Acute lymphoblastic leukemia (ALL) affects the quality of life of many children in the world and particularly in Mexico, where a high incidence has been reported. With a proper financial investment and with well-organized institutions caring for those patients, together with solid platforms to perform high-throughput analyses, we propose the creation of a Mexican repository system of serum and cells from bone marrow and blood samples derived from tissues of pediatric patients with ALL diagnosis. This resource, in combination with omics technologies, particularly proteomics and metabolomics, would allow longitudinal studies, offering an opportunity to design and apply personalized ALL treatments. Importantly, it would accelerate the development of translational science and will lead us to further discoveries, including the identification of new biomarkers for the early detection of leukemia.
La leucemia linfoblástica aguda (LLA) afecta la calidad de vida de una gran cantidad de individuos en edad pediátrica en todo el mundo; particularmente en México, donde se ha reportado una alta incidencia. Con un apropiado fondo de inversión financiera, así como instituciones adecuadamente organizadas al cuidado de los pacientes con LLA, en conjunto con plataformas sólidas para llevar a cabo análisis globales y de alto rendimiento, se propone la creación de un repositorio para la conservación de suero y células provenientes de médula ósea y sangre derivadas de pacientes pediátricos con LLA al diagnóstico. Estos recursos, en combinación con las tecnologías ómicas, en particular la proteómica y la metabolómica, podrían permitir el establecimiento de estudios longitudinales y ofrecer una oportunidad para el diseño y aplicación de tratamientos personalizados para la LLA. Esta estrategia permitiría acelerar el desarrollo de la ciencia traslacional, favoreciendo el hallazgo de importantes descubrimientos, incluyendo la identificación de nuevos biomarcadores para la detección temprana de la leucemia.
Non-communicable diseases occur more frequently in low- and middle-income countries. Besides cancer, cardiovascular diseases such as coronary heart diseases as well as diabetes represent major causes of death.1 Cancer represents one of the biggest challenges regarding morbidity and mortality in the world. In 2012, there were around eight million deaths related to cancer and 14 million new cases were diagnosed.2 Therefore, it is important to distinguish between differences in cancer care and cancer outcome, acknowledging that outcomes are inextricably linked to the quality of care. It is important to plan integrated, evidence-based, and cost-effective interventions across the cancer continuum, which includes research, prevention, early detection, treatment, and palliative care to reduce the burden of cancer in a specific population.3 Therefore, it is essential to analyze the health systems that are working in the world, and also the actual development of the science and technology. Furthermore, how each society can produce health actions aimed at reducing the burden of cancer, particularly in the developing world which includes low- and middle-income countries, where the progress in the development of treatments for cancer is poor.
A possible novel strategy for those countries facing these diseases is to collect a large number of disease tissues, along with proper control samples. This collection would constitute a Biobank,4 which may allow developing countries to build a new scientific and technologic system to identify novel targets and to focus on the early detection of cancer.
Hispanics (Mexicans, Cubans, Puerto Ricans, and South or Central Americans) account for over 40% of pediatric leukemia cases in North America.5 Mexico itself has a high incidence of acute lymphoblastic leukemia (ALL).6,7 The objective of this work is to review how Mexico is addressing this disease in children, and what are the opportunities for applying new technologies to benefit these patients.
2Health investment in Mexico in comparison with other countriesIn a middle-income country like Brazil, 9.3% of the gross domestic product (GDP) is used for health expenditures,3 representing approximately 1042 USD per capita of GDP in 2013. On the other hand, high-income countries have higher health care expenditures per capita regarding the percentage of GDP. For example, in 2014, the United States spent 8895.1 USD; Germany, 4683.2 USD, and Italy, 3032.5 USD.8 Finally, Kenya, as an example of a low-income African country, had total health expenditures of 4.7% of GDP, representing approximately 58.5 USD per capita.3
At the global level, financing of health care comes from a mix of public and private sources. Across the Organisation for Economic Co-operation and Development (OECD) countries, the main financing agent is the public sector, particularly in some European areas such as Scandinavia and the United Kingdom. In contrast, the United States, and some Latin American countries have less than 50% of public spending on health; the former has a high proportion of spending financed by private insurance, while the latter directly by households (out-of-pocket spending).9 Nevertheless, it is known that 5-year survival rates for most cancers are higher in richest countries than in low- and middle-income countries, probably because developed countries spend five to ten times more in cancer control on a per capita basis than low- and middle-income countries.3
The situation in Mexico is not very different. Considered a middle-income country, only around 5-7% of Mexicans have private medical health insurance.7 Despite the fact that health insurance coverage is becoming more universal, it is still one of the OECD countries with the lowest health expenditures, representing around 1048 USD of GDP per capita in 2012.10 With this investment the country has managed to provide health coverage to 49% of the population through a federal government program. However, the poorest Mexicans, who are non-salaried workers, rural residents and the unemployed, represent close to 50% of the total population from 2006-2010. Government efforts were designed to include people without access to the Social Security system in a program called Popular Medical Insurance (PMI). Importantly, children up to 18 years old suffering with any cancer, including ALL, are eligible to receive coverage under PMI.7
Scarce of resources should be recognized as the most important limiting factor for increasing the public health spending in developing countries. However, the design of a health policy system that links both the research focused on health priorities and the investment in strategic technology would deliver more cost-effective care for emergent diseases. This factor is critical since developing countries represent a potentially valuable source of clinical samples research for the use in efforts aimed at the identification of novel biomarkers and therapeutic targets.1 Omics techniques, which involve universal detection of genes (genomics), mRNA (transcriptomics), proteins (proteomics) and metabolites (metabolomics) in a specific biological sample represent a powerful way to explore new research options in a non-targeted and non-biased manner.11
3Acute lymphoblastic leukemia in MexicoChildhood cancer represents between 0.5% to 4.6% of the total number of cases in the population. However, children living in countries with low Human Development Index (HDI) are proportionally more affected than those living in countries with high HDI.2 In Mexico, childhood cancer is the second cause of death among children aged 4 to 15 years. ALL represents the most common cancer among children and the most frequent cause of death from cancer before 20 years of age. ALL corresponds to 23% of cancer diagnoses among children under 15 years, having a peak incidence around 3-5 years of age. The most common cells present in ALL patients are B-cell precursors (B-ALL) that account for about 80-85% of the cases, whereas T-cell ALL is the second most common type and it is frequently associated with poor prognosis.12,13
The survival rate of childhood ALL has improved significantly over the last few years. In developed countries, 5-year survival in childhood ALL is around 90%. In Mexico, survival is about 67%, and efforts to improve this include studies applying novel medical treatments to Mexican children, like the one developed by the Dana Farber Cancer Institute 00-01 (Boston, MA).5,14,15 These efforts sometimes generate mixed results, possibly due to other circumstances during the treatment that may be related to poverty or unrecognized differences in the genetic background in the treated population. Other factors that may affect outcomes include malnutrition, infections, or toxicity of the treatment. On the other hand, these experiences are also leading to the improvement of therapies and clinical care, resulting in effective and low-cost treatments (compared to developed countries). Accordingly, around 7,260 USD are spent in treatment per child (no monoclonal antibodies included) in a period of one year, as shown in a study by the Children's Hospital Federico Gómez.16 Those efforts are also being performed at other Mexican institutions such as hospitals of the Mexican Institute for Social Security, the Security and Social Services for State Workers, and from the Mexican Military.14
4Omics technology in MexicoSince there is not an accurate screening test to diagnose ALL, the disease can easily spread in the body. Once signals related to ALL appear, the patient must wait to be transferred to a medical center with adequate equipment, not just a facility to take blood and bone marrow samples but also equipment to perform a set of analyses, as microscopy and flow cytometry, to reach a final diagnosis. It is apparent that basic research focused on the identification of biomarkers associated with early stage disease should be encouraged.
Proteomics, defined as the set of all expressed proteins in a cell, tissue or organism,17 may allow the identification of novel targets. This new area brings research opportunities in promising fields, including the delineation of altered protein expression (not only at cell or tissue levels but also in subcellular structures, protein complexes, and biological fluids); the development of new targets for therapeutics; and the discovery and development of novel biomarkers for diagnosis and early detection.18 It is worth mentioning that the comprehensive analysis of cell molecules—genes, mRNA, proteins, and metabolites—combined with the use of mathematical models and bioinformatics is globally referred as systems biology or high-dimensional biology, and the technologies used to map and characterize the identified novel molecules, including alterations or modifications, constitute the so-called omics.11,19
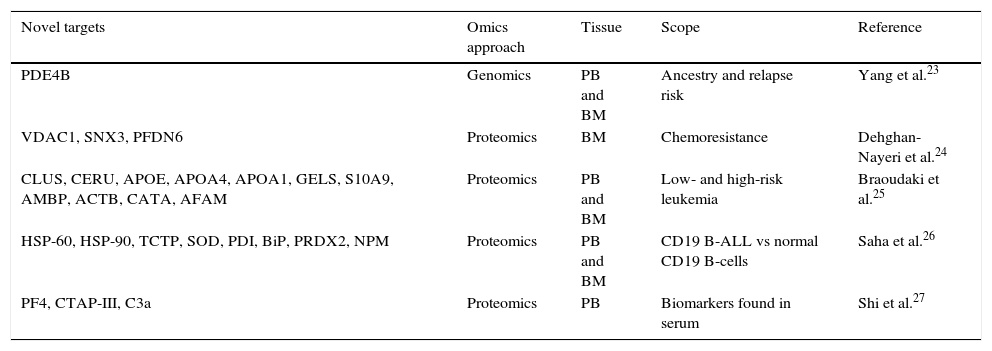
Efforts to apply omics technologies for the discovery of new biomarkers with the potential to be used for early diagnosis of some diseases are still in progress, including those aimed at ALL. In several countries, efforts are underway using leukemic cell lines and analyzing human samples, focusing on three different tissues: blood, bone marrow and cerebrospinal fluid.20–22 Interestingly, the scope of the research has been wide since the focus is not just on finding novel biomarkers that could be used for diagnosis, but also on prognostic factors—molecules involved in chemoresistance, profiles related to high or low-risk leukemia, single nucleotide polymorphisms (SNPs), among others (Table 1).23–27
Omics technologies application for the discovery of new acute lymphoblastic leukemia targets.
| Novel targets | Omics approach | Tissue | Scope | Reference |
|---|---|---|---|---|
| PDE4B | Genomics | PB and BM | Ancestry and relapse risk | Yang et al.23 |
| VDAC1, SNX3, PFDN6 | Proteomics | BM | Chemoresistance | Dehghan-Nayeri et al.24 |
| CLUS, CERU, APOE, APOA4, APOA1, GELS, S10A9, AMBP, ACTB, CATA, AFAM | Proteomics | PB and BM | Low- and high-risk leukemia | Braoudaki et al.25 |
| HSP-60, HSP-90, TCTP, SOD, PDI, BiP, PRDX2, NPM | Proteomics | PB and BM | CD19 B-ALL vs normal CD19 B-cells | Saha et al.26 |
| PF4, CTAP-III, C3a | Proteomics | PB | Biomarkers found in serum | Shi et al.27 |
PB: peripheral blood; BM: bone marrow; ALL, acute lymphoblastic leukemia.
In Mexico, the development of high-dimensional biology is starting to rise. Two important events have helped to establish the idea that this country could start carrying out world-class research by applying omics techniques. In 2004, the National Institute of Genomic Medicine (INMEGEN) was established, supporting new areas of research where genomic medicine technologies could be applied, including the development of pharmacogenomics. This institution also encouraged the association with the business sector to favor a translational approach to clinical research derived from genomic medicine that could eventually contribute to the healthcare of Mexicans.28 The second event occurred in 2005 with an initial investment of 50 million USD: the birth of the National Laboratory of Genomics for Biodiversity (LANGEBIO). LANGEBIO main focus is on exploiting the potential of Mexico's natural diversity. It has developed a unit capable of doing research on the biotechnological utilization of Mexican biodiversity, the provision of genomic services to national research organizations and enterprises, an effective program for intellectual property protection and technology transfer, and a solid program of awareness of biotechnology.29
The foundation of the Mexican Proteomics Society has helped to strengthen and promote the field in this country and to build a community among emerging national proteomics laboratories.30 At present, some public institutions, including universities and hospitals, have started to apply omics technologies, which allow high-quality research. For example, the first full-scale sequencing project focused on the symbiotic plasmid of Rhizobium etli.31 Also, the sequencing of the approximately 2-gigabase genome of the Mexican landrace Palomero Toluqueño (Palomero), which revealed differences with the modern inbred line B73.32 Of remarkable value is the first high-throughput study of human genetics, which helped to determine whether the genetic variation in indigenous and mestizo populations could affect disease risk and the accuracy of diagnostic tools.33 However, even after several years of experience in applying cutting edge technology for research, the country is still far from goals achieved by other developing nations like Brazil or India. In fact, the development of bioinformatics, which is a sensitive field of omics research since it is essential for the analysis of thousands of data generated by a high-throughput study, is largely undeveloped in Mexico. This fact is evidenced by the difference in the number of publications: 95 papers in bioinformatics published from 1987-2012, compared with 388 for Brazil and 742 for India.34 Additionally, the use of omics technologies focused on the discovery of new biomarkers or therapeutic targets to solve the challenges that some diseases represent for Mexico, lately became an important topic. Recently, the Children's Hospital Federico Gómez invested more than 2 million USD, to jump-start a research unit focused on the analysis of proteomics and metabolomics of tissue samples from different childhood diseases.
Progress is being made in different areas of research in Mexico: proteomics of neglected tropical diseases, venomics, peptidomics, plant proteomics and cancer proteomics. These efforts have triggered Mexican proteomics research to be recognized at an international level.30
5Biobanks as promising platforms of omics studies focused on ALLBetween 1975 and 1999, ∼1% of newly marketed drugs were aimed at tropical diseases and tuberculosis.4 Currently, since cancer is one of the leading causes of death worldwide, the possibility of using omics technologies, particularly proteomics, for the identification of biomarkers and therapeutics targets would have profound consequences in pharmaceutical markets. For example, the market of monoclonal antibodies for cancer treatment was 23 billion USD in 2012 and it is expected to grow to 33 billion USD in 2017. On the other hand, in 2012, antibody drug conjugates (ADC) were estimated to be worth 138 million USD.35 Therefore, establishment of repositories of biological samples or biobanks, linked with extensive medical data of patients and infrastructures for sustained research may lead to improve the development of vaccines, drugs, and diagnostics, and constitute platforms that should be built in developing countries to address their health problems.4
With recent advances in the technology used for high throughput analyses, particularly the increased sensitivity of the tests and the ability to use less tissue, biobanks could allow large assays with different types and large numbers of human samples, allowing the collection of data from a specific region or country. These analyses would permit to understand the relationship at the genetic, mRNA, protein and metabolic levels.
For these reasons, biobanks have become universal research infrastructures that can be accessed by researchers from various fields, sectors, and nations. Biobanks provide researchers with an opportunity to maximize the research and clinical and public health translation potential from the new high-throughput research technologies, which require such repositories to generate important new health knowledge. However, the majority of those sources are found in developed countries and designed to address the health problems relevant to the minority of people living in wealthy nations, mostly the complex chronic non-communicable diseases of late onset.36,37 Nevertheless, efforts have been made in developing countries to build their biobanks. The establishment and proper running of a biobank may represent an overwhelming task since researchers have to consider a series of ethical, legal and social issues, including informed consent, benefit sharing, confidentiality, ownership, commercialization and public participation. However, the collection of human tissues is taking place in many countries, such as China, Gambia, Jordan, Mexico and South Africa.38 Mexico started to build a biobank in 1994 to assess the association between risk factors and common causes of death. So far, it is the only one in the country with public information available. It is documented that blood samples from 155,487 individuals are in storage, and available for research.39
We conclude that Mexico has the necessary infrastructure to begin a collection of tissues derived from pediatric patients diagnosed with ALL. The identification of biomarkers within this collection may permit the early detection of this disease and represents a good example of translational science. It will also allow the discovery of new potential therapeutic targets. Furthermore, it will also enrich the information available for the explanation of leukemogenesis.
FundingThis work was supported by Fondos Sectoriales-FOSISS, grant 261227; Grant HIM 2016-023 SSA, 1263; and Grant HIM 2015-007 SSA, 1176 (for RVR). Conacyt, grant 426295 (for WAAG).
Conflict of interestThe authors declare no conflicts of interest of any nature.