Introducción: Se sabe que la vitamina D (25(OH) D) tiene un efecto directo sobre la salud del hueso y músculo. Se ha relacionado también con enfermedades reumatológicas de origen autoinmune. Los estudios en niños con este tipo de enfermedades son escasos, sobre todo en artritis idiopática juvenil. El objetivo de este trabajo fue determinar las concentraciones de 25(OH) D en pacientes con lupus eritematoso sistémico (LES) y artritis idiopática juvenil (AIJ), y compararlas con las concentraciones en individuos sanos.
Métodos: Se determinaron las concentraciones de 25(OH) D por medio de espectrometría de masas con cromatografía liquida por tándem (ID-LC-MS/MS), las concentraciones de la hormona paratiroidea por análisis inmunorradiométrico (IRMA), y las concentraciones de calcio, fósforo y fosfatasa alcalina por métodos colorimétricos en 37 pacientes con LES, 37 pacientes con AIJ y 79 controles sanos.
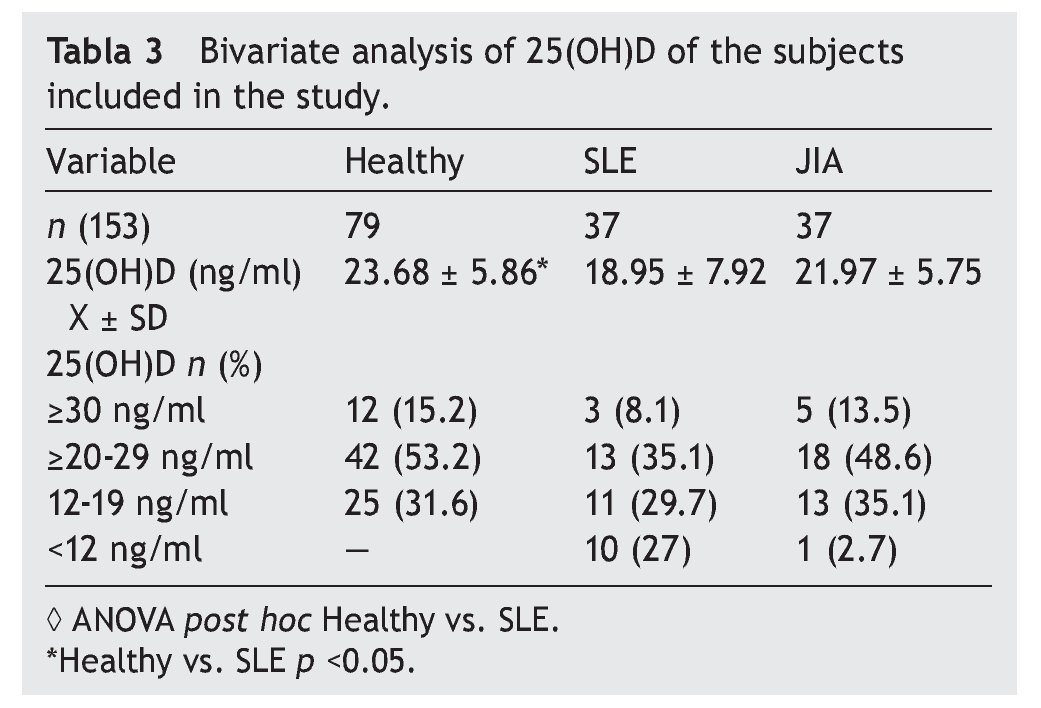
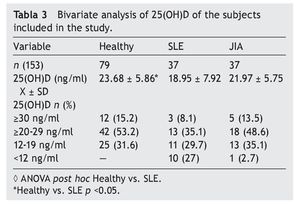
Resultados: Las concentraciones séricas de 25(OH) D fueron de 18.9 ± 7.92 ng/ml en LES, 21.97 ± 5.55 ng/ml en AIJ y 23.6 ± 3.07 ng/ml en controles sanos. Hubo una diferencia significativa al comparar las concentraciones de 25 (OH) D entre los pacientes con LES y los controles sanos (p <0.05). El 29.7% de pacientes con LES, el 35.1% con AIJ y el 31.6% de sujetos sanos cursaron con niveles deficientes de vitamina D en este estudio.
Conclusiones: Una tercera parte de los niños estudiados en los tres grupos mostraron deficiencias de vitamina D. La más severa fue en los niños con LES.
Background: It is well recognized that vitamin D has a direct effect in bone and muscle and has been associated as well with some rheumatologic diseases. Reports in children are scarce. The aim of this study was to determine the concentration level of 25(OH)D in a group of patients with systemic lupus erythematosus (SLE) and juvenile idiopathic arthritis (JIA) and compare them with healthy controls.
Methods: Vitamin D (25(OH)D) was measured with isotope-dilution liquid chromatographytandem mass spectrometry (ID-LC-MS/MS), PTH with immunoradiometric assay (IRMA), calcium, phosphorus and alkaline phosphatase by colorimetric assay in 37 patients with SLE, 37 patients with JIA and 79 healthy controls.
Results: Mean 25(OH)D concentration levels were as follows: SLE 18.9±7.92 ng/ml, JIA 21.97±5.55 ng/ml and 23.6±3.07 ng/ml in healthy controls. There was a significant difference between SLE patients vs. healthy controls (p <0.05); 29.7% of SLE patients, 35.1% of JIA patients and 31.6% of healthy controls had deficient levels of vitamin D.
Conclusions: One third of the total sample of children in this study had deficient levels of vitamin D. Patients with SLE presented a significant difference compared with healthy controls.
Pagina nueva 1
1. Introduction
Vitamin D (25(OH)D) has a role in bone pathology and its deficiency has important effects in the skeletal system. It is currently attributed to functions in the immune system because of its recognized participation in the growth and differentiation of multiple cell types (macrophages, dendritic cells and B and T cells).1-4 In addition to the few food sources rich in this vitamin, several risk factors related to low concentrations of vitamin D have been demonstrated such as latitude and little exposure to the sun, skin color, age, season, type of clothing, obesity, environmental pollution, and use of corticosteroids as well as use of sunscreen.5-7 It has been identified that vitamin D deficiency is present in different chronic degenerative diseases such as type 1 diabetes mellitus, cardiovascular disease, and some types of cancer as well as some autoimmune diseases like multiple sclerosis.4,8-14 Systemic lupus erythematosus (SLE) and juvenile idiopathic arthritis (JIA) are two rheumatological diseases considered to be a risk for the presence of vitamin D deficiency. Children with SLE present a higher risk of vitamin D deficiency due to steroid treatment, use of sunscreen and strict recommendation to not be exposed to the sun.15-17 JIA is also an autoimmune disease in which, in addition to the inflammatory process, steroids are indicated.18,19 Information about the vitamin D status in pediatric patients with SLE is rare. With regard to JLA, reports are even more limited. Therefore, the objectives of the present study were to determine the concentrations of 25(OH)D in a group of patients with SLE and JLA and compare them with healthy controls in order to determine the relationship between vitamin D concentrations with parathyroid hormone (PTH), calcium (Ca), phosphorus (P) and alkaline phosphatase (AP).
2. Methods
Children and adolescents <18 years of age with diagnosis of SLE or JIA from the Rheumatology Department of the Hospital Infantil de México, Federico Gómez (HIMFG) and of the Instituto Nacional de Rehabilitación (INR) in Mexico City were included in the study. Diagnosis of SLE was made according to the criteria of the American College of Rheumatology20,21 and for JIA according to the criteria of the Committee on Pediatric Rheumatology from the International League against Rheumatism (ILAR).22 Healthy children and adolescent control group was age- and gender-matched with sick subjects and recruited from the central zone of the country. Excluded from the study were subjects who were taking any vitamin D supplement. For the controls, we excluded those with a family history of diseases such as SLE and JIA.
2.1. Questionnaire
In each case, a questionnaire was applied to gather information on demographic variables, disease history, time of exposure to the sun (quantified in hours per week on a schedule of 10-16 h), use of steroids, skin color (using a color scale) and use of sunscreen.
Disease activity in subjects with SLE was assessed with the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI).23,24 In the case of subjects with JIA, a functional capacity questionnaire was applied (CHAQ).25-27 Functional class was determined using the Steinbrocker scale.28
2.2. Anthropometry
For measuring weight and height, a Seca stadiometer was used (model 700) with capacity for 220 kg and 200 cm height. BMI was calculated using data from the CDC 2000 Growth Chart (CDC 2000).
2.3. Vitamin D, PTH, Ca, K and AP
Concentrations of 25(OH)D were analyzed with the isotope-dilution liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS) method in the Tufts University Metabolism Laboratory in Boston, Massachusetts. PTH was measured in the Bone Mineral Metabolism Laboratory of the HIMFG using the immunoradiometric assay technique (IRMA). Ca and P concentrations and AP were determined in the central laboratory of the same hospital.
2.4. Statistical analysis
Statistical analysis was done using the SPSS v.17.0 statistical package; p <0.05 was considered significant. Mean difference between groups was assessed using ANOVA. Differences between proportions were evaluated with χ2. Pearson correlation was used between serum concentrations of 25(OH)D and Ca, P, AP and PTH levels. Multiple logistic regression analysis and covariance (ANCOVA) were performed to adjust the 25(OH)D levels with the variables of diagnosis, gender, age, sun exposure and sunscreen use.
The protocol was approved by the Ethics Committee of the HIMFG. Informed consent from parents or guardians was obtained for all subjects and informed consent for all children >8 years of age.
3. Results
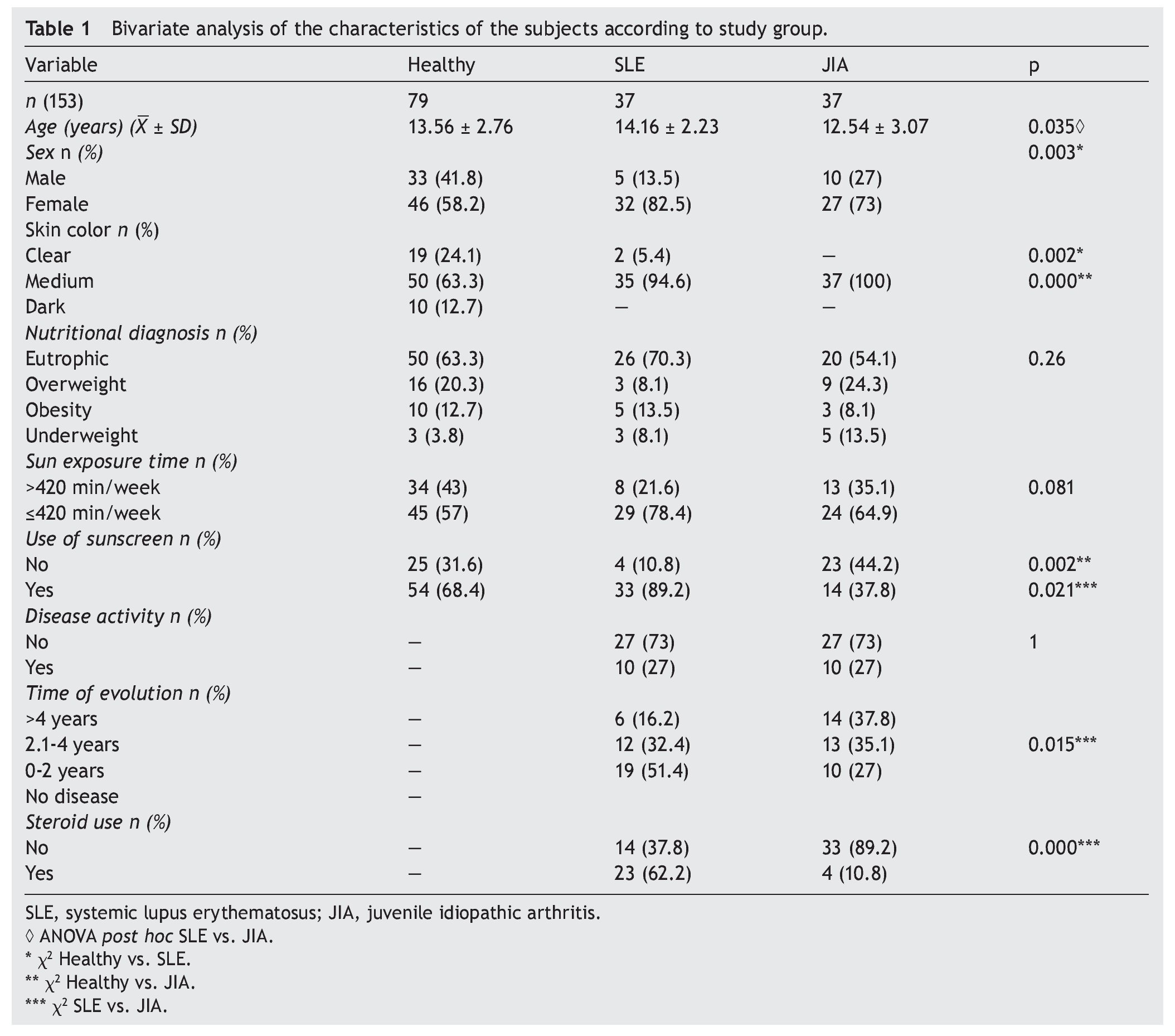
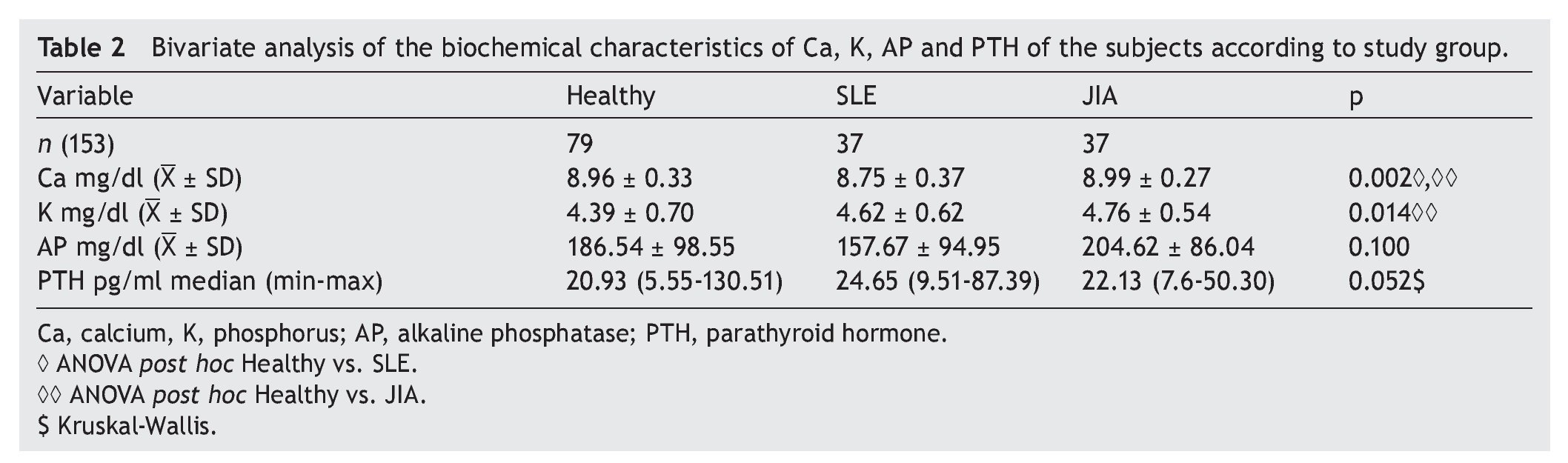
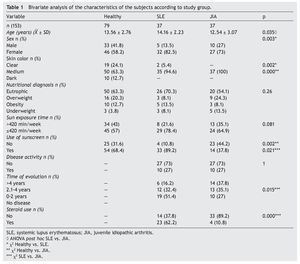
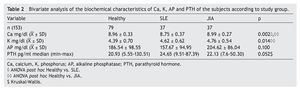
A total of 153 subjects were included, 37 with SLE, 37 with JIA and 79 healthy controls. There were no significant differences in nutritional diagnosis and time of exposure to the sun among the three groups (Table 1). In the group of subjects with disease, no differences were found in disease activity (73% were found active). Table 2 shows the mean of Ca, P, and AP concentration and the median PTH values for the three groups. There were differences in Ca values between the healthy control group and the sick group. P showed significant differences between the healthy group and the group with JIA. PTH and AP values showed no significant difference.
3.1. Vitamin D
Results of the mean concentrations of 25(OH)D by study group are shown in Table 3. In SLE, mean serum concentrations of 25(OH)D were significantly lower (18.9 vs. 23.6 ng/ ml, p <0.05) compared with the controls but without significant differences when compared with JIA (21.9 ng/ml). Comparing the different cut-off points, it was found that 31.6%
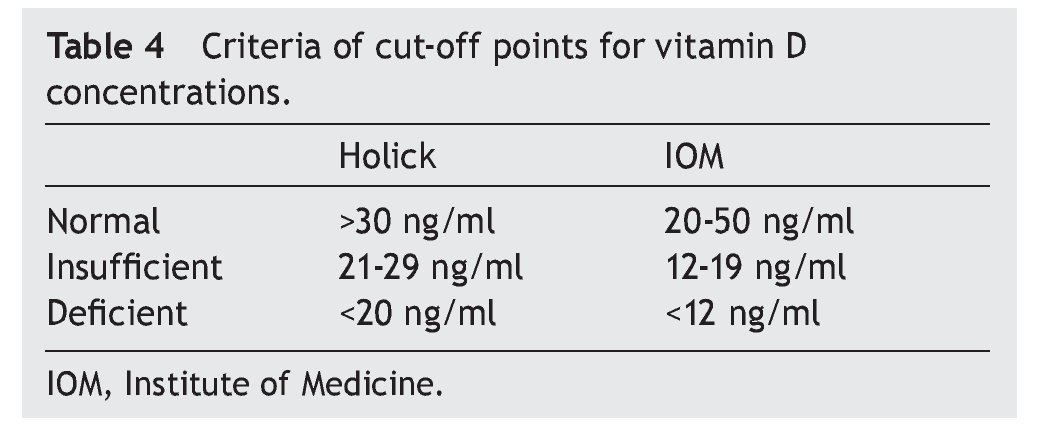
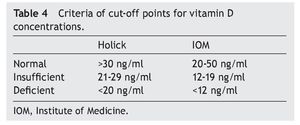
of healthy subjects had a deficiency in accordance with the parameters established by Holick29 (<20 ng/ml) and the same percentage for failure with the Institute of Medicine (IOM)30 (≥12-19 ng/ml) parameters (Table 4). As for the group of sick subjects, 29.7% of patients with SLE and 35.1% with JIA had levels within a range of ≥12-19 ng/ml.
3.2. Cut-off point criteria for vitamin D
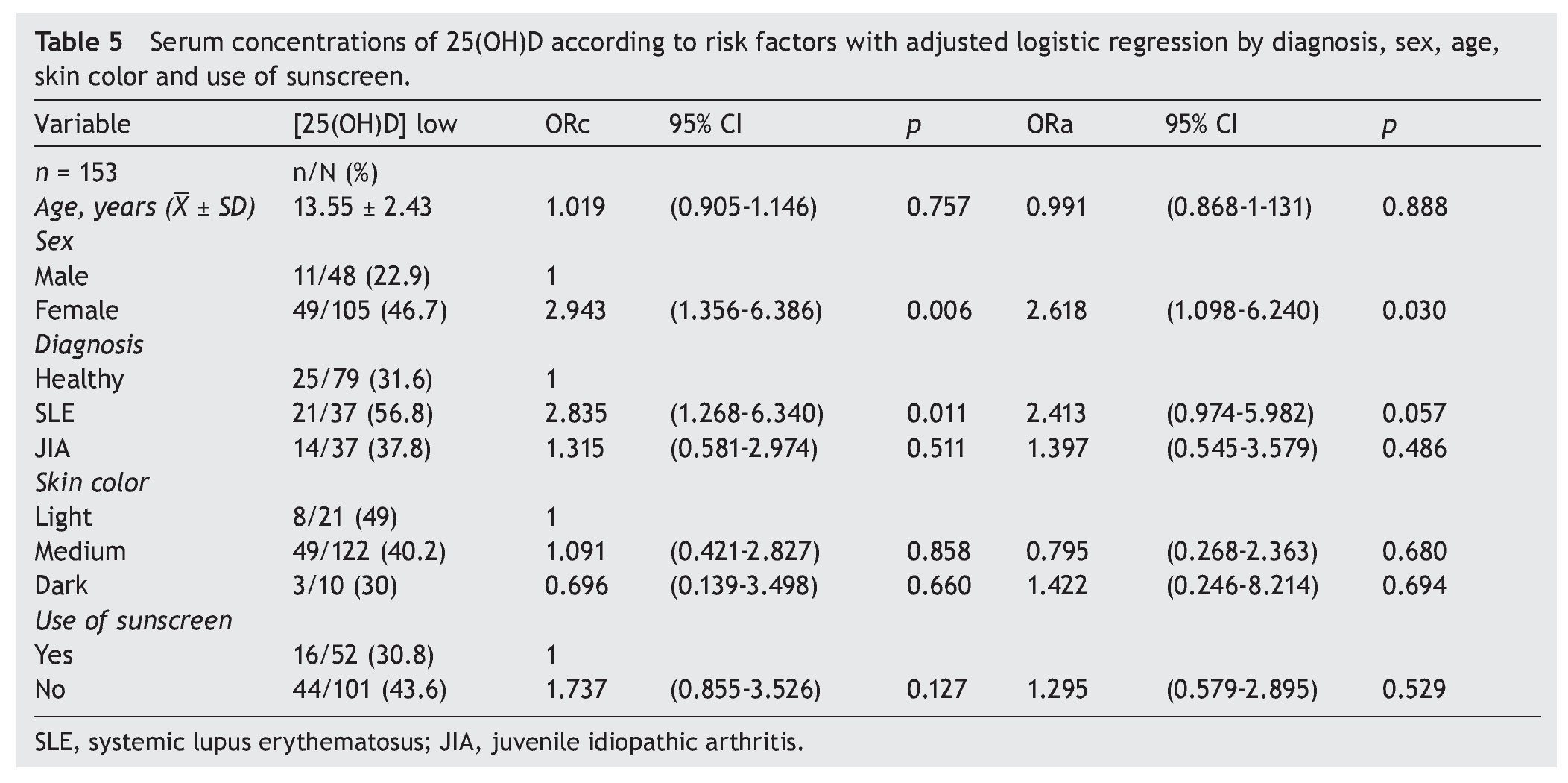
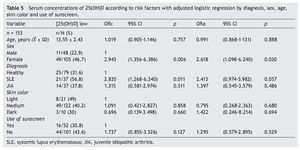
There were no differences found when analyzing the mean ± SD between sick subjects taking steroids and patients without steroids (19.23 ± 7.99 ng/ml vs. 21.17 ± 6.41 ng/ml, p = 0.25). Sick patients with disease activity had lower concentrations compared with subjects without disease activity (20.12 ± 8.79 ng/ml vs. 20.59 ± 6.36 ng/ml, p = 0.8). Subjects with AU had CHAQ applied; 54% had a mild-moderate category and moderate category, and the medians for vitamin D were 21.6 and 24.2 ng/ml, respectively. In respect to the Steinbrocker functional class, 97.3% of the population was between classes I and II, which correspond to a patient capable of performing daily activities (personal care, professional and entertainment) with median concentrations of vitamin D 21.3 and 20.8 ng/ml, respectively. On analyzing the correlation of the concentrations of 25(OH)D with the PTH log, a negative correlation was seen, which was statistically significant (—0.210, p=0.009), different from the correlations with the Ca, AP and P levels that were positive. Ca and AP correlations were statistically significant (p <0.05) but not so with P (p=0.441). Upon performing the logistic regression for risk factors (diagnosis, age, gender, skin color and use of sunscreen) for hypovitaminosis, it was observed that subjects with diagnosis of SLE had a higher OR for the presence of hypovitaminosis 2.41 (95% CI: 0.97-5.98; p=0.057) and the OR for females was 2.61 (95% CI: 1.09-6.42; p <0.05). No significant differences were observed between the levels of vitamin D and skin color and use of sunscreen (Table 5).
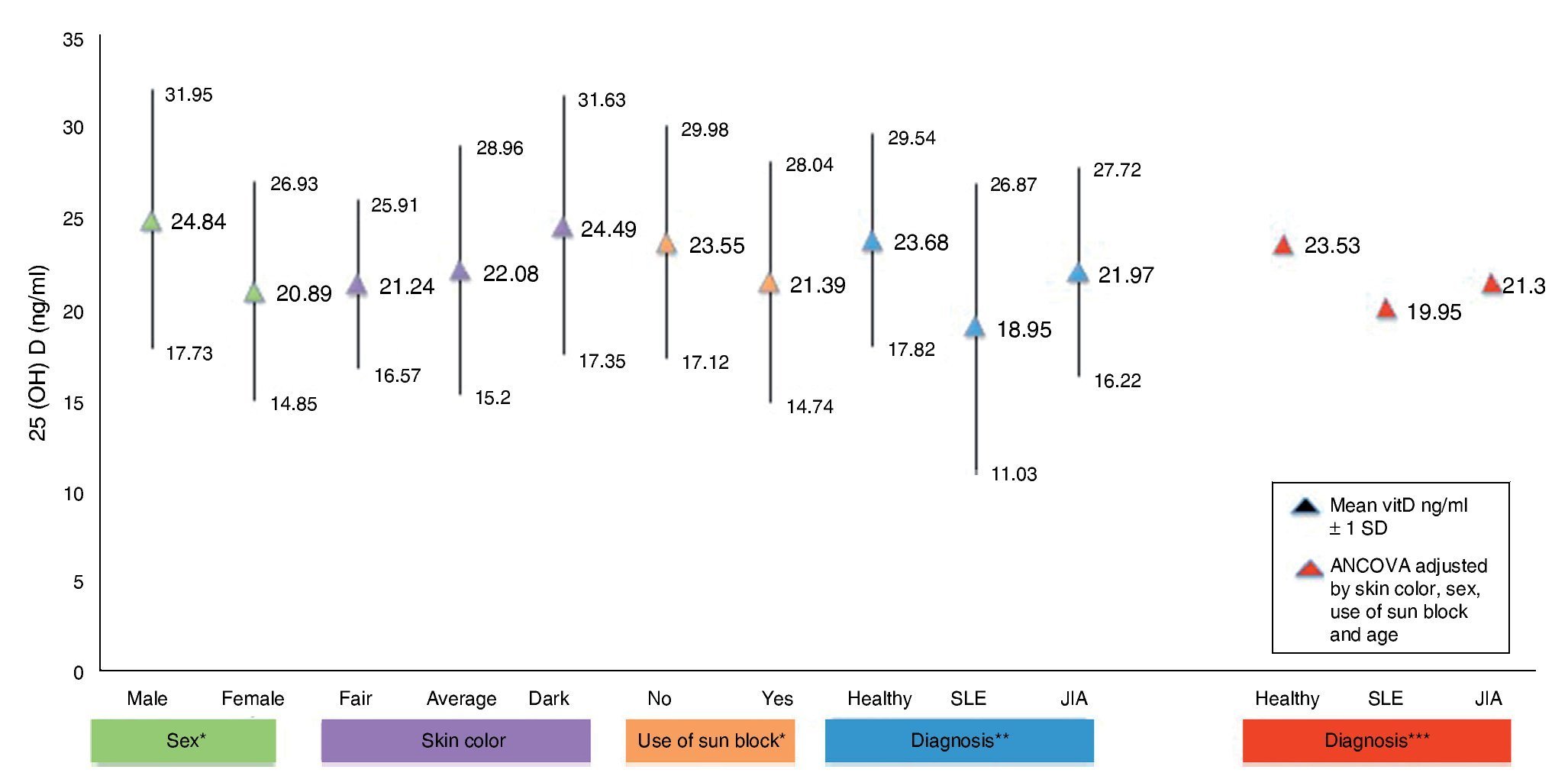
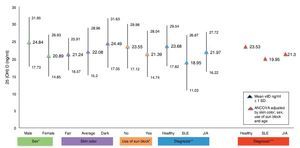
Figure 1 shows the mean concentrations of 25(OH)D divided by risk factors. Significant differences are observed in the variables for gender, use of sunscreen and diagnosis (p <0.05). After completing the covariance analysis adjusted for risk factors, it was noted that the mean values of 25(OH) D in the SLE group remained low (19.95 ng/ml) when compared with the healthy control group (p <0.05).
Figure 1 Concentrations of 25(OH)D with risk factors for hypovitaminosis. *p < 0.05. **SLE vs. Healthy ANOVA p < 0.05. ***SLE vs. Healthy ANCOVA p < 0.05.
4. Discussion
Results obtained in this work demonstrate a difference between mean concentrations of vitamin D of patients with SLE compared with healthy subjects (18.95 vs. 23.68 ng/ml), which was significant (p <0.05). This finding is consistent with the results of the study by Wright et al. in pediatric patients with SLE (n=38) and healthy subjects (n=207). When the results are compared, lower levels of vitamin D concentration were reported in sick subjects (18 ng/ml) than in healthy controls (22.3 ng/ml), with a p=0.004 difference.16
The literature shows that vitamin D deficiency with concentrations <20 ng/ml is a prevalent problem in the pediatric population.31-36 In accordance with the cut-off points established by the IOM with respect to 25(OH)D concentrations (normal 20-50 ng/ml, insufficient 12-19 ng/ml and deficient <12 ng/ml),30 the present study reports mean vitamin D concentrations in the normal ranges for the JIA group (21.97 ng/ml) and healthy controls (23.68 ng/ml) compared with the SLE group who had ranges of insufficiency (12-19 ng/ml). With respect to the values established by Holick (normal >30 ng/ml, insufficient 20-29 ng/ml and deficient <20 ng/ml),29 mean concentrations for the SLE group are deficient and for the JIA group and healthy controls were insufficient.
Risk factors for presenting low vitamin D concentrations in this type of disease are multiple, among which are skin color, use of sunscreen, sun exposure, use of steroids, and disease activity. It has been reported that skin color plays an important role in the concentrations of vitamin D. Specifically, higher pigmentation inhibits the synthesis and skin bioavailability of vitamin D.2,6,16,17 It was found in this study that subjects with a higher pigmentation correspond to 6.5%, and lower 25(OH)D concentrations were found in subjects with light and medium skin color, which corresponded to 93.5% of the population. These results differ from the study performed by Wright et al. in which subjects with a higher pigmentation color corresponded to 47% and lower vitamin D values were observed.16
Patients with SLE have photosensitivity, one of the diagnostic criteria for the disease, which promotes the production of antibodies and for worsening of the inflammation. For this reason, use of sunscreen is indicated, which reduces skin synthesis of vitamin D up to 99%.6 In this study it was observed that 89.2% of the subjects with SLE used sunscreen and that 78% have less exposure to the sun (≤420 min/week). In contrast, in subjects with JIA, sun exposure was 65% and in healthy subjects 57%. When evaluating vitamin D concentrations it was found that subjects who used sunscreen had lower concentrations compared with those who did not use sunscreen (p=0.05). In a study by Robinson et al. in which 37 subjects aged 4–21 years with diagnosis of SLE were evaluated, sunscreen use was reported by 57% of the subjects, which was associated with an increase of 6 ng/ml in the concentrations of 25(OH)D. It is worth mentioning that sun-screen users were subjects taking vitamin D supplements as opposed to this study in which subjects did not take these supplements.37
It has been reported in the literature that steroid use may have an adverse effect on the metabolism and bioavail-ability of vitamin D.6,29 In the population studied here, 62.2% of the subjects with SLE and 10.8% of the subjects with JIA were treated with steroids. However, no significant differences were found between medication and vitamin D concentrations. In the previously mentioned study by Robinson et al., 66% of the population with SLE took some type of steroid. No association was found between steroid use and vitamin concentrations.37
Disease activity apparently plays an important role on vitamin D concentrations. It was observed that sick subjects with disease activity had lower vitamin D concentrations, although without significant differences. In the study by Wright et al. it was reported that the patients with disease activity had concentrations <20 ng/ml.16 Robinson et al. found that subjects with SLE also had low concentrations of vitamin D, which were significantly associated with disease activity.37
Likewise, as reported in th literature, a statistically significant negative correlation was found between 25(OH)D and PTH concentrations. As vitamin D concentrations decrease, PTH activity increases to maintain calcium metabolism.38
It is important to mention that the cut-off points with greater validity are those established by the IOM because the technique with which they were defined has been considered up to now as the gold standard.39 It is paramount to have cutoff points established worldwide because author differences are the subject of criticism when making decisions about supplementation when there are such wide ranges.
Ethical responsibilities
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors must have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Funding
None.
Conflict of interest
The authors declare no conflict of interest of any nature.
Received 16 December 2014;
accepted 29 January 2015
http://dx.doi.org/10.1016/j.bmhimx.2015.05.002
* Corresponding author.
E-mail:osteoclark@gmail.com (P. Clark).