More than 90% of the population is infected by Epstein-Barr virus (EBV), which has sophisticatedly evolved to survive silently in B cells for the life of infected individuals. However, if the virus-host balance is disturbed, latent EBV infection could be associated with several lymphomas. The age at primary infection varies substantially worldwide, and exposure to EBV is likely to be due to socioeconomic factors. In Argentina, EBV infection is mostly subclinical and 90% of patients are seropositive by the age of 3 years; therefore, its epidemiological characteristics resemble those of an underdeveloped or developing population. EBV-positive Hodgkin lymphoma (HL) in young adults from developed populations has been attributed to delayed primary EBV infection as suggested by the association with recent mononucleosis development. EBV-associated Burkitt lymphoma and Hodgkin lymphoma in children from Argentina display frequencies similar to those observed in developed countries, whereas EBV presence in pediatric diffuse large B-cell lymphoma is slightly increased compared to those populations. However, EBV presence is statistically associated particularly with patients < 10 years of age in all three entities. Therefore, a relationship between low age of EBV seroconversion and B-cell lymphoma development risk could be suggested in children from Argentina.
Más del 90% de la población se encuentra infectada con el virus de Epstein-Barr (VEB), que ha evolucionado sofisticadamente para sobrevivir de por vida de manera silenciosa en las células B de individuos infectados. Sin embargo, si el balance entre el virus y el huésped se altera, la infección latente por VEB se podría asociar con linfomas severos. La edad de la infección primaria varía sustancialmente a escala global, y la exposición al VEB al parecer se relaciona con factores socioeconómicos. En Argentina, la infección por VEB es mayormente subclínica, y el 90% de los pacientes son seropositivos a la edad de 3 años. Por lo tanto, las características epidemiológicas se asemejan a aquellas de una población subdesarrollada o en vías de desarrollo. El linfoma de Hodgkin (LH) positivo para VEB en adultos jóvenes se ha atribuido a una infección por VEB tardía en economías desarrolladas, como lo sugiere la asociación con el desarrollo de mononucleosis. En Argentina, el linfoma de Burkitt y el linfoma de Hodgkin asociados con VEB en ninos presentan frecuencias similares a las observadas en países desarrollados, mientras que la presencia de VEB en el linfoma difuso de células B pediátrico se encuentra con un ligero aumento comparado con estas poblaciones. Sin embargo, la presencia de VEB en los tres padecimientos se asocia estadísticamente, en particular, con pacientes menores de 10 años. Por ello, se podría sugerir una la relación entre la menor edad de seroconversión y el riesgo de desarrollo de linfoma de células B en niños de Argentina.
Epstein-Barr virus (EBV) isa ubiquitous double-stranded DNA virus that belongs to the Herpesviridae family and Gammaherpesvirinae subfamily. EBV is characterized by a tropism for B-lymphocytes displaying latent infection in the host and the capacity for transforming B-lymphocytes. More than 90% of the population worldwide carries the virus,1 which has sophisticatedly evolved to survive in B cells for the life of infected individuals. The result is a finely balanced relationship that allows the virus to be acquired silently early in life and carried thereafter as a lifelong asymptomatic infection in the B lymphoid system. However, the virus-host balance can be disturbed in various ways, and one of a range of virus-associated diseases may then ensue.2 Latent EBV infection is linked to many human malignancies. In immunocompetent persons, EBV is associated with ~20% of Burkitt lymphoma (BL) in the developed world, almost all African BL, 50% of Hodgkin lymphoma (HL), 10% gastric carcinomas, almost all endemic nasopharyngeal carcinoma (NPC), certain fractions of diffuse large B-cell lymphoma and T-cell lymphoma. In the absence of normal T-cell immune responses, EBV-infected B-lymphocyte proliferations can cause lymphoproliferative disease (LPD), similar to post-transplant LPD. The persistence of EBV genomes in cells of these malignancies, even in subjects with otherwise normal immune response, is consistent with the notion that EBV genomes are important for malignant cell growth.3
The age at primary infection varies substantially worldwide, and exposure to EBV is likely to be due to socioeconomic factors. Young children most likely acquire primary EBV infection due to close contact that involves exchange of oral secretions via shared items such as toys, bottles, and utensils. Before the age of 10 years, primary infection is usually asymptomatic. In adolescents and young adults, however, primary EBV infection frequently presents as infectious mononucleosis (IM). Seroprevalence of EBV varies widely by geographic location. Data indicate that primary EBV infection occurs at a younger age among persons from lower vs. higher socioeconomic backgrounds, which has been attributed to crowded living conditions.4 A delay in acquiring a primary EBV infection at an older age in childhood or adolescence, which usually occurs in more developed countries, can manifest itself as infectious mononucleosis, occurring in ~25–75% of EBV-infected persons.5 The severity of primary EBV infection in adults increases with age, and patients > 40 years of age are especially prone to serious illness. EBV infections in children < 10 years are often overlooked, either because they are entirely asymptomatic or because they do not present with a typical IM syndrome.4
There is a hypothesis that proposes that the clinical presentation variability related to the age of EBV primary infection is linked to the different magnitude of the viral dose received by a child or a young adult through salivary contact.6 Another possibility is that IM in adolescents may reflect the global CD8+ T-cell lymphocytosis, with a great proportion of activated EBV-specific CD8+ T-cells.7 In contrast, it was recently described in children from Africa that asymptomatic EBV infection elicits a virus-specific CD8+ T-cell response that can control the infection, without CD8+ T-cells over-expansion.8 Moreover, it was suggested that preexisting NK cell populations in children may provide an explanation for why IM occurs more frequently in adolescents and adults than in children.9 In Argentina, EBV infection is mostly subclinical and 90% of patients are seropositive by the age of 3 years; therefore, its epidemiological characteristics resemble those of an underdeveloped or developing population.10
2EBV infection in relation to lymphoma developmentAs previously mentioned, EBV is among the infectious agents whose clinical manifestations vary according to age at primary infection. Moreover, its epidemiological behavior is similar to that of HL. The relatively few cases of EBV-positive HL in younger adults may be attributed to delayed primary EBV infection as suggested by the association with mononucleosis.11 In fact, IM was associated with an increased risk of EBV-positive HL, along with the particularly pronounced risk in younger adults. Furthermore, IM-associated lymphomas occurred with a median of 2.9 years after infection in patients from a developed population. Although the idea that EBV is merely an etiologically innocent passenger in the neoplastic HL cells cannot easily be dismissed, it remains more likely that the observed association indeed reflects causality,12 at least in cases with recent IM development. In contrast, no associations between EBV serology status, elevated antibody titers against EBNA-1, EBNA-2, VCA, or EA, and risk of non-Hodgkin lymphoma (NHL) overall was demonstrated in immunocompetent individuals, with the possible exception of chronic lymphocytic leukemia (CLL). Therefore, it was proposed that the biological mechanism for the causal role of EBV in the pathogenesis of NHL would predominantly involve immune system deregulation.13
The temporal risk variation in HL after IM suggests that the predilection for the younger adult age group may simply result from the combination of age and time since IM rather than from other mechanisms particular to HL in younger adults.14 It is therefore tempting to speculate that a similar association may exist between primary EBV infection and HL risk at any age, e.g., in childhood.12 EBV epidemiology in Argentina reflects a typical pattern of a developing country with primary infection in early childhood and the epidemiology of EBV-related lymphomas differs from that observed in developing countries. Therefore, EBV infection in our pediatric patients shows a complex epidemiological pattern, very attractive to investigate.
3EBV in pediatric Hodgkin lymphomaIn the United States and most European countries, HL shows an annual incidence of 4.5–6.0 per million and its incidence increases with age and peaks between 15 and 30 years. In contrast, in developing countries, it shows a high incidence in children exceeding 7 per million per annum and 70% of cases occur in children < 10 years of age.15 EBV presence in HL varies with geographic location, age, sex, clinical stage, and histologic type. EBV is detected in Hodgkin Reed-Sternberg (HRS) cells in ~40% of classical HL in the Western world, mostly in cases of mixed cellularity (MC) and lymphocyte-depleted subtypes, and less frequently in nodular sclerosis and lymphocyte-rich classical subtypes. EBV is rarely found in nodular lymphocyte predominant HL (NLPHL).16 Developing countries have an increased incidence of EBV-positive cases, which may be attributed to the existence of underlying immunosuppression.17 In addition, in developed populations EBV-positive HL also affects more Asians and Hispanics than whites or blacks and in the UK is more common in South Asian children compared with non-South Asian children.15
Childhood HL is defined as affecting those patients ≤16 years of age. It represents ~10% of all diagnosed HL and typically shows a significant prevalence of male gender and MC subtype (30–35%) in comparison with HL in adolescent/young adults or adults. Like its adult counterpart, ~40–50% of HL cases are associated with EBV in developed countries.18 HL is more likely to be EBV-associated to boys < 10 years of age than to young adults. This led to the suggestion that HL consists of three disease entities: pediatric HL (EBV-positive, mixed cellularity type), HL of young adults (EBV-negative, nodular sclerosis type), and HL of older adults (EBV-positive, mixed cellularity type).15
In Argentina, our group previously reported an epidemiological pattern II together with an EBV association of 31% for adult HL, which rose up to 54% in pediatric patients.19–21 In pediatric HL in Central and South America, several groups demonstrated an EBV-association that could be up to 90%.16 Therefore, EBV presence in childhood HL from our series resembles the scenario observed in developed countries. In spite of this, EBV was statistically associated with MC subtype and males (p < 0.05, χ2 test), typical of an underdeveloped or developing population. In addition, when only children < 10 years of age were analyzed, they displayed distinctive characteristics. EBV was statistically distributed in 74% of patients < 10 years of age vs. 34% observed in patients 11–16 years (p < 0.0001, χ2 test). Furthermore, EBV+ cases had a median age of 8 years vs. a median of 12 years observed in EBV- cases (p = 0.006, Mann-Whitney test) (authors’ unpublished data). These paradoxical epidemiological characteristics make our population a quite interesting group for analysis. In contrast, in southeast Brazil, where both EBV primary infection and EBV-associated pediatric HL reveal the same pattern as in Argentina, EBV+ HL was not significantly distributed in patients < 10 years of age or MC subtype.22,23 However, childhood HL from northeast Brazil display 87% of EBV+ cases together with a prevalence of MC subtype,24 as in underdeveloped populations. In line with this, Mexico and Peru also showed a high frequency of EBV-associated HL, 70% and 94%, respectively.25,26
EBV-associated tumors display three classical patterns with differential expression of latency proteins, in part as a consequence of host immune response to EBV antigens. Latency III expresses EBNA1, -2, -3A, -3B, -3C, -LP together with LMP1 and -2 and the non-coding RNAs (EBERs, microRNAs, and BARTs), typically associated with post-transplant lymphoproliferative disorders. All other latency types involve increasing degrees of viral gene silencing. In latency I, observed in BL, only EBNA1 and the non-coding RNAs are expressed, whereas EBNA1, LMP1 and -2 and the non-coding RNAs are expressed in the latency II program, related to HL.27 It was proposed that LMP1 rescue proapoptotic germinal center B-cells with crippling mutations in the heavy chain immunoglobulin locus from apoptosis,28 whereas LMP2A may act as an indispensable BCR-mimic in BCR-positive and BCR-negative germinal center B-cells,29 pointing to its crucial role in the rescue of these cells from apoptosis and in the HL transformation process as well. On the other hand, Thorley-Lawson et al. suggested a modest signaling role for LMP1 and LMP2A in GC B-cells, based on the presence of high affinity antibodies in LMP1 and LMP2A positive germinal center B-cells.30 In Argentina, we found latency II pattern in our HL series, defined by LMP1 expression in cytoplasm membrane of HRS cells. Given that HRS cells derive from crippled, pre-apoptotic germinal center B-cells,16 LMP1 expression in our cases could be responsible for rescuing those cells from apoptotic stimuli. In a preliminary study of LMP1 expression in tonsils from pediatric EBV+ patients, we found that LMP1+ cells were prevalent outside the germinal centers (authors’ unpublished data). This fact could imply that LMP1 presence in only a small number of cases might prevent apoptosis and lead to first steps in malignant transformation towards HRS cells.
The risk of HL after IM suggests that the predilection for the younger adult age group may simply result from the combination of age and time since IM. Therefore, given the fact that in Argentina primary infection occurs at early age and is mostly subclinical, and HL prevail in patients < 10 years old, a similar association may exist between primary EBV infection and HL risk in childhood.
4EBV in pediatric Burkitt lymphomaBurkitt lymphoma (BL) can be classified into three forms, which differ in geographic distribution and EBV association: endemic (eBL), sporadic (sBL) and HIV-associated BL.31 The high-incidence “endemic” form typically presents as a jaw or abdominal tumor in children in areas of equatorial Africa and Papua New Guinea where malaria is holoendemic and is almost 100% EBV+. Elsewhere, BLoccursin “sporadic” form, again mainly in children, at intermediate to low incidence and with different degrees of EBV association depending upon the area. Western countries show the lowest incidence rates and the weakest virus association, with only 15% to 20% tumors being EBV+. Remarkably, a third, adult form of the tumor, AIDS-BL, proved to be very common among HIV-infected individuals, often appearing as one of the first symptoms of AIDS; 30% to 40% of these tumors carry EBV.32 The hallmark of all BL tumors is the translocation between the MYC gene and one of the immunoglobulin (Ig) heavy or light chain loci.31 BL cells phenotypically resemble centroblasts. Moreover, many BL cells and also some BL-derived cell lines show evidence of active somatic hypermutation, a further hallmark of germinal-center B cells. The structure of the chromosome breakpoints derived from the analysis of MYC-Ig translocations strongly indicates that the translocations occurred either as a mistake of class switch recombination or somatic hypermutation, both processes that occur in germinal-center B cells.33 There is some evidence that the original cell may derive from a post-GC or memory B cell re-entering the GC and may differ in EBV-positive and -negative tumors; however, it is clear that germinal center involvement is critical for the pathogenesis of this disease both in terms of MYC translocation events and the contribution of co-factors such as EBV, malaria or HIV infection.31
In Argentina, our group demonstrated an EBV association with pediatric BL in 37% of cases, including immunocompetent and immunocompromised patients. All HIV+ patients (100%) were EBV+, whereas 29% of the immunocompetent patients displayed EBV presence in tumor cells. EBV presence in BL biopsies was analyzed according to age, and the virus was statistically distributed in patients < 5 years old (p < 0.0001, χ2 test). Furthermore, as also demonstrated for EBV-associated HL, the median age of EBV-positive patients was lower (3 years old) than that observed for EBV negative ones (8 years old) (p = 0.0027, Mann–Whitney test).34 Remarkably, a sporadic EBV association with BL (29%) in immunocompetent patients was observed in our series. In contrast, variable regionally EBV association with BL was described in Brazil, varying from 29% in the South to 76% in the North, but higher in pediatric groups from all regions.35 The scenario for BL in HIV-infected patients was quite different from that described previously because these series showed an interesting 100% EBV association with pediatric BL patients infected with HIV, higher than the typical 30–40% reported for this entity.32
Latency I pattern seen in most endemic BL tumors is characterized by the restricted expression of a single latent antigen EBNA1 transcribed from Qp viral promoter, different from the latency III EBNA transcripts, which are driven by either of two promoters, Cp or Wp. However, another type of latency, termed Wp-restricted latency, characterized by expression of EBNA1, EBNA3A, EBNA3B, EBNA3C, EBNA-LP and BHRF1in the absence of EBNA2 and the LMPs, was also demonstrated in a subset of endemic BL tumors that carry EBV genomes deleted for the EBNA2 gene.33 For that reason, we studied viral latency profiles by LMP1 immuno-histochemistry and RT-PCR. Most EBV+ cases (80%) displayed the typical latency I profile described in BL, whereas the remaining 20% showed also LMP1 immunohistochemical expression, remarkably those cases were HIV-infected patients. This pattern did not match either with latency I or Wp-restricted pattern described for Burkitt lymphoma or with latency III pattern associated with EBV-positive lymphomas in immunosuppressed patients. In addition, LMP2A latent gene as well as BZLF1 and BHRF1 lytic genes were not detected by RT-PCR, whereas EBNA1 transcripts driven from Qp promoters were demonstrated in the absence of Wp/Cp promoter usage.34 Only one group has previously reported LMP1 expression without LMP2A by RT-PCR in 2 eBL patients from Africa.36 It can be hypothesized that LMP1 expression in BL patients infected with HIV could arise from a germinal center cell that failed to turn-off selectively LMP1 expression to achieve the full BL latency I phenotype, promoted by HIV presence. Alternatively, the presence of HIV-associated immunosuppression allows the unchecked proliferation of EBV-infected lymphocytes expressing latent antigens different from typical latency I.34
5EBV in pediatric diffuse large B-cell lymphomaWorldwide, diffuse large B-cell lymphoma (DLBCL) represents the most common subtype of NHL, accounting for 30% to 40% of all newly diagnosed cases. Two distinct subtypes have been identified, termed germinal center cell and post-germinal center, which are believed to represent lymphomas arising from different stages of lymphoid differentiation.37 The 2008 World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues has recognized a new provisional entity designated as EBV-positive DLBCL of the elderly. It has been postulated that EBV-positive DLBCL of the elderly might be caused by the senescence of the immune system as a part of the normal aging process, based largely on shared features with immunodeficiency-LPDs.38 The age cutoff for EBV-positive DLBCL was set as > 50 years. However, recent reports show that EBV-positive DLBCL can affect younger patients,39 including our study.40 Furthermore, in some previous reports, patients < 50 years old were even excluded from the studies.39 Therefore, it is not evident that EBV-positive DLBCL in young adults has distinct clinical features and outcome compared to their elderly counterpart.
DLBCL is rarely diagnosed in children < 4 years of age, but the proportion of patients with DLBCL increases throughout childhood toward the second decade of life.41 The role of EBV in pediatric DLBCL pathogenesis remains uncertain; therefore, we assessed EBV association as well as viral gene expression in the DLBCL pediatric population from our hospital. EBV association with DLBCL rose up to 40%, using 20% of EBERs+ cells as cut-off value; nevertheless, when excluding immunosuppressed patients, this association decreased to 26%.42 There are only few studies dealing with EBV presence in DLBCL. Our overall percentage was very similar to those observed in previous reports restricted to African children, which analyzed series of NHL that included a subset of DLBCL and described an association of 43–44% between EBV and this lymphoma subtype, even though the immunological status of these patients was not clearly specified.43,44 EBV presence in pediatric DLBCL patients was sorted in two age groups: less than 10 years (> 10 years) and 11–16 years (≤10 years). The number of EBV+ cases was significantly higher among patients ≤ 10 years than among patients > 10 years (p < 0.018, Fisher’s exact test). Moreover, median age of EBV-positive patients was 6 years vs. 11 years observed in the negative patients (p = 0.005, Mann Whitney test). This observation together with the high association in childhood HL as well as BL confirms that EBV could be an important cofactor in B-cell lymphomagenesis in younger children in Argentina.42
Previous studies suggested that EBV+ DLBCL in elderly patients generally showed a viral latency type II or III pattern,45,46 whereas viral latency pattern was not previously described in pediatric and young adults patients. In our pediatric DLBCL series, latency pattern III was the most frequently observed together with lytic gene expression, followed by latency II pattern.42 BZLF1 lytic gene expression could mirror a percentage of cells undergoing lytic phase, as previously observed for BL.47 A mixture of latency II or III and a lytic EBV gene was also observed in our adult lymphoma series from Argentina, in both patients younger and older than 50 years.40 This finding suggests that the entity EBV+ DLBCL of the elderly can occur in pediatric and young immunocompetent patients and might not be an age related-EBV association. In addition, latency III pattern together with lytic viral antigen expression in DLBCL from Argentina suggest that lytic cycle may play an unexpected role in virus-mediated oncogenesis.
In order to reinforce the hypothesis of EBV-association not exclusively related to elderly patients, DLBCL microenvironment was studied in pediatric, younger and older than 50 years old patients. CD3, CD8, CD4 and Foxp3 positive cells were compared in those three groups and in relation to EBV presence. Remarkably, we did not observe major differences linked with age of the relative numbers of any T-cell population analyzed.40,42 This fact, together with EBV association with both age groups, might confirm previous statements that the definitional cutoff of 50 years proposed by the WHO could be arbitrary48 because cases < 50 years of age can be found and suggest that, at least for T-cell populations analyzed, immunosenescence could be ruled out. Concerning EBV status, no significant difference was found among each cellular marker investigated.40,42
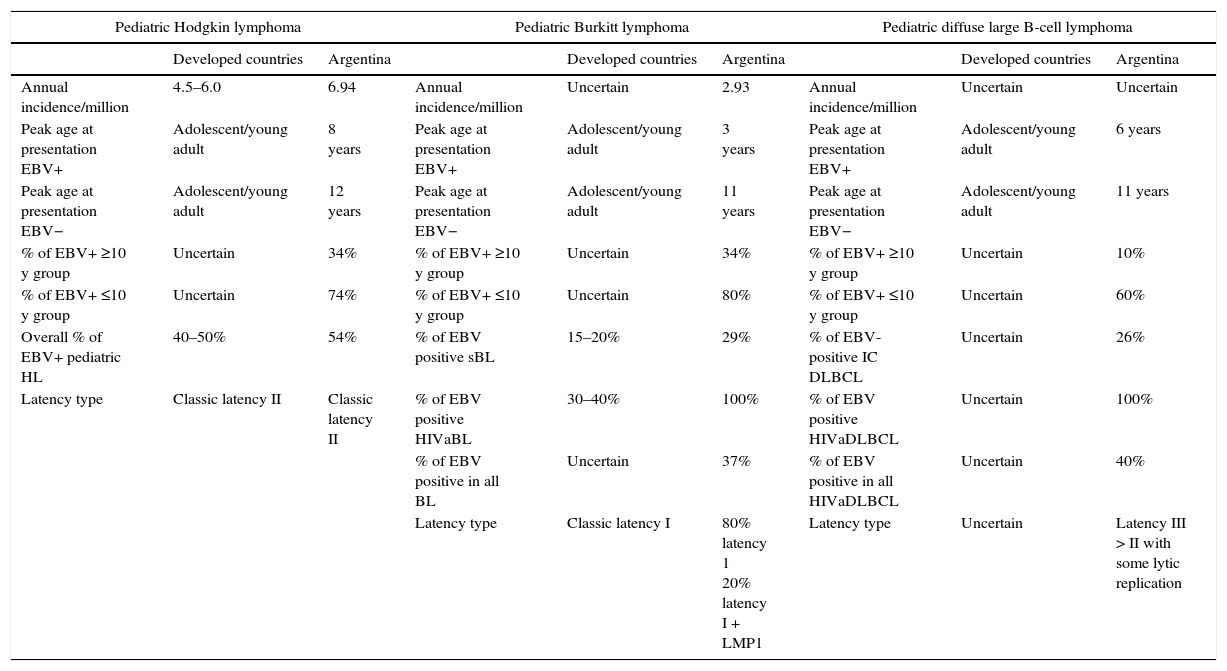
6Concluding remarks and future perspectivesFrom the first discovery of EBV particles in sub-Saharan BL, the virus has now been found in many more tumors of B, T, NK and epithelial cell origin. Overall, EBV seems to contribute to ~2% of all tumor burdens in man. Therefore, the question that drives most research into EBV since its discovery is why some individuals develop EBV-associated tumors, whereas most control the virus without symptoms.49 In fact, it remains to be elucidated whether EBV-association with lymphoma reflects causality. The increased risk of HL development a few years after IM leads to speculate that a similar association may exist between primary EBV infection and HL risk at any age, for example, in childhood. A higher prevalence of EBV in lymphomas diagnosed in children < 10 years old was demonstrated by our research group not only in pediatric HL,20,21 but also in BL34 and DLBCL.42 Therefore, in our country, EBV infection shows interesting epidemiological characteristics to study viral pathogenesis. Given that in Argentina children are infected quite early in their life and nearly 70% of children are seropositive by the age of 2 years,10 we hypothesize that infection with EBV early in life increases the risk of developing EBV-associated lymphoma and may be as a late complication of primary infection. A comparison of EBV-positive pediatric lymphomas between developed countries and our own data from Argentina is presented in Table 1. It is well accepted that IM often precedes HL and that IM is an adolescent/young adult disease, but to our knowledge there is not a clear consensus of the median age of presentation in developed countries. Still more confusing is the literature about BL and DLBCL because most studies do not distinguish between EBV-positive and -negative cases and because of the multiple presentation forms; for instance, BL sporadic, endemic or HIV associated and DLBCL of immunocompetent or immunosuppressed patients.
Comparison between EBV positive and negative pediatric lymphomas between developed countries and Argentina.
| Pediatric Hodgkin lymphoma | Pediatric Burkitt lymphoma | Pediatric diffuse large B-cell lymphoma | ||||||
|---|---|---|---|---|---|---|---|---|
| Developed countries | Argentina | Developed countries | Argentina | Developed countries | Argentina | |||
| Annual incidence/million | 4.5–6.0 | 6.94 | Annual incidence/million | Uncertain | 2.93 | Annual incidence/million | Uncertain | Uncertain |
| Peak age at presentation EBV+ | Adolescent/young adult | 8 years | Peak age at presentation EBV+ | Adolescent/young adult | 3 years | Peak age at presentation EBV+ | Adolescent/young adult | 6 years |
| Peak age at presentation EBV− | Adolescent/young adult | 12 years | Peak age at presentation EBV− | Adolescent/young adult | 11 years | Peak age at presentation EBV− | Adolescent/young adult | 11 years |
| % of EBV+ ≥10 y group | Uncertain | 34% | % of EBV+ ≥10 y group | Uncertain | 34% | % of EBV+ ≥10 y group | Uncertain | 10% |
| % of EBV+ ≤10 y group | Uncertain | 74% | % of EBV+ ≤10 y group | Uncertain | 80% | % of EBV+ ≤10 y group | Uncertain | 60% |
| Overall % of EBV+ pediatric HL | 40–50% | 54% | % of EBV positive sBL | 15–20% | 29% | % of EBV-positive IC DLBCL | Uncertain | 26% |
| Latency type | Classic latency II | Classic latency II | % of EBV positive HIVaBL | 30–40% | 100% | % of EBV positive HIVaDLBCL | Uncertain | 100% |
| % of EBV positive in all BL | Uncertain | 37% | % of EBV positive in all HIVaDLBCL | Uncertain | 40% | |||
| Latency type | Classic latency I | 80% latency 1 20% latency I + LMP1 | Latency type | Uncertain | Latency III > II with some lytic replication | |||
sBL, sporadic Burkitt lymphoma; HIVaBL, HIV-associated Burkitt lymphoma; IC DLBCL, immunocompetent diffuse large B-cell lymphoma; HIVaDLBC, HIV-associated diffuse large B-cell lymphoma.
Our preliminary results in tonsils from pediatric carriers show that a latency III pattern prevails and, based on the known oncogenic potential of EBV in vitro, we can propose that the synergism of oncogenic viral proteins could occur in these cases (personal observations). This finding suggests that there could be an implication of EBV infection involved in B-cell lymphoma development in our country, and emphasizes the need for epidemiological studies involving different centers from the Latin American region to confirm this hypothesis.
Conflict of interestThe authors declare no conflict of interest of any nature.