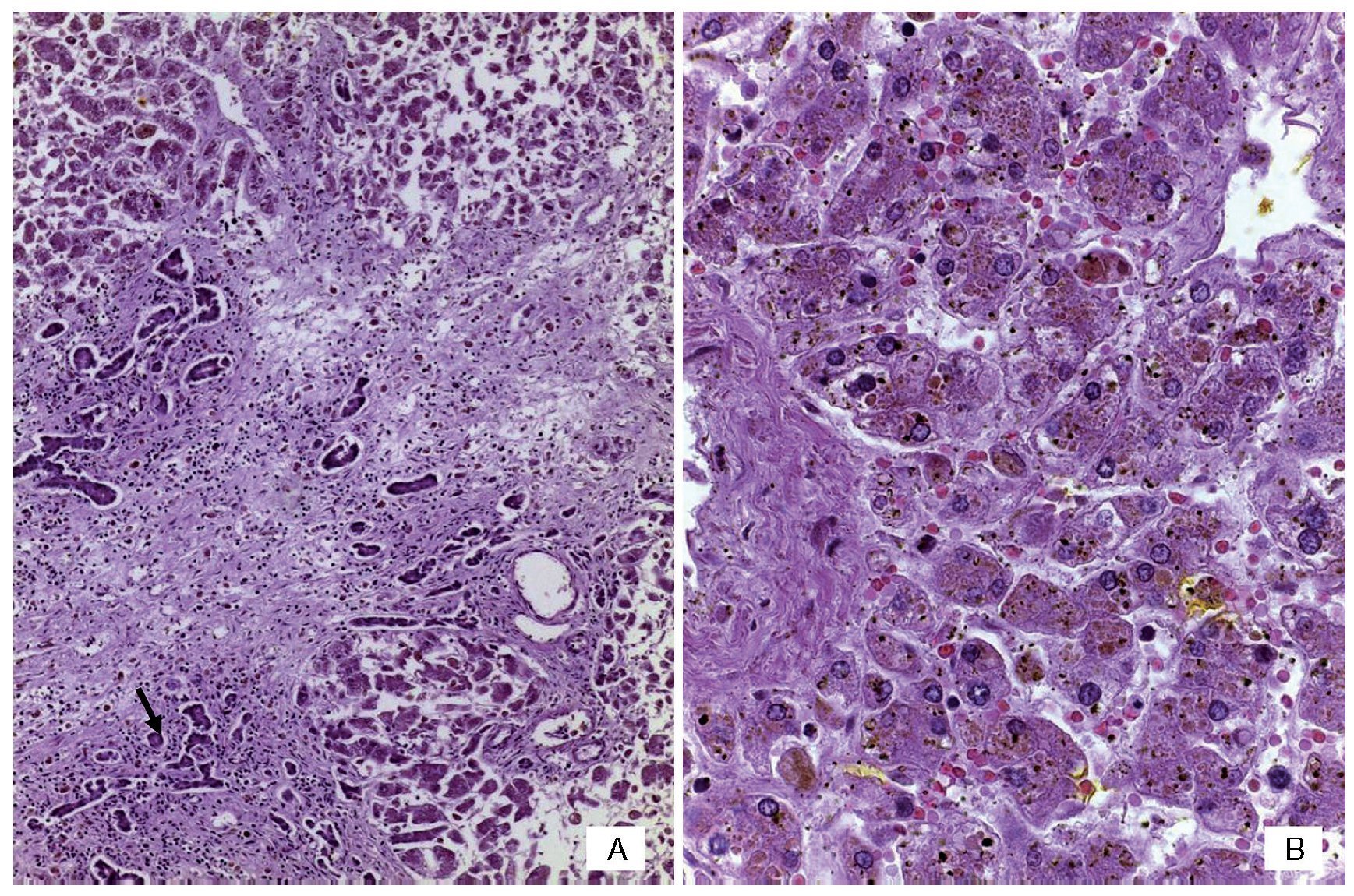
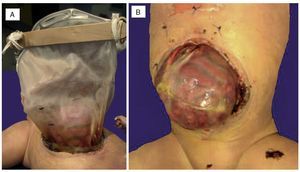
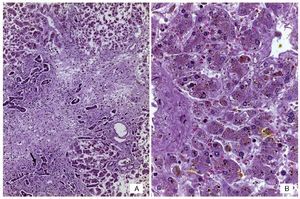
An autopsy was done of a newborn with edema of the skin and soft tissues as well as a 12-cm diameter abdominal wall defect covered with plastic silo with intact sutures (Figure 2). When the silo was removed it was noted that the small bowel loops were outside the abdominal cavity due to viscero-abdominal disproportion and were covered by a whitish fibrin deposit, an edematous appearance and joined by adhesions. Histopathological study of the digestive apparatus showed submucosal edema of the stomach and intestinal serosa and acute extensive chronic fibroadhesive peritonitis (Figure 3). The liver was soft, increased in size, and parenchyma was of brown-yellow color. Microscopically, recent panlobular parenchymal bridging necrosis was observed with the proliferation of neocholangioles and collapse as well as hepatocellular and ductal cholestasis (Figure 4). No viral inclusions or microorganisms were identified; therefore, the necrosis was considered to be secondary to shock. Since the patient’s admission, she presented a picture of severe hypoalbuminemia, which has been associated with a poor prognosis. It has been reported that the hypoalbuminemia is due to the loss of fluid from the amniotic cavity secondary to the abdominal defect and exposure of the abdominal loops. However, the liver necrosis that the patient developed towards the end also contributed to worsening of the hypoalbuminemia due to the lack of synthesis.7,8
Figue 2 (A) Complete silo. (B) A 12-cm diameter wall defect, small intestine outside the abdominal cavity with extensive peritonitis and dark red coloration.
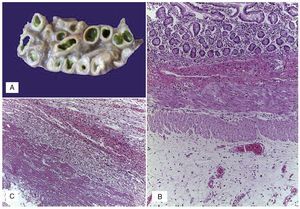
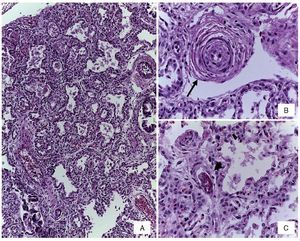
Figure 3 (A) Dilated small bowel loops with meconium in the lumen, wall edema and interase adhesions. (B) Serosal edema. (C) Thickening of the peritoneum due to fibrosis, mixed inflammatory infiltrate and fibrin.
Figure 4 (A) Panoramic view of the liver with bridging necrosis of hepatocytes and proliferation of neocholangioles (arrow). (B) Hepatocellular cholestasis and bile deposits in Kupffer cells (H/E 400x).
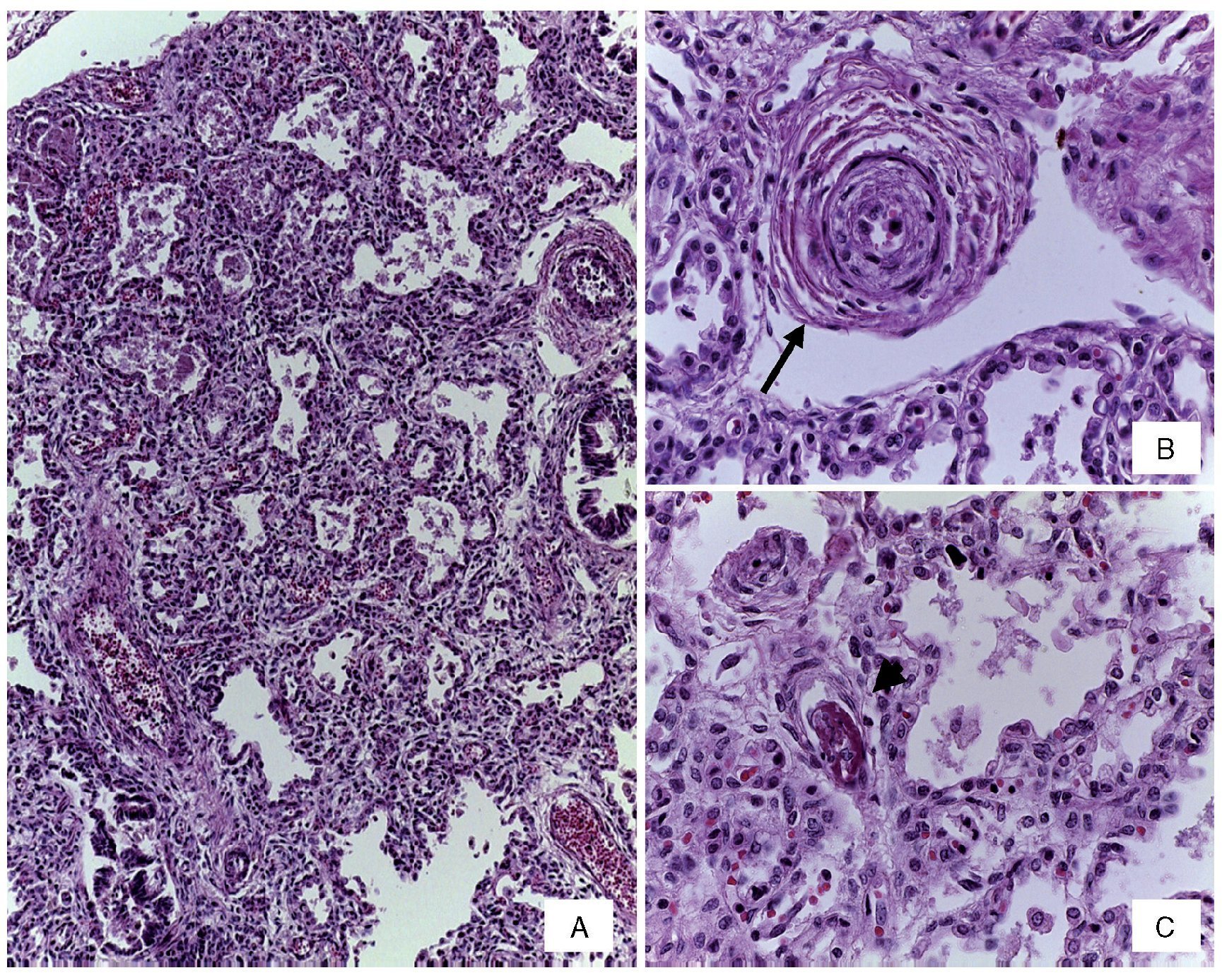
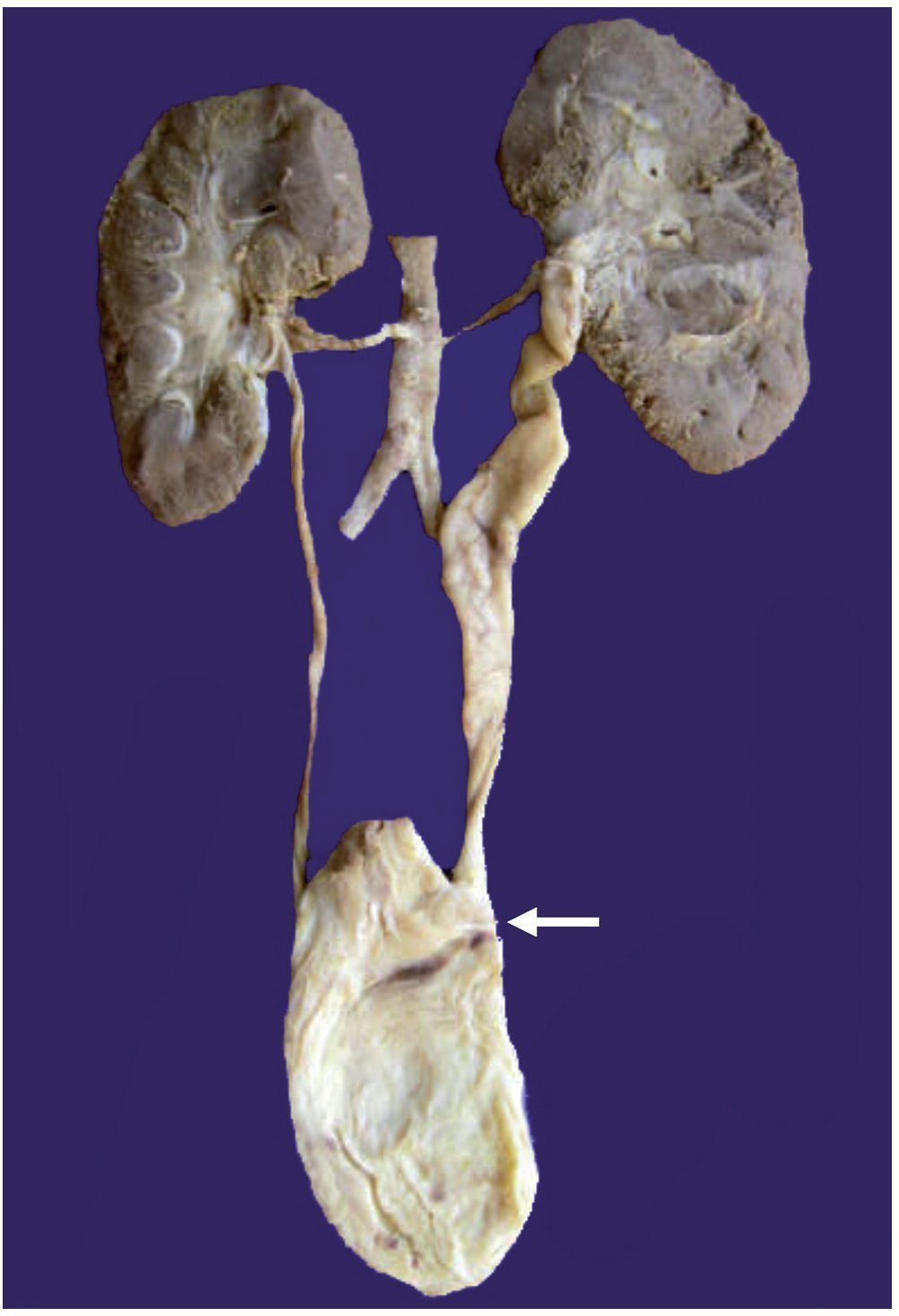
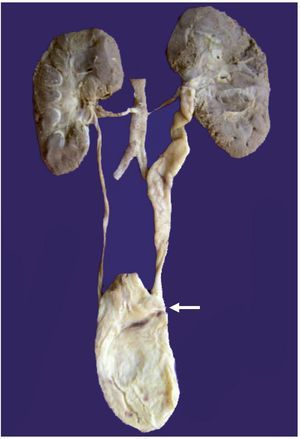
Changes were seen in the arterial pulmonary vasculature that corresponded to morphological data from grade B vascular hypertension and thickening of the septum from lymphocyte infiltrate due to pneumonitis (Figure 5). No fungi, bacteria or viral inclusions were identified in cuts or special stains. An associated malformation, an anomalous implantation from the ureter to the vagina, was found, which caused dilation and tortuosity of the lumen (Figure 6). The brain presented data of hypoxic encephalopathy and no intraventricular hemorrhage was found. This was the last patient with gastroschisis who had a postmortem study performed in the HIMFG. In the Pathology Department of this institution there were 1388 autopsies performed from 1997–2011, of which 29 were for abdominal wall defects. Of these, 22 were described as gastroschisis (Table 2). An increase in the frequency of gastroschisis in the offspring of young women has been reported in Europe. It is expected that the number of cases that arrive at third-level care hospital would be higher.9
Figure 5 (A) Thickening of the interalveolar septa by lymphocyte infiltrate. (B) Pre-acinar artery with grade B pulmonary vascular disease (arrow). (C) Capillary fibrin thrombus (arrowhead) (H/E stain).
Figure 6 The left kidney is increased in size with tortuous and dilated ureter due to abnormal implantation in the bladder (arrow).
4.1. Final diagnoses
Gastroschisis with exposure of the small intestine and stomach.
4.2. Concomitant alterations
• Viscero-abdominal malproportion
• Acute and chronic peritonitis with adhesions
• Status post-silo placement
• Severe hypoalbuminemia
• Anasarca
• Soft tissue edema
• Submucosal edema in the stomach and intestinal serosa
• Pulmonary vascular disease grade B
• Submassive liver necrosis
• Hepatomegaly (300 g vs. 127 g)
• Severe mixed hepatic cholestasis
• Hypoxic-ischemic encephalopathy
• Hydronephrosis and left hydroureter secondary to anomalous implantation
The immediate cause of death was attributed to hypoalbuminemia and sepsis. Autopsy cultures were positive for Staphylococcus epidermidis in the blood culture, right lung, spleen, and liver.
5. Final comments
5.1. Department of Surgery (Dr. Eduardo Bracho Blanchet)
Since 2014, a new management protocol for patients with gastroschisis has been implemented at our institution. This consists of the following steps:
1. Early referral and safe transfer
2. Surgical management with pre-armed silo placed at the bedside
3. Multidisciplinary medical management
4. Early parenteral feeding
With this strategy, mortality has decreased. The patient presented here was the last to expire in the HIMFG due to gastroschisis before 2014. From 2014-2015 there have been 35 patients with gastroschisis treated with closure of the defect without the use of sutures. Since then only one patient has died, but no autopsy was performed.
5.2. Gastroenterology Department (Dr. Salvador Villalpando Carrión)
Current management of patients with gastroschisis is based on the following treatment:
1. Early parenteral nutrition and of short duration
2. Management of cholestasis
3. Introduction of new lipids
4. Early enteral nutrition (5 days)
5. Parenteral glutamine
A case of gastroschisis wall defect is reported, with a poor and rare prognostic factor, severe hypoalbuminemia. The patient died in 2012 and was the last patient with this disease who had a postmortem study performed because beginning in 2014 we implemented a new management protocol with very good results. The importance of early referral and safe transfer of these patients to a tertiary-level care hospital for advanced multidisciplinary management should be emphasized.
Ethical disclosure
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflict of interest
The authors declare no conflict of interest of any nature.
Received 20 August 2015;
accepted 24 August 2015
☆ Please cite this article as: Zalles-Vidal C, Vega González MG, Valadez Reyes MT, Cabrera-Muñoz ML. Prematuro tardío con gastrosquisis e hipoalbuminemia grave. Bol Med Hosp Infant Mex. 2015. http://dx.doi.org/10.1016/j.bmhimx.2015.08.004
* Corresponding author.
E-mail:cabreramalu@aol.com (M.L. Cabrera-Muñoz).