In mammals, apoptosis is the main mechanism to eliminate unwanted cells, securing tissue homeostasis and consequently maintaining the health in the organism. Classically, apoptosis culminates with the activation of caspases, which are enzymes that display cysteine protease activity to degrade specific substrates implied in essential cellular processes. This process is highly regulated. A key regulation mechanism is mediated by the Inhibitor of Apoptosis Proteins (IAPs) family members, which inhibit the activated forms of caspases through physical interaction with them. Smac/DIABLO, a mitochondrial protein that is translocated to the cytoplasm in apoptotic conditions, derepresses the IAP-mediated caspase inhibition through physical interaction with IAPs. The first four amino acids (AVPI) of Smac/DIABLO mediate the interaction with IAPs and subsequent apoptosis induction. This interaction has lead to the creation of small molecules mimicking the AVPI segment for potential anticancer therapy. Nevertheless, several studies have pointed out the existence of AVPI-independent functions of Smac/DIABLO. The aim of this review was to provide a landscape of these underestimated AVPI-independent biological functions that have been observed using different approaches, such as the study of endogenous splice variant isoforms and truncated and mutated artificial proteins.
La apoptosis es uno de los principales mecanismos en los mamíferos para eliminar células no deseadas, asegurando la homeostasis de los tejidos y, consecuentemente, la salud de los mismos. De forma clásica, la apoptosis finaliza con la activación de las caspasas, enzimas que despliegan actividad de proteasas de cisteína, involucradas en la degradación de sustratos específicos implicados en procesos celulares esenciales. El proceso apoptótico se encuentra altamente regulado. Un mecanismo de regulación es el mediado por los miembros de la familia de las Proteínas Inhibidoras de la Apoptosis (PIA), las cuales inhiben a las formas activas de las caspasas a través de la interacción física con estas. Smac/DIABLO, proteína mitocondrial que es translocada al citoplasma en condiciones apoptóticas, antagoniza la inhibición de las caspasas mediante su interacción física con las PIA. Los cuatro primeros aminoácidos (AVPI) de Smac/DIABLO intervienen en su asociación con las PIA y la subsecuente inducción apoptótica. Esto ha guiado a la generación de pequeñas moléculas miméticas del segmento AVPI para el uso potencial como una terapia anti-cancerígena. Sin embargo, varios estudios han indicado la presencia de funciones en Smac/DIABLO independientes del AVPI. El objetivo de esta revisión fue proporcionar un panorama de estas funciones biológicas desestimadas —independientes al AVPI— las cuales se han observado utilizando diferentes aproximaciones, como el estudio de las isoformas generadas por el procesamiento alternativo del gen y la síntesis de proteínas artificialmente mutadas.
Apoptosis, or programmed cell death, is a natural mechanism that secures tissue homeostasis through the elimination of both damaged and unwanted cells. The apoptotic program culminates with the degradation of specific proteins implied in key cellular processes by a group of enzymes termed caspases. Caspases are expressed as zymogens, the inactive configuration of their cysteine protease activity. These enzymes are divided into two groups: initiator caspases (caspase-2, -8, -9 and -10) and effector caspases (caspase-3, -6 and -7). As their name indicates, initiator caspases are involved in the activation of the apoptotic pathway, while effector caspases mediate the signaling amplification loop leading to the generation of the morphological apoptotic features.1
Apoptosis initiates through two pathways: the extrinsic pathway (also known as death receptor pathway) and the intrinsic pathway (mitochondrial signaling pathway). The extrinsic pathway is mediated by the engagement of death receptors—transmembrane proteins belonging to the tumor necrosis factor receptor superfamily (TNFRs)—with their associated ligands (e.g. the association of TNF-α with its cognate receptor). After this, proapoptotic cellular transduction signaling is initiated by the formation of DISC (death-inducing signaling complex), which is formed by the recruitment of adapter proteins, such as FADD, TRADD or RAIDD, to the intracellular domain of the activated death receptor. After that, the initiator caspase-8 associates with the DISC and undergoes autocatalytic activation. Conversely, the intrinsic pathway is initiated by several stimuli, such as irradiation, growth factors withdrawal, and antineoplastic drugs, among others. These stimuli compromise the integrity of the mitochondrial outer membrane, fomenting the mitochondrial outer membrane permeabilization (MOMP), and releasing several proapoptotic mitochondrial proteins such as cytochrome-C (Cyt-c). This point is known as the point of no return due to the loss of mitochondrial energy-generating function. Once Cyt-C is relocated to the cytosol, it can associate with APAF1, caspase-9, and ATP, assembling the apoptosome. Similar to caspase-8 in the extrinsic pathway activation, caspase-9 is activated by autocatalysis in the apoptosome. Once initiator caspases are activated (from the extrinsic or intrinsic pathway), they induce the activation of effector caspases through their cysteine protease activity. Finally, the effector caspases are implied in the generation of both biochemical and morphological apoptotic features such as cellular shrinkage, bleeding, chromatin condensation, DNA fragmentation and cellular fragmentation into membrane-bound apoptotic bodies.1,2
2Apoptosis regulation is lost in cancerThe caspase activity threshold dictates several cell fate decisions. In some cases, suppression of apoptosis through caspase inhibition is needed for tissue and organism health. For example, it is important in cells with low regenerative potential such as neurons and cardiomyocytes.3 On the other hand, higher levels of caspase activity balance the cellular choice toward apoptotic program targeting unwanted cells, or damaged ones, for a clean elimination. Thus, apoptosis inhibition in damaged cells is a feature in several diseases such as cancer.4,5 Since cancer is a group of diseases that accounts for more than 100 distinct pathologies from different organs,6 the understanding of the underlying mechanisms implied in apoptosis inhibition is needed for the development of new antineoplastic options. In this sense, it is important to describe the apoptotic brakes.
The apoptotic pathway is a highly regulated process at different levels. If we focus on the inhibition or repression of caspase activation, there are at least three levels of regulation, taking MOMP as a reference point. First, in the extrinsic pathway, which is upstream to MOMP, cFLIP mediates the inhibition of caspase-8 activation through its displacement of the DISC. Recently, it has been described that cFLIP isoforms regulate caspase-8 activation in a co-operative and hierarchical fashion.7 Second, at the mitochondrial level, Bcl2 family members modulate the release of mitochondrial proapoptotic proteins into the cytoplasm by the regulation of MOMP. Briefly, Bcl2 family structure is characterized by the presence of 1-4 Bcl2-homolgy domains (BH). This family can be divided into two groups: antiapoptotic members (BCL2, BCL-w, BCL-XL, MCL1 and BOO/DIVA) and proapoptotic members (BAX, BID, BAD, BIK, BAK, BOK, BCL-Xs, PUMA, NOXA, NIX and BIM). Two subfamilies of proapoptotic members can be differentiated: the Bax subfamily (BAX, BAK, and BOK), which proteins are implicated in the pore formation at the mitochondrial outer membrane;8–10 and the proapoptotic Bcl2 proteins, also called BH3-only proteins (BID, BAD, BIM, NOXA, and PUMA).11 It has been proposed that BH3-only proteins induce BAX activation through two mechanisms. The first mechanism triggers conformational changes that allow the homodimerization and translocation of Bax family members to the outer membrane of mitochondria (OMM) for the induction of pore formation through direct interaction.12 The other mechanism is the derepression of Bax/Bax dimers from Bcl-2 antiapoptotic proteins.11 Importantly, p53 protein has a relevant role in this scenario using the transcriptional induction of Bcl-2 proapoptotic genes.13 Finally, the inhibitor of apoptosis proteins (IAP) family members directly inhibit the activated forms of both effector and initiator caspases. Thus, IAPs have a regulatory role both upstream and downstream of the apoptotic pathway.
3Inhibitor of apoptosis protein family in cancerAs stated before, IAP family members target activated caspases for inhibition through physical interaction with them. These proteins structure is characterized by the presence of 1 to 3 BIR (baculoviral IAP repeats) domains that mediate physical interactions. In addition, some IAPs have a RING domain that displays an E3-ligase activity. From the three enzymes involved in the ubiquitination process, E3-ligase activity has been well described as the responsible for the substrate specificity of the posttranslational modification. By this way, the RING domains of IAPs target caspases for proteasome-dependent degradation.14 The members of the IAP family in mammals are X-chromosome-linked IAP (XIAP), cellular-IAP1 (c-IAP1), cellular-IAP2 (c-IAP2), ML-IAP/Livin, neuronal apoptosis inhibitory protein (NAIP), Bruce/Apollon and Survivin. XIAP exerts the most potent inhibitory effect over caspases and, consequently, it is the most studied IAP member. XIAP has three BIR and one RING domains. The linker region between BIR1 and BIR2 of XIAP is needed for both caspase-3 and -7 inhibition.15,16 The BIR3 domain is involved in caspase-9 inhibition.17 As expected, apoptosis inhibition through IAP overexpression has been described. Specifically, XIAP over-expression has been related with both cancer initiation and progression and anticancer therapy resistance.18,19 Concordantly, XIAP degradation mediated by different approaches induced tumor growth retardation and chemosensitization in mouse xenograft models.20
4Smac/DIABLO negatively regulates IAPsSmac/DIABLO favors apoptosis culmination through IAP antagonism. The canonical Smac/DIABLO isoform, also known as Smac-α, is a mitochondrial-compartmentalized protein due to the presence of a mitochondrial targeting sequence (MTS) in its coding sequence. Once in the mitochondria, the MTS is cleaved to generate the mature protein that displays the IBM (IAP-binding motif) at its N-terminus.21–23 Following apoptotic insults that induce MOMP, Smac/DIABLO is released from the mitochondria into the cytoplasm. In this respect, the efflux of Smac/DIABLO seems to follow a distinct mechanism than Cyt-C release.24–26. Several explanations have been formulated to explain these phenomena: from technical issues, such as buffer composition for the analysis of mitochondrial proteins released to cytosolic compartment,27 to regulatory mechanisms achieved by JNK28 or XIAP29,30 in stress-induced apoptosis.
Once Smac/DIABLO is located in the cytoplasm, it can interact and inhibit the antiapoptotic actions of IAPs through its IBM motif. The four N-terminus amino acid residues (Ala-Val-Pro-Ile) form this IBM motif that is known as the AVPI segment. In turn, IBM interaction with BIR3 domain of XIAP releases the IAP-inhibition of caspase-9.22,31 The release of caspase-9 from XIAP is a consequence of the mutually exclusive interaction and the larger affinity of Smac/DIABLO to the BIR3 domain.32 Smac/DIABLO also interacts with the BIR1 and BIR2 domains of XIAP releasing effector caspases previously retained by XIAP.21,33 Smac/DIABLO can also interact with other IAP family members, such as cIAP-1,34,35 cIAP-2,35 Survivin,36–38 and Livin39,40 by its AVPI segment. However, Smac/DIABLO can also be targeted for proteasome degradation by IAPs. This ubiquitination has been described for XIAP,41 c-IAP1 and c-IAP2,42 Livin,39 and BRUCE.7,43 On the other hand, Smac/DIABLO can also modulate the protein half-life of some IAPs by inducing its RING-mediated auto-ubiquitination targeting them for proteasome degradation, as observed for c-IAP1 and c-IAP2.35 Although Smac/DIABLO is unable to induce degradation of XIAP,35,44 it favors XIAP auto-ubiquitination.35
Structural and thermodynamic studies indicate that Smac/DIABLO is one of the highest stability proteins. This feature is explained by the ability of Smac/DIABLO to form homodimers.45 At physiological conditions, it is very unlikely that the homodimeric structure of Smac/DIABLO can be dissociated.45 Functional studies have revealed that dimeric Smac/DIABLO is needed to relieve XIAP-mediated caspase inhibition.33 It has been demonstrated concordantly that artificial mutant versions of Smac/DIABLO with the loss of apoptotic effects are unable to associate themselves.46,47 It has also been observed that the dimeric interface of Smac/DIABLO could be a subject of posttranslational modifications; thus, compromise its ability to form homodimers and, consequently, its proapoptotic actions.46 Interestingly, it has been described that JNK3 can phosphorylate Smac/DIABLO decreasing its ability to interact with XIAP.48 Therefore, it would be interesting to address under which biological conditions Smac/DIABLO is post-translationally modified, and the biological extent of those modifications.
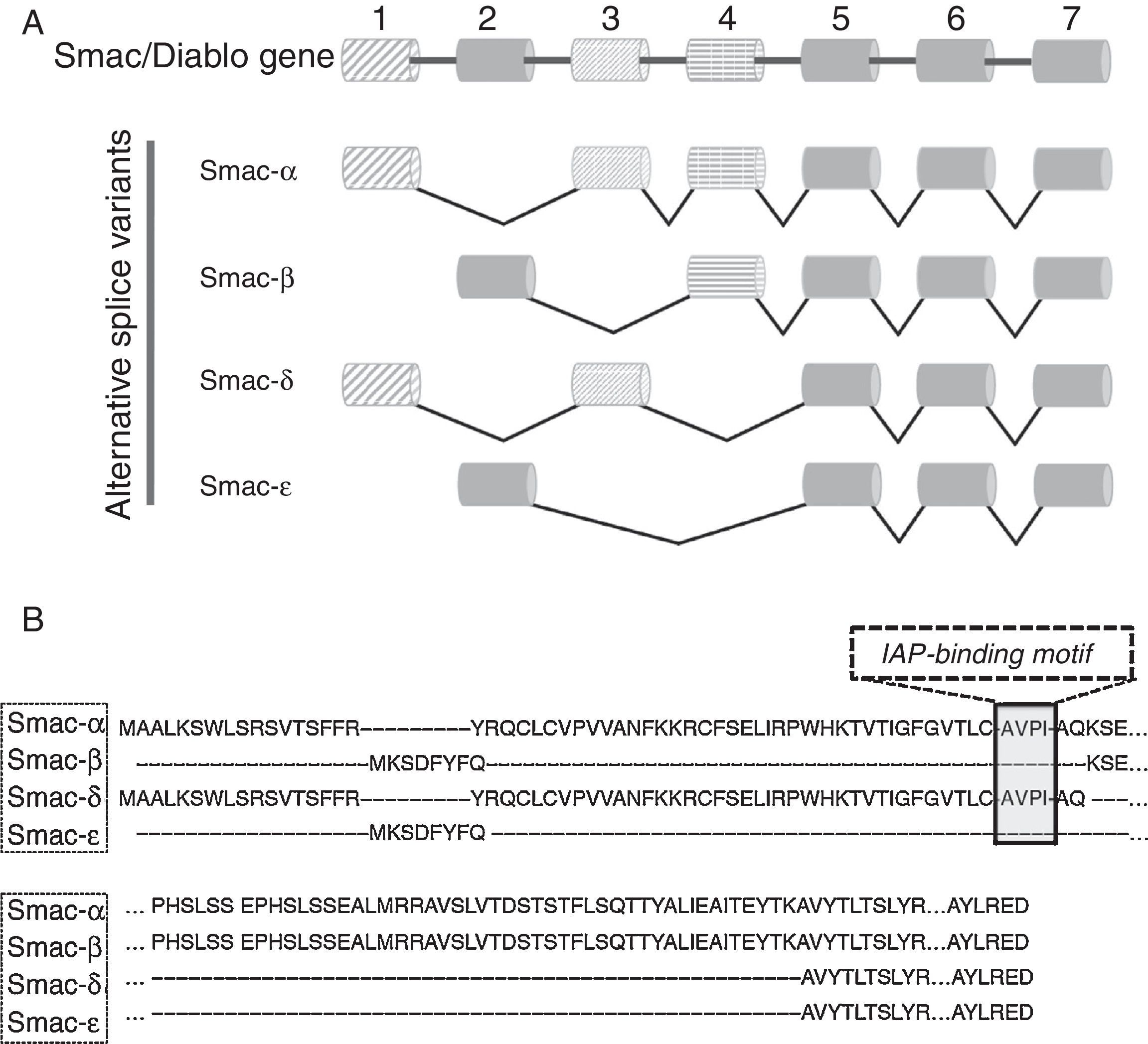
5Alternative splice variants of Smac/DIABLO geneThe Smac/DIABLO gene is formed by seven exons that are located in the long arm of chromosome 12 (12q). Exon 1 and the first part of exon 3 encode for the mitochondrial targeting sequence (MTS). The AVPI segment is encoded in the latest part of exon 3. Isoforms of Smac/DIABLO having these exons in its coding sequences are translocated to the mitochondrial compartment. After that, the MTS is eliminated by proteolytic cleavage, exposing the AVPI segment at its NH2-terminus. Four splice variant isoforms have been described (Figure 1A). The canonical Smac/DIABLO, Smac-α, lacks exon 2 from its coding sequence. Smac-β is another splice variant that lacks exons 1 and 3 but incorporates exon 2.21,49 The transcription start site for this splice variant is located in exon 2. In the splice variant Smac-δ, exons 2 and 4 are missing, but it retains both exon 1 and exon 3.46,50 Our group described the latest splice variant, Smac-¿, that lacks exons 1, 3 and 4 (Figure 1B).51
It would be expected that the splice variants retaining exons 1 and 3 encoding both the MTS and AVPI segments would share some of Smac-α’s biological functions. Smac-δ is similar to Smac-α since it presents both exons 1 and 3 (Figure 1). In apoptotic conditions, Smac-δ translocates from the mitochondria to the cytosolic compartment where it can interact with the BIR2 and BIR3 domains from XIAP through its AVPI sequence,50 acting as Smac-α. Importantly, Smac-δ but not Smac-α is able to induce the auto-ubiquitination of XIAP, thus, leading to its proteasome-mediated degradation.50 Since Smac-α induces apoptosis through its AVPI segment, it could be inferred that those isoforms lacking this element could not have biological actions. Neither Smac-β nor Smac-¿ present MTS or AVPI segments, thus display a cytosolic localization.21,49,51 Unexpectedly, both Smac-β and Smac-¿ demonstrated biological activity.49,51 Since Smac-β description, it has been suggested the existence of an uncharacterized AVPI-independent apoptotic mechanism residing within the Smac/DIABLO carboxy-terminus. Experimental evidence that points toward AVPI-independent biological functions present in Smac/DIABLO is addressed in the following sections.
6Smac/DIABLO splice variants lacking both MTS and AVPI segments have biological functionsRT-PCR assays have demonstrated that Smac-β is expressed constitutively in different cell lines.21,49 The ectopically-expressed isoform shows a cytosolic localization, which can be explained by the absence of the MTS in its coding sequence.21,49 Since Smac-β also lacks the AVPI segment it would be unexpected that this splice variant could interact with IAPs. Surprisingly, recombinant Smac-β interacts with XAIP, c-IAP1 and c-IAP2.49 In concordance, an independent study using cell-free systems showed that recombinant Smac-β derepresses caspase inhibition by XIAP, although in a lesser extent than canonical Smac/DIABLO.21 A weak association of Smac-β with the isolated segment formed by the BIR1/BIR2 domains of XIAP explained this result.21 Unexpectedly, ectopically-expressed Smac-β undergoes N-terminus cleavage, losing at least 20 amino acids and thus impairing the weak association with XIAP.49 Importantly, the presence of Smac-β sensitizes cancer cell lines to apoptosis induced by different antineoplastic drugs to a similar extent than canonical Smac/DIABLO.49 On the other hand, Smac-¿ is another splice variant that also lacks both MTS and AVPI elements.51 The only difference between Smac-¿ and Smac-β is the absence of exon 4 in Smac-¿ (Figure 1). Smac-¿ shares some similarities with Smac-β, such as the proteolytic processing at its NH2-terminus, and the inability to interact with XIAP in vivo.49,51 Intriguingly, we demonstrated that Smac-¿ has a short half-life time due to proteasomal degradation.51 Interestingly, using gene expression microarrays, it was observed that the ectopic expression of this isoform modulates distinct pathways in an IAP-independent manner.51 These results were consistent with those reported by Roberts et al., which showed that Smac/DIABLO has additional AVPI-independent biological functions.49 In this respect, determining the interacting proteins with either Smac-β or Smac-¿ could provide some highlights in the mechanism involved in the AVPI-independent actions of Smac/DIABLO.
7Smac-α exerts proapoptotic effects that do not depend on its IAP-binding motifIt has been demonstrated that Smac-α interacts with other proteins with a region distinct from its IAP-binding motif. BRUCE, an IAP member, has been associated with apoptosis evasion.52 BRUCE is able to interact with both the precursor and the processed forms of Smac-α inducing its proteasome-dependent degradation. Importantly, BRUCE interacts with Smac-α in an AVPI-independent manner.7 In apoptotic conditions, derepression of Smac-α is observed when the proteasome pathway degrades BRUCE. Therefore, BRUCE increases the threshold of apoptosis activation through decreasing the half-life time of Smac-α.7 In another study it was demonstrated that Smac-α is able to interact with NADE in an AVPI-adjacent region.53 Previously, it was described that XIAP induces ubiquitination and proteasome-dependent degradation of Smac-α through its RING-domain, favoring apoptosis evasion.41 However, the interaction between NADE and Smac/DIABLO inhibits XIAP-mediated Smac-α ubiquitination.53 Concordantly, co-expression of both Smac-α and NADE potentiates TRAIL-induced apoptosis.53 Overall, these studies suggest that Smac/DIABLO protein has additional functions in regions apart from its AVPI motif.
8Artificial mutants with point mutations in the AVPI segments of Smac-α have biological functionsIt has been demonstrated that the AVPI segment of Smac-α interacts with residues 241 to 351 (the binding pocket) from the XIAP BIR3 domain.31 Alanine, the first NH2-residue, interacts with this XIAP binding pocket through five hydrogen bonds.22 As a consequence, the interaction between Smac-α and XIAP is compromised by any amino acid substitution in the AVPI segment.31 Additionally, any mutation in the following NH2-terminus residues also decreases the capacity of Smac-α to associate with XIAP and other IAPs even though to a lesser degree.22,31,47 As mentioned above, mutations in the AVPI segment do not always eliminate the proapoptotic effects of Smac-α. The addition of a methionine residue in the NH2-terminus of Smac-α hides its first amino acid residue alanine (M-AVPI-Smac-α), and inhibits its association with IAPs, such as Survivin.36 However, M-AVPI-Smac-α sensitizes cancer cells to Taxol-induced apoptosis, pointing toward the existence of an uncharacterized alternative mechanism by which M-AVPI-Smac-α exerts its proapoptotic actions.36 The first NH2-terminus amino acid in Smac-δ was substituted by methionine (M-VPI-Smac-δ) using a similar approach, generating a protein that is still able to interact with the BIR2 domain from XIAP.50 Intriguingly, in an independent study, Smac-δ and Smac-α displayed comparable proapoptotic activities regarding PARP cleavage and caspase-3 activation. However, the association between Smac-δ with no mutations and XIAP was scarcely detected.46 It seems that the presence of the AVPI segment in Smac-δ is dispensable for its proapoptotic action in certain biological settings. On the other hand, mutation of the AVPI sequence to AAAI in Smac-α induced a complete loss of its ability to interact with XIAP. Surprisingly, this mutant version is able to interact with other IAP members, such as BRUCE. Qui and Goldberg (2005) demonstrated that the interaction between Smac-α and BRUCE in an AVPI-independent fashion is an important mechanism to evade apoptosis in unstimulated conditions.7
9Deletion of the NH2-terminus AVPI sequence of Smac-αSome studies have demonstrated that the proapoptotic functions of Smac-α can be compromised by the absence of the AVPI-segment on having lost its ability to interact with IAPs.21,22 However, some studies contradict this finding. A version of Smac-α without the first NH2-terminus amino acid (Smac-ΔA) is unable to interact with XIAP or Livin, but still, induces the auto-ubiquitination of these proteins to a similar extent to that induced by wild-type Smac-α.35 Because Smac-α induces auto-ubiquitination of IAPs by its association with them, it seems that Smac-ΔA needs additional factors to exert IAPs auto-ubiquitination. Regarding this, it is important to mention that XIAP interacts with TAK1 which, in turn, induces NFKB activation.54 Smac-α is able to inhibit this association, thus, negatively regulates NFKB activation.51 Smac mimetics that resemble the IBM motif can induce NFKB inhibition through autoubiquitination and proteasomal degradation of c-IAP1 and c-IAP2.18 Additionally, in glioblastoma cancer cell lines, Smac mimetics are able to induce NFKB activation, reducing the pool of cancer stem cells that are implied in the acquisition of anticancer therapy resistance.17 Although the biological relevance of those posttranslational modifications (PM) induced by Smac-ΔA is still unaddressed, we could envision a possible cross-talk with the NFKB pathway. Since XIAP has been associated with several cellular events such as invasion, motility, and migration55, it would be important to study the biological consequences of those PM-induced by Smac-ΔA in an apoptosis-independent context. Following this line of thinking, it has been described that Smac-α regulates tumoral suppression in an apoptosis-independent way.56
Artificially truncated proteins that completely lack the AVPI-segment have been associated with sensitization to cell death induced by antineoplastic drugs. Smac-Δ60 and Smac-Δ75 lack the first four and twenty amino acid NH2-terminus residues, respectively. Overexpression of these proteins sensitizes cancer cell lines to chemotherapeutic agent-mediated apoptosis to a similar extent than Smac-α.49 Interestingly, Smac-Δ60 still showed some interaction with XIAP, which suggested that the AVPI segment might be dispensable under certain conditions.46 Under similar conditions, Smac-Δ60 displays major caspase-3 activation and PARP cleavage both cellular events related to apoptosis culmination, following proteasome inhibition-mediated apoptosis similar to Smac-α.46 Similarly, it was demonstrated that the overexpression of Smac-Δ73, which lacks the first 18 NH2-terminus amino acid residues, sensitizes cells to Taxol-induced apoptosis. Under this condition, Smac-Δ73 is unable to interact with Survivin, an IAP member.36 An additional report showed that activation of the extracellular lymphotoxin-β receptor by its cognate ligand-induced the recruitment of several proteins to its cytoplasmic tail, one of them being Smac-α. Overexpression of Smac-α potentiated lymphotoxin-β receptor-mediated apoptosis. Curiously, a similar proapoptotic effect was observed with Smac-Δ76, which lacks 21 NH2-terminus amino acid residues. Nevertheless, Smac-Δ76 was unable to associate with the identified complex.57 Overall, these studies point toward an AVPI-independent mechanism present in the Smac/DIABLO protein.
10PerspectivesThe role of endogenous Smac/DIABLO isoforms generated by alternative splicing and artificial proteins that lack the IAP-binding motif as sensitizers for antineoplastic drug-induced apoptosis have led to the notion of the presence of AVPI-independent proapoptotic motif. However, the signaling basis of this AVPI-independent mechanism remains unaddressed. In this respect, the structural analyses of these proteins could help to highlight this structural motif implied in the uncharacterized mechanism. Once identified, this AVPI-independent proapoptotic motif could be used to design new small molecule anticancer drugs for therapy.
Conflict of interestThe authors declare no conflicts of interest of any nature.