Background: Obesity, a worldwide health problem, is associated with the increase of noncommunicable diseases. Excess adipose tissue above what is expected produces a cytokine imbalance decreasing adiponectin—an anti-inflammatory cytokine—and increasing those proinflammatory cytokines such as resistin, IL-6 and IFN-γ. This imbalance elicits a low-degree systemic inflammation associated with insulin resistance (IR). Therefore, the aim of this study was to determine the relationship between pro- and anti-inflammatory cytokines levels with IR in eutrophic and obese Mexican children.
Methods: A cross-sectional study was conducted in 183 school-age children classified as obese and 186 children classified as eutrophic. Adiponectin, resistin, IL-6 and IFN-γ, glucose, insulin, high-density lipoprotein cholesterol and triglycerides were determined from a fasting blood sample. Height, weight, waist circumference, and systolic and diastolic blood pressures were measured. Spearman correlation and linear regression analysis were used to assess the association between cytokines and IR.
Results: Anthropometric and metabolic measurements as well as adiponectin concentrations were statistically different between eutrophic and obese children (p <0.001). Adiponectin concentrations were 12.5 ± 5.0 and 10.8 ± 4.2 μg/mL (p <0.018) for obese subjects without IR and obese subjects with IR. Resistin concentrations were 11.7 ± 7.5 and 14.2 ± 7.8 ng/mL (p =0.026), respectively. Linear regression showed that the HOMA-IR decreased -0.04 units (p =0.003) by unit of change of adiponectin. Whereas the association with resistin was opposite, the HOMA-IR units increased 0.02 by unit of change in resistin (p =0.018).
Conclusions: In this sample of eutrophic and obese Mexican children, adiponectin concentrations were inversely related with IR contrary to resistin, whose levels were directly related.
Introduction
Obesity in children is a worldwide health problem.1 In México, the prevalence of obesity has increased in the last 20 years.2 The National Health Survey and Nutrition (ENSANUT, 2006) reported, between 1999 and 2006, a dramatic increase of obesity in school-age children (77% in boys and 47% in girls).3 These figures remain almost the same in the Mexican ENSANUT, 2012.4 It is well known that obesogenic environments and shifts in lifestyle where energy ingested is greater than energy expended are at the core of this problem.5 Obesity is the most common cause of insulin resistance (IR) which, in turn, represents the common pathophysiological mechanism for metabolic alterations that are associated with the subsequent chronic illnesses such as hypertension and type 2 diabetes (T2D).6,7 In the recent past, these were typically adult diseases, but nowadays are appearing during childhood.
When adipose tissue increases more than expected, a cytokine imbalance appears, increasing the release of pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β, resistin, and leptin, whereas adiponectin—an anti-inflammatory cytokine—decreases.8,9 This imbalance produces a chronic low-grade inflammation.10,11
It is currently accepted that alterations such as IR, endothelial dysfunction, atherosclerosis12-14 and subsequent cardiovascular events are consequences of this low-grade state of inflammation.15 Adiponectin protein is exclusively produced by adipocytes and plays a role in energy homeostasis and fatty acid oxidation as well as in reducing plasma triglycerides.16 Although it negatively correlates with body fat,17,18 the relationship with metabolic impairments in obese children remains controversial.19,20
On the other hand, resistin, whose levels augment in diet-induced obesity as well as in genetic models of obesity and insulin resistance, has proposed to link obesity with diabetes due to its insulin antagonism. However, these results are inconsistent in humans. Some studies did not find differences in the concentration of resistin between lean and obese children or an effect on insulin resistance.21,22 Other groups did not find consistent associations,23 whereas others reported that adults with high resistin levels have a higher risk for heart failure.24
Likewise, human adipose tissue is the main site of IL-6 production, although it is also released by cells such as T lymphocytes, macrophages, and muscle cells. IL-6 is the major determinant of the hepatic production of C-reactive protein, a mechanism that can explain the link between low-grade inflammation during obesity and IR. Similarly, IL-6 secretion is induced during exercise. In this case, IL-6 acts as a myokine, functioning as an energy sensor by activating AMP-activated protein kinase and enhancing glucose disposal and, in turn, regulates metabolic programming such as lipolysis and gluconeogenesis in adipose tissue and liver, respectively.25
IFN-γ is a major T-cell cytokine. It has been postulated that a shift of T helper 1 over T helper 2 cytokines is observed in obesity and can promote inflammation in fat tissue. Also, IFN-γ induced sustained loss of insulin-stimulated glucose uptake in human adipocytes, coincident with downregulation of the insulin receptor.23,26 However, this relation has been scarcely studied. Notwithstanding, this cytokine has been related to obesity as well as to IR in children.24
Despite the obesity pandemic, which has led to a parallel rise in the prevalence of pediatric forms of chronic diseases such as T2D, there is not an abundance of studies that show the participation of cytokines linking obesity and IR in children. The purpose of the study was to explore which of the aforementioned cytokines are able to identify IR and the metabolic alterations in eutrophic and obese Mexican children.
Subjects and methods
This epidemiological study was conducted in nine middle socioeconomic class schools of Mexico City. Prior to the study, ethical clearance was obtained by both the ethics and research institutional boards of our hospital and school authorities; likewise, written informed consent was obtained from the participants and their parents in each of the two stages of the study.
The first stage was aimed to identify obese school-age children. For this purpose and following anthropometric international guidelines, a trained team of nurses measured the children's weight and height without shoes and wearing light clothing. Weight was taken using a digital scale (Seca, Hamburg, Germany) to the nearest 0.1 kg. Height was measured using a Seca 225 stadiometer to the nearest 0.1 cm. This screening was performed in 1,441 children between 6 and 12 years of age and eutrophic or obese status was assessed using the body mass index (BMI) percentiles according to the Centers for Disease Control and Prevention 2000 references (Atlanta, GA). Eutrophic status was considered when BMI was between the 25th and 75th percentile and obesity was considered when BMI was ≥95th percentile for the child's age and gender. To establish a contrasting nutritional status, those children with BMI between 76 and ≤95 pc were purposely excluded.
After classifying the participants, 200 obese and 200 eutrophic children were identified. From these, 186 eutrophic and 183 obese children accepted to participate in the following stage and complete blood samples were collected.
During the second stage, blood pressure was measured by the auscultatory method using a sphygmomanometer (ALPK2, Tokyo, Japan) with appropriate cuff size for arm width, following 2004 North American guidelines.27 Four blood pressure readings were taken for each participant on the right arm in a sitting position, resting 1 min between each measurement and considering the blood pressure level as the mean of the last three readings. Waist circumference (WC) was measured at the midpoint between the lowest rib and the iliac crest after a normal exhalation with children in the standing position. In addition, after 12 h of fasting, blood was drawn to determine insulin by chemiluminescence immunoassay (IMMULITE 2000, Euro, DPC, Llanberis, UK), glucose, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (ILAB 350, Instrumentation Laboratory, Barcelona, Spain).
Cytokines—IL-6, IFN-γ, and resistin—were determined by ELISA method using R&D kits (Minneapolis, MN), and total adiponectin was determined with a Millipore kit (St. Charles, MO). Insulin resistance was assessed through HOMA-IR using the following equation:28
[fasting glucose (mg/dL) × fasting insulin (μU/mL)/405]
A 3.4 HOMA-IR value was the cut-off point to accept insulin resistance, corresponding to the 90th percentile of a population of healthy children. Values above this cut-off point were considered as cardiovascular risk factors.29
Statistical analysis
Mean and standard deviation were obtained from the demographic, anthropometric and metabolic variables. Using BMI, subjects were categorized as eutrophic or obese and, in turn, obese subjects were classified according to those who have or do not have IR. All measurements in each of the groups were compared using Student t test. Bivariate Spearman correlation analysis was performed for IR and metabolic and inflammatory factors. Using robust linear regression analysis with inflammatory markers as independent variables, the effect over HOMA-IR was assessed. For all analyses, p <0.05 values were considered statistically significant. Data were processed with STATA, SE v.11.0, and EPIINFO 3.3.2 according to the CDC 2000 reference.30
Results
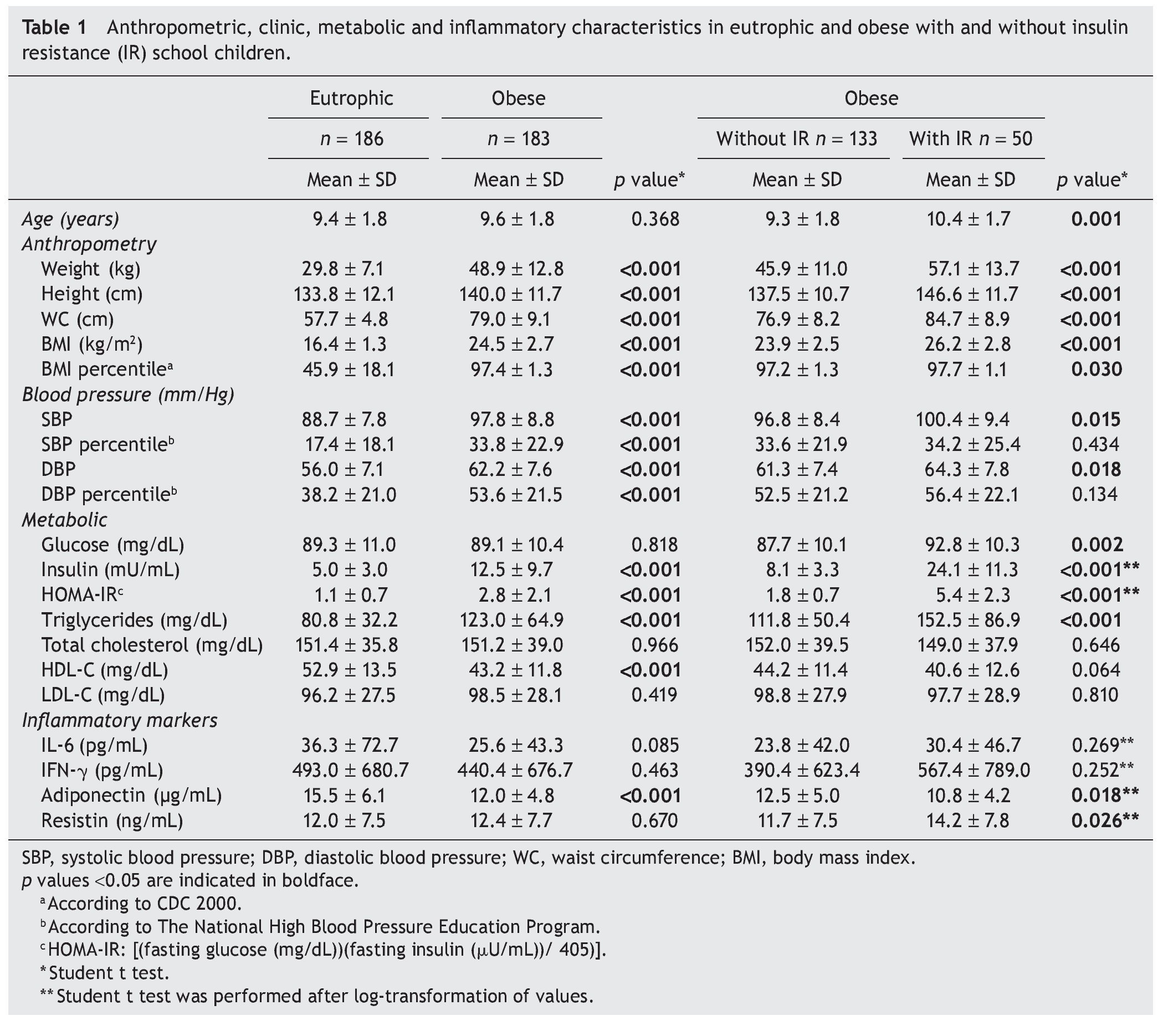
Anthropometric features, blood pressure, metabolic profile and cytokine concentrations of eutrophic and obese children with or without IR are shown in Table 1. Mean age of the participants was 9.5 years. The prevalence of IR in obese populations was 27.3%. Anthropometric and blood pressure values were different between eutrophic and obese participants and also between obese children with or without IR (p <0.05). In terms of metabolic variables, obese children with IR exhibited higher values of glucose, insulin, HOMA-IR and triglycerides than obese children without IR and eutrophic children. Another parameter showing differences was HDL-C in which the obese group showed the lowest values (p <0.001).
Adiponectin values were inversely correlated with nutritional status. Eutrophic children had higher values than obese children (p <0.001). Interestingly, the obese group with IR showed lower concentrations of adiponectin and higher concentrations of resistin than the obese group of children without IR (p <0.05).
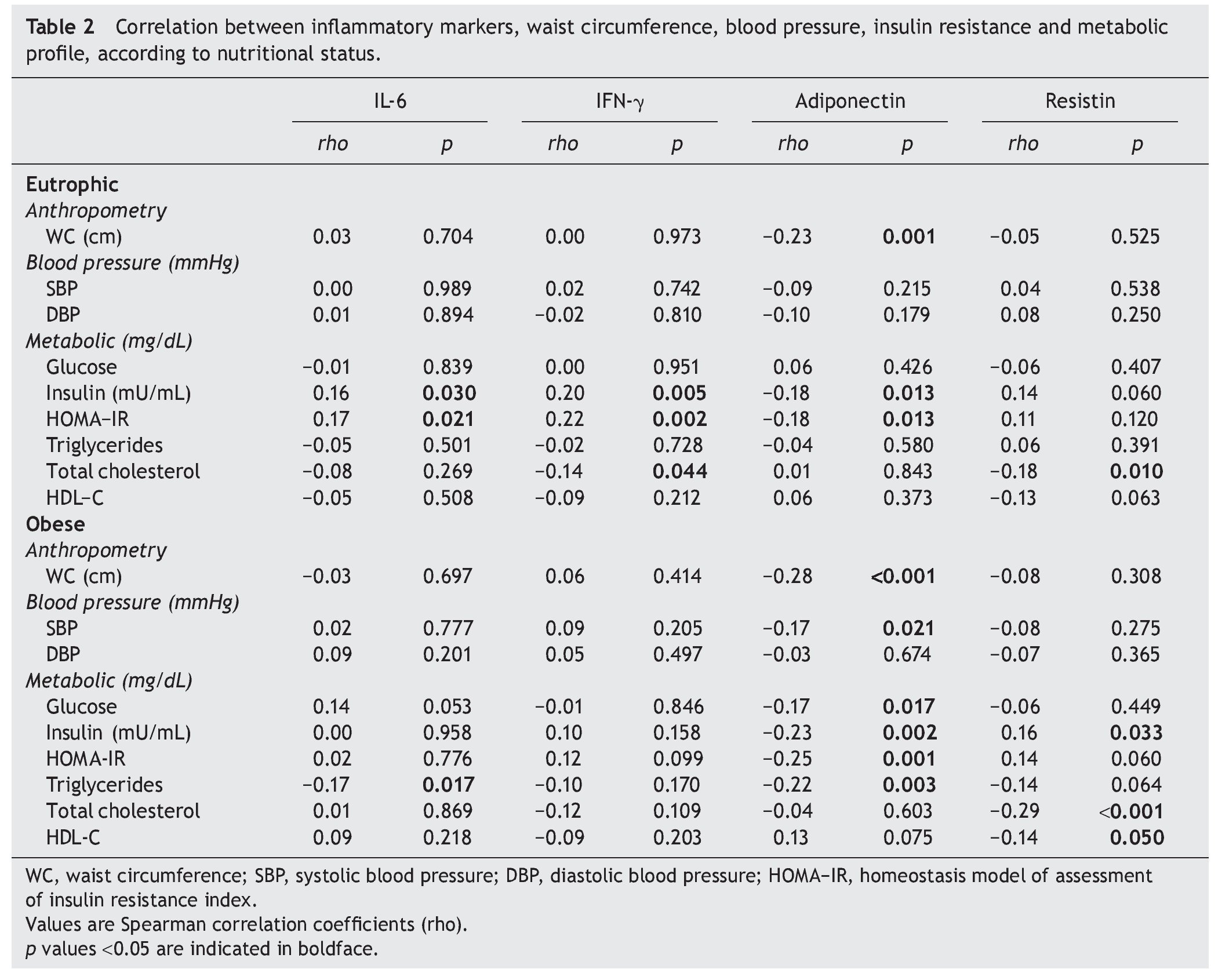
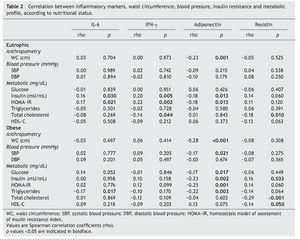
Table 2 shows the correlations between the levels of inflammatory markers with metabolic and anthropometric features in both eutrophic and obese children. It describes that adiponectin values in eutrophic children were inversely related with insulin and HOMA-IR, whereas IL-6 and IFN-γ showed a direct association (p <0.05). The correlation between impairment of IR components and adiponectin, although observed in both eutrophic and obese children, was stronger in obese children. Likewise, systolic blood pressure and triglycerides showed a significant inverse association in these groups. On the contrary, a direct correlation was observed between resistin and insulin (rho = 0.16, p = 0.033). Adiponectin in both eutrophic and obese children showed inverse correlations with WC values (p =0.001).
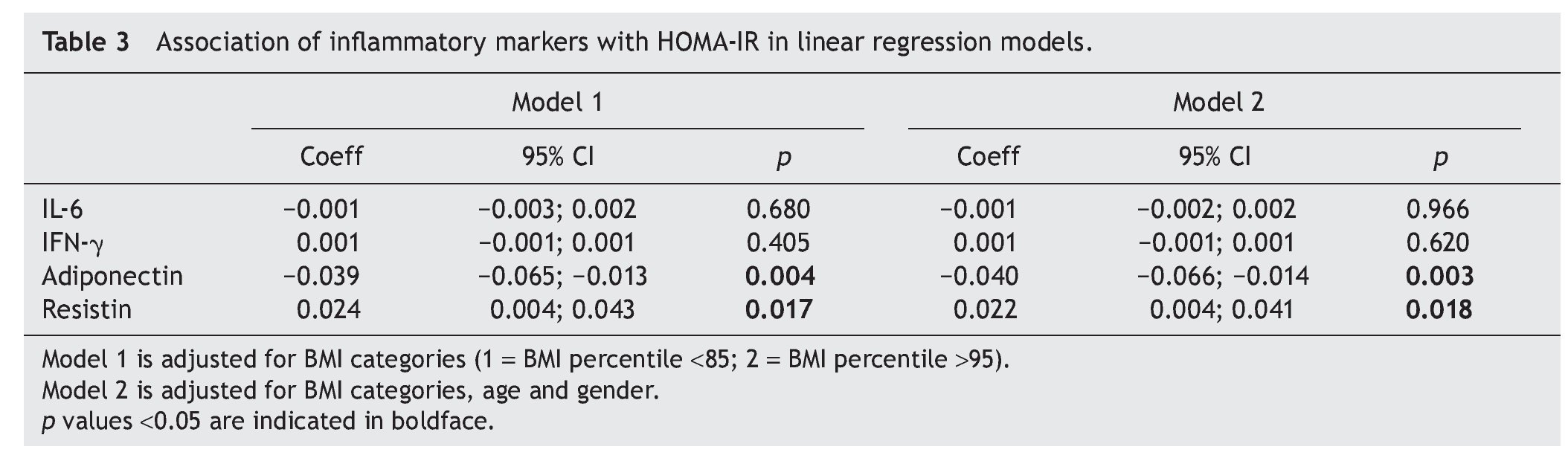
Table 3 shows, in whole populations, how HOMA-IR coefficients decrease, whereas adiponectin units increase. These change remained significant after adjusting by gender, age, and BMI categories. HOMA-IR decreased 0.04 units (95% CI 0.004; 0.041) for unit change of adiponectin. Whereas the association with resistin was opposite, HOMA-IR units increased 0.02 by unit of change in resistin (p =0.018).
Discussion
The results of this study, which explored the effects of inflammatory cytokines on IR in children with contrasting nutritional status, showed that HOMA-IR values were inversely related with adiponectin concentrations and directly associated with resistin. However, the adiponectin effect was observed in both obese and eutrophic children, whereas resistin was observed only in obese children.
Although there are reports in which adiponectin is not useful for prediction of IR and metabolic syndrome features,19,20 results of the present study agree with most literature reports17,18 that show that adiponectin is consistently negatively correlated with body weight and HOMA-IR values in obese children. Although to a lesser degree than obese children, the same effect was seen in eutrophic children.
Additionally, studies in obese children addressed for weight reduction have shown that adiponectin concentrations increase, whereas IR improves in those patients who lose weight.22,31-34 Likewise, due to the fact that low plasma concentrations could precede changes of IR not only in obese subjects but also in eutrophic children, this supports the idea that adiponectin could be used as a biomarker to asses metabolic changes and to predict IR in both obese and eutrophic children8 as well as in metabolic syndrome8 and T2D.35
In addition, high adiponectin values had an inverse association with triglycerides, whereas they had a direct association with HDL-C (data not shown) as reported by other authors.36
Regarding resistin, which has been proposed as a hormone linking obesity with T2D in humans, it currently remains controversial because support for the above-proposed information comes from experimental rodent studies37 that show a strong correlation with IR and obesity that upregulates their production, whereas amelioration of obesity produces a downregulation.38
Currently, the role of resistin on IR in children is not well understood due to contradictory study results.39 Some find positive associations,40,41 whereas others do not.21,42-44
Our results show that resistin concentrations are higher in obese than in eutrophic children and also are higher in obese children with IR than in obese children without IR. Likewise, in these children this cytokine has a strong association with HOMA IR even after adjustment for gender and nutritional status, confirming that resistin is positively associated with IR.
According to this theory, it has been shown in longitudinal interventions for weight reduction that in those patients who remain at the same weight, resistin increases,22 which could be interpreted as the persistence of low-grade inflammation. Additionally, when obese patients with glucose intolerance receive metformin to ameliorate their problem, it is observed that resistin concentration reduces, probably because metformin affects the resistin gene expression as well as activating the AMPK/LKB1 pathway, eliminating some of the abnormal resistin regulation.45
IL-6 and IFN-γ have been related with IR in both adults and children.11,46,47 Other studies have shown that after weight reduction in children, IL-6 decreases, with improvement of insulin sensitivity.48,49
Likewise, results from the present study show that both IL-6 and IFN-γ are correlated with HOMA-IR values. However, when each was correlated, there was a lack of effect even after adjusting the model by age and gender. This has also been reported elsewere.50
The present study is observational and cross-sectional, with the limitation that by using between study variables it is not possible to establish causality. However, given the close relationship between obesity, chronic low-grade inflammation and IR with the attendant risk of developing comorbidities such as T2D even in children, it is imperative to deal with the problem by looking for early biomarkers with the aim of recognizing the problem.
Preventing IR means preventing obesity from the early stages of life through modification of lifestyles and obesogenic environments. The low grade of inflammation produced by obesity is a common way for developing, in the short term, IR and diseases such as T2D which, in turn, are the leading causes of mortality in adults. It is necessary to prioritize and to understand and prevent these alterations from early ages.
This study has confirmed that among obese children, regardless of age and gender, increasing figures of resistin or decrease of adiponectin are associated with the presence of IR and metabolic disorders. Therefore, these could be used as reliable biomarkers for predicting IR in obese children.
Additionally, when adiponectin values increase in eutrophic children, it is possible to observe a decrease in insulin concentrations and HOMA-IR values. This observation may support the idea that adiponectin is an early biomarker of IR, not only in obese subjects but also in eutrophic subjects.
In conclusion, among cytokines, adiponectin, which is an anti-inflammatory, was negatively associated with IR in both obese and eutrophic children, although to a lesser magnitude in eutrophic subjects. On the other hand, the pro-inflammatory IL-6 and IFN-γ were directly associated with obesity and IR. Resistin was directly associated with obese children, particularly those who had IR, supporting the idea that this cytokine is involved in the inflammatory process produced by obesity. Finally, the plasma behavior of this adipocytokine could be used to monitor the previously mentioned metabolic alterations.
Conflict of interest
The authors declare no conflict of interest of any nature.
Received 2 September 2013;
accepted 2 October 2013
* Corresponding author.
E-mail address:floreshuertamd@gmail.com (S. Flores-Huerta).