Introducción: El raquitismo dependiente de vitamina D tipo I es una enfermedad hereditaria rara debida a una mutación en el gen CYP27B1 que codifica la enzima 1α-hidroxilasa. Se caracteriza por la presentación de raquitismo hipocalcémico grave desde la edad de la lactancia debido al déficit de producción del metabolito activo de la vitamina D, la 1α,25-dihidroxivitamina D3.
Caso clínico: Presentamos el caso de un paciente con raquitismo diagnosticado a los 11 meses de edad y el seguimiento hasta los 9 años.
Conclusiones: Se discute la fisiopatología de la enfermedad y la importancia del diagnóstico y tratamiento oportunos.
Background: Vitamin D dependent rickets type I is a rare hereditary disease due to a mutationin CYP27B1 encoding the 1α-hydroxylase gene. Clinically, the condition is characterized by hypocalcemic rickets in early infancy due to a deficit in the production of the vitamin D active metabolite 1,25-dihydroxy-vitamin D3.
Case report: We report the case of a patient diagnosed at 11 months with follow-up until 9 years of age.
Conclusions: The pathophysiology of the disease and the relevance of early diagnosis and management are discussed.
Pagina nueva 1
1. Introduction
In 1961, Prader et al. described a form of hereditary rickets, which they called “pseudo-deficiency rickets”.1 This type of rickets can be differentiated from the hypophosphatemia linked to chromosome X due to different mode of transmission to the presence of severe hypocalcemia and to the possibility of remission with high doses of vitamin D. In 1973, Fraser et al. treated a patient with this type of rickets with physiological doses of the active form of vitamin D, 1α-25-dihydroxyvitamin D3 [1α,25(OH)2D3]. They suggested that this disease was the consequence of a defect of the 25(OH)D3-1α-hydroxylase enzyme in the kidney.2 Subsequently in 1978, Brooks et al. described a patient with similar clinical characteristics but with elevated levels of 1α,25(OH)2D3 in plasma before and during treatment. Because of this, these authors suggested that vitamin D-dependent rickets could be classified as type I (deficient production 1α,25(OH)2D3) or type II (lack of response of the target organ to 1α,25(OH)2D3).3
Vitamin D-dependent type I rickets is a very rare disease. In the experience of the Nephrology Department of the Hospital Infantil de México Federico Gómez (HIMFG), four patients were studied in the last 38 years.4,5 For this reason we believe it is of interest to present the clinical characteristics, treatment response and long-term progress of a child who was diagnosed with vitamin D-dependent type I rickets at the age of 11 months and whose treatment and follow-up was continued until 9 years of age.
2. Clinical case
We present the case of an 11-month-old infant. The father was 30 years of age and the mother 33 years of age, and both were apparently healthy. There is a healthy 10-year-old brother and a brother who died at 1 year of age due to lung infection and diagnosis of rickets. The patient is the product of a vaginal delivery with a birth weight of 3.6 kg. He was breast fed until 7 months of age with weaning at 6 months. He was able to support his head at 3 months and was sitting at 9 months of age.
The patient was admitted due to a clinical picture characterized by cough of 2 weeks evolution, respiratory difficulty at 7 days and fever of 3 days evolution. On admission his weight was 7.6 kg (Z-score –3.33), height of 65 cm (Z-score –3.72) and blood pressure 90/60 mmHg. He had generalized muscular hypotonia, normal skull formation, bird-like chest, bilateral rachitic ribs resembling rosary beads, pulmonary fields with crepitant rales, xiphoid retraction, liver palpable 3 cm below the costal margin, and widening of the elbows, wrists, knees and ankles joints.
Chest x-ray demonstrated bilateral parahilar infiltrate with the diagnosis of community-acquired pneumonia and was treated with antibiotics. The patient progressed favorably.
Blood tests performed upon admission showed the following results: hemoglobin 12 g/dl, glucose 98 mg/dl, sodium 139 mmol/l, potassium 4.1 mmol/l, CO2 total 22 mEq/l; total proteins 6.6 g/dl, albumin 3.6 g/dl, calcium 6.0 mg/dl, phosphorus 1.9 mg/dl, magnesium 1.8 mg/dl, alkaline phosphatase 466 U/l, creatinine 0.1 mg/dl; normal bilirubin and aminotransferase, parathyroid hormone 571 pg/dl (normal 9-25 pg/dl); 1α,25-(OH)2D3 <5 pg/ml (normal 15-90 pg/ml); 25-hydroxycholecalciferol [25(OH)D3] 23.3 ng/ml (normal 17-54 ng/ml).
Urine and renal function tests showed calciuria of 0.71 mg/kg/d (normal <4 mg/kg/day); calcium/creatinine ratio 0.1 mg/mg (normal for age <0.6 mg/mg); phosphaturia 25.6 mg/kg/day (normal <20 mg/kg/day); tubular reabsorption of phosphates 87% (normal >80%). Urinalysis reported pH 7.0, density 1.024, negative albumin, and negative glucose with the remainder being normal. Renal ultrasound showed normal dimensions of the renal silhouette for age without evidence of nephrocalcinosis.
Radiographic study of the long bones, both in upper as well as lower extremities showed bone demineralization, widening of the epiphysis and irregularities of the metaphysis with fraying; in some of them there was evidence of “inverted cup”. In the upper extremities a pathological fracture was also seen of the right humerus. Calcium, phosphorus, and alkaline phosphatase studies done in the parents were normal.
Ten days after admission with the diagnosis of type I vitamin D-dependent rickets already made, treatment was initiated with calcitriol (1α,25(OH)2D3) 0.25 μg every 24 h, orally and phosphate solution 2 mL (60 mg) every 6 h (35 mg/kg/ day). One week later, due to persistent severe hypocalcemia (5.9 mg/dl), calcitriol was increased to 0.5 μg every 24 h, phosphate solution 4 ml every 6 h and calcium carbonate 195 mg every 6 h was begun. Various intravenous infusions of calcium gluconate were started due to persistent hypocalcemia.
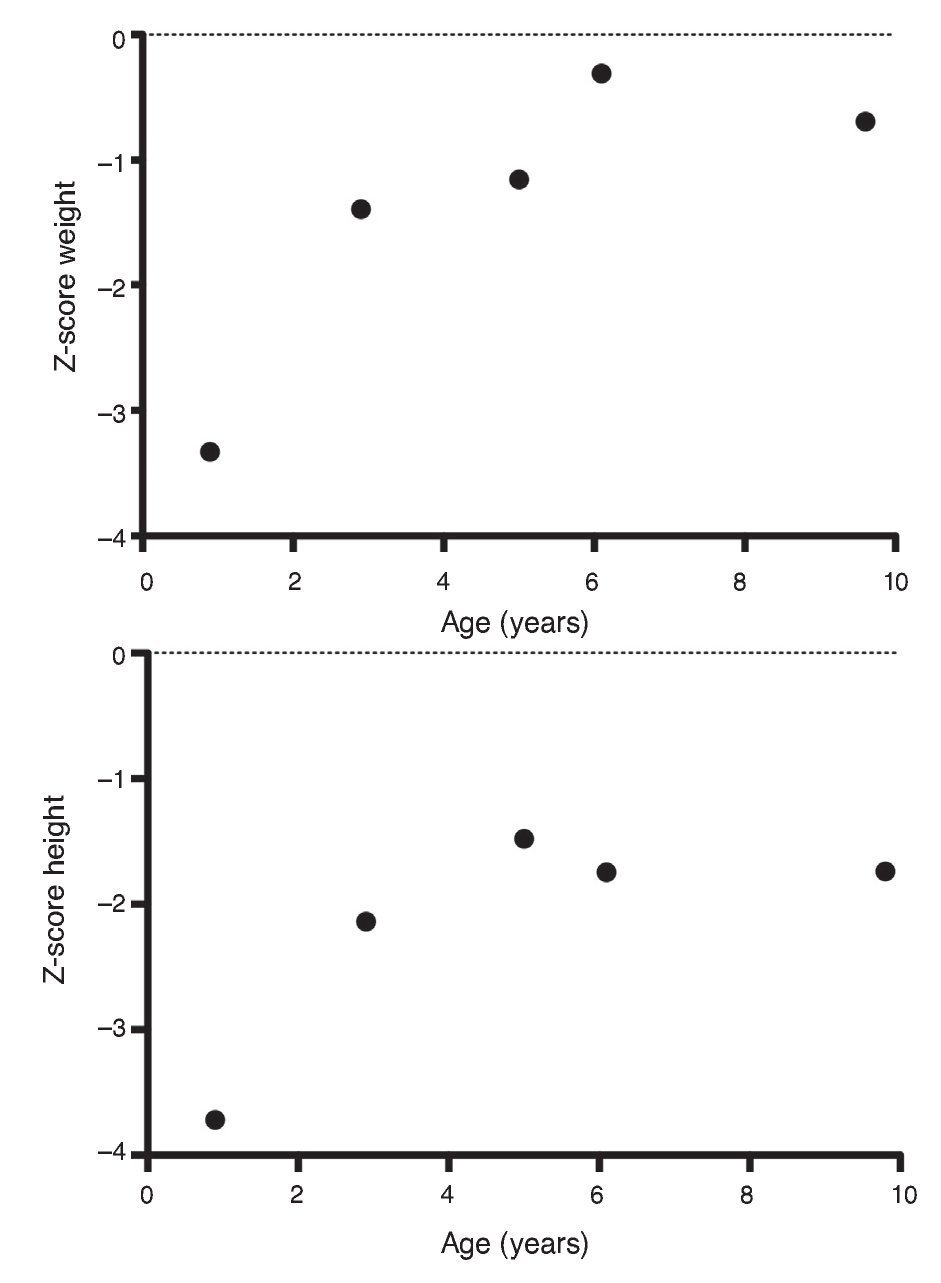
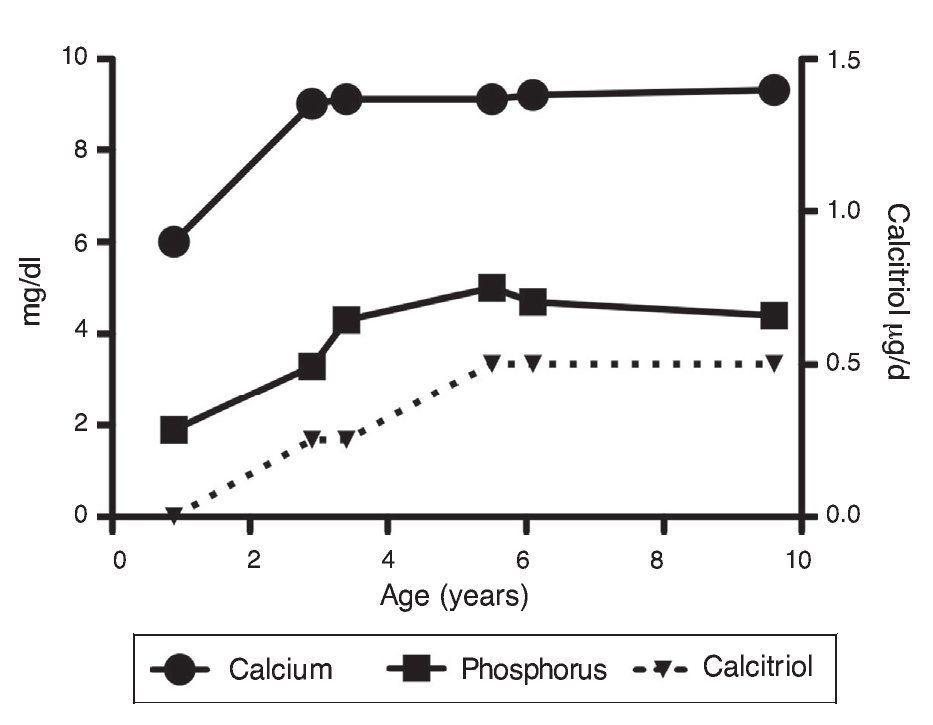
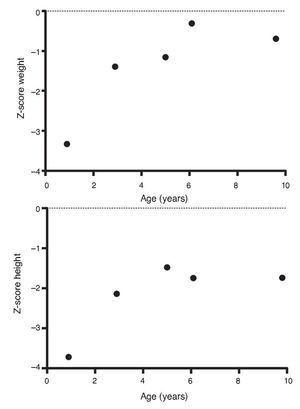
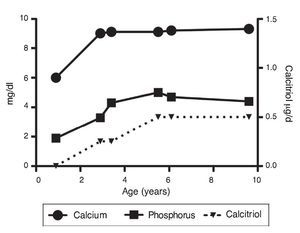
Upon the patient’s discharge 40 days later, blood tests showed sodium 139 mmol/l, potassium 4.5 mmol/l, calcium 7.1 mg/dl, phosphorus 3.3 mg/dl and magnesium 2.2 mg/dl. At that time the patient was receiving calcitriol 0.75 μg every 12 h, phosphate solution 6 ml every 6 h and calcium carbonate 1 g every 6 h. There was notable improvement in his growth during the first year after initiation of treatment (Fig. 1), which has been maintained throughout the follow-up period with calcitriol adjusted so as to maintain normal serum levels of calcium and phosphate (Fig. 2).
Figure 1 Evolution of the Z-score of weight and height.
Figure 2 Evolution of serum calcium and phosphorus as well as the dose of calcitriol.
Subsequent follow-up recorded initiation of walking at 15 months of age. At 2 years 9 months of age the patient weighed 12.8 kg (Z-score –1.39) and height was 80 cm (Z-score –2.14). He was receiving treatment only with calcitriol at a dose of 0.25 μg/day. Laboratory results showed calcium 9.8 mg/dl, phosphate 2.9 mg/dl, and creatinine 0.2 mg/dl. Urinalysis showed pH 6.5 and urine density 1.028. The remainder was negative. Follow-up x-ray of the right wrist was done and showed significant improvement of the bone lesions with bone age of 1 year 9 months.
At 5 years of age the patient weighed 16.9 kg (Z-score –0.9) and his height was 102 cm (Z-score –1.16). Due to the progressive decrease of plasma calcium concentration (8.0 mg/dl), calcitriol dose was increased to 0.5 μg/day. Serum calcium normalized to 9.1 mg/dl after 6 months.
At the age of 9 years, the patient weighed 25.1 kg (Z-score –1.36) and had a height of 126 cm (Z-score –1.74). Laboratory studies showed creatinine 0.4 mg/dl, calcium 9.4 mg/dl, phosphorus 4.2 mg/dl, alkaline phosphatase 175 U/l and parathyroid hormone 25.1 pg/ml. X-ray studies of the long bones did not show signs of rickets. Treatment with calcitriol was indicated to be maintained at a dose of 0.5 μg/day.
3. Discussion
Type I vitamin D-dependent rickets (OMIM 264700)6,7 has also been called “hereditary type I vitamin D-dependent”, “hereditary vitamin D pseudo-deficiency ” and “selective hereditary deficiency of 1α,25(OH)2D3”.8 This type of rickets is transmitted in an autosomal recessive manner. The gene responsible has been identified as CYP27B1. It is located on chromosome 12q13.1-13.3 and encodes the enzyme 25(OH) D3-1α-hydroxylase.6,9,10 The availability of the information on the sequence of the gene CYP27B1 has allowed investigations of the mutations in patients with this disease and obligate carriers.11,12 More than 60 mutations have been identified in different family groups studied.12-16 The presence of these mutations causes suppression of the activity of 25(OH)D3-1α-hydroxylase and therefore the deficit in the synthesis of the active form of vitamin D, 1α,25(OH)2D3.9,12
Patients affected with this form of rickets have early clinical manifestations related with severe hypocalcemia.5 Thus, this patient with normal appearance at birth, at 11 months of age had bone disorders that included pathological fractures of the right arm, deformity of the rib cage (“bird-like chest”), rachitic ribs in “rosary bead formation” and widening of the bone epiphysis, especially in the wrist joints but also apparent in the elbows, knees and ankles. The rib cage deformity in these patients could cause a reduction in respiratory capacity resulting in a life-threatening infectious complication of the pulmonary parenchyma. In fact, the clinical history reported that a brother also had clinical manifestations of rickets and died at 2 years of age due to pneumonia. Moreover, the studied patient was admitted to the hospital due to lung infection.
Other patients studied present convulsions at an early age (before 1 year of age) because of severe hypocalcemia. In these cases and in the presence of manifestations of rickets the possibility of vitamin D-dependent rickets should be suspected.5,16,17
As noted in the patient studied, the presence of muscular hypotonia is described, which causes a delay in the development of motor skills in these children.7,17 In children with vitamin D-dependent rickets, in addition to the presence of severe hypocalcemia, there may also be an elevation in serum concentration of parathyroid hormone with development of secondary hyperparathyroidism.6 As a consequence of this and as seen in this patient, serum levels of phosphate are decreased. In addition, in some patients may also show proximal tubular loss of bicarbonate (with development of hyperchloremic metabolic acidosis) and generalized aminoaciduria.18 These disorders require the differential diagnosis to be made with two renal disorders: X-linked hypophosphatemia and Fanconi syndrome, especially in its infant nephropathic cystinosis variety.19 These renal tubular disorders usually normalize in the first 2 months after initiating treatment with calcitriol in relation with the normalization of serum levels of parathyroid hormone.18
It has been mentioned that in patients with type I vitamin D-dependent rickets, defects have been demonstrated in the production of 1α,25(OH)2D3.9 Subsequently, the diagnosis of type I vitamin D-dependent rickets is based on the findings of very reduced levels or non-detectable levels of 1α,25(OH)2D3.16 This allows for the differential diagnosis to be made with type II in which high levels of this metabolite are found.20 The latter type of rickets has been called hereditary vitamin D-resistant rickets.20 On the other hand, 25(OH) D3 levels may be normal or mildly increased16 as was the case in the patient presented in this report.
X-ray study of long bones demonstrated widening and distortion of the growth plate with irregularity in the area of mineralization of the metaphysis with fraying appearance (“inverted cup” shape). The bony cortex was found to be very thin with few trabecular areas. Pseudofractures are common. In some severely affected children, chest deformity can be seen (“bird-like chest”).
Patients with type I vitamin D-dependent rickets have been treated with calciferol analogues such as α-calcidiol or 1α-hydroxycholecalciferol [1α-(OH)D3], which converts to 1α,25(OH)2D3 by action of 25-hydroxylase in the liver.4,6,16,17 On the other hand, another treatment used is the administration of the active metabolite of deficient vitamin D, 1α,25(OH)2D3.5,7,18,20 Usually two high initial doses are required of 1α,25(OH)2D3 (between 0.5 and 3 μg/day). These doses should be adjusted subsequently to maintain serum calcium levels in the normal range, as was done in the patient studied. In this manner, by maintaining normal activity of the main active metabolite of vitamin D the vitamin receptors will be adequately activated in its target organs. The nuclear receptor of vitamin D, one of the nuclear phosphoproteins of 50 kDa, belongs to the superfamily of nuclear receptors that includes the receptors of the steroid and thyroid hormones.21,22 The renal loss of phosphate found on diagnosis is due to secondary hyperparathyroidism caused by the reduction in the intestinal absorption of calcium.23 Treatment with 1α,25(OH)2D3 is indicated permanently.
The patient studied required high doses of vitamin D at the beginning to correct the hypocalcemia and the secondary hyperparathyroidism, in addition to the oral calcium supplements in the form of calcium carbonate.18 Currently, normal serum calcium levels have been maintained with a dose of 0.50 μg/day. In addition, because of the development of hypophosphatemia, the patient required a phosphate solution supplement in the initial stages of treatment.
In conclusion, the finding of an infant with manifestations of rickets due to severe hypocalcemia in whom a deficiency rickets has been ruled out, the diagnostic possibility of rickets should be kept in mind, whether it is dependent due to a lack of production of the vitamin D metabolite by the kidney or due to resistance to its effects in target organs. In the first case, which is the main feature in the type I vitamin D-dependent rickets, appropriate treatment will lead to the correction of the clinical, biochemical and bone alterations of affected children, improving their growth.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors must have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Funding
None declared.
Conflict of interest
The authors declare no conflicts of interest of any nature.
Received 23 March 2015;
accepted 5 May 2015
☆ Please cite this article as: Velásquez-Jones L, Medeiros M, Valverde-Rosas S, Jiménez-Triana C, del Moral-Espinosa I, Romo-Vázquez JC, Franco-Alvarez I. Seguimiento a largo plazo de un paciente con raquitismo dependiente de vitamina D tipo I. Bol Med Hosp Infant Mex. 2015. http://dx.doi.org/10.1016/j.bmhimx.2015.03.008
* Corresponding author.
E-mail:medeiro.mara@gmail.com (M. Medeiros).