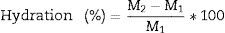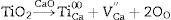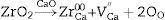Contrary to the many benefits of dolomite refractories, their usage has been limited due to poor hydration resistance. For this reason, the aim of this research work is a comparative study of the influence of trivalent and tetravalent nano-sized oxides [iron (Fe2O3), alumina (Al2O3), zirconia (ZrO2), and titania (TiO2)] on the performance and microstructure of dolomite refractories with an affirmation their hydration resistance improvement. After preparation and firing the specimen's, physical properties such as bulk density, apparent porosity, and hydration resistance tests were examined. Furthermore, XRD and SEM/EDX analysis were used to evaluate the ceramic phases and their microstructure, respectively. Results revealed that specimens with trivalent and tetravalent nano-sized oxides have improved densification and hydration resistance of the specimens through the liquid phase mechanism and solid state firing mechanism, respectively. Also, the specimens with tetravalent nano-sized oxides have higher hydration resistance than the specimens with trivalent nano-sized oxides. Generally, the hydration resistance improvement trend of specimens with various nano-sized oxides presence is Al2O3<Fe2O3<TiO2<ZrO2.
Contrariamente a los muchos beneficios de los refractarios de dolomita, su uso ha sido limitado debido a la mala resistencia a la hidratación. Por esta razón, el objetivo de este trabajo de investigación es un estudio comparativo de la influencia de óxidos nano-dimensionales trivalentes y tetravalentes [Hierro (Fe2O3), Alúmina (Al2O3), Zirconia (ZrO2) y Titania (TiO2)] en el rendimiento Y la microestructura de refractarios de dolomita con una afirmación de su mejora de la resistencia a la hidratación. Después de la preparación y la cocción de la muestra, se examinaron las propiedades físicas tales como la densidad aparente, la porosidad aparente y los ensayos de resistencia a la hidratación. Además, se utilizaron análisis XRD y SEM/EDX para evaluar las fases cerámicas y su microestructura, respectivamente. Los resultados revelaron que los especímenes con óxidos nano-dimensionados trivalentes y tetravalentes han mejorado la densidad y la resistencia a la hidratación de los especímenes a través del mecanismo de fase líquida y el mecanismo de disparo en estado sólido, respectivamente. Además, los especímenes con óxidos nano-dimensionados tetravalentes tienen mayor resistencia a la hidratación que los especímenes con óxidos nano-dimensionados trivalentes. En general, la tendencia de mejora de la resistencia a la hidratación de especímenes con diversos óxidos nano-tamaño presencia es Al2O3<Fe2O3<TiO2<ZrO2.
Dolomite refractories are considered as one kind of Cr2O3-free refractories that are appropriate for replacement the magnesia-chrome and magnesia-spinel bricks [1–4]. Due to represent an outstanding properties such as high chemical resistance against alkaline environments at high-temperature, good thermal shock resistance, slight vapor pressure, thermodynamic durability in the existences of carbon, high refractoriness, and an appropriate abrasion resistance, dolomite refractories are extensively consumed in argon oxygen decarburization (AOD) furnaces in the metallurgy industry and cement rotary kilns [1,5–7]. Furthermore, dolomite refractories are useful to eliminate impurities such as sulfur, phosphorus, etc. from molten steels; thus, they have been considered to be one of the impressive refractory kinds for processing clean steel products [3,5,8,9]. However, notwithstanding these primary advantageous properties, the utilization of dolomite refractories has not been prevalent due to its high tendency to moisture absorption when subject to the atmosphere [10–12]. This tendency led to lime (CaO) and magnesia (MgO) phases change to Ca(OH)2 and MgO(OH)2 phases according to following reactions:
These reactions causes to a volume expansion and generation many cracks in the structure. Lately, much attempt has been done by several researcher to ameliorate the efficiency of dolomite bricks by the addition of various additives such as V2O5[8], CuO [9], FeTiO3[10], ZrO2[4,7,11], Fe2O3[1,3,4,12], NiO [13], BaO [14], Cr2O3[15], and CaF2[16]. It has also been mentioned that physical properties of these bricks could be improved by using pitch, tar, flake, and vein graphite minerals [1,3,12]. Also, the hydration resistance of dolomite refractories can be enhanced by treating in a CO2 atmosphere which results in the generation of the dense layer on the surface of calcia (CaO) and protect CaO grains from contact with moisture [17]. On the other hand, nano-technology was offered to the refractory industry some year ago, and nowadays it is a significant tool subjected in many research projects [18–20]. The aim of this research work is to offer a comparative study of the impact of trivalent and tetravalent nano-sized oxides addition (Fe2O3, Al2O3, ZrO2, and TiO2) on the performance and microstructure of dolomite refractories with an affirmation of enhancement on the hydration resistance of dolomite refractories and determination of the most effective nano-sized oxide. This research work can be a useful guide for producer of these type refractory bricks in choosing the best additives according to their function.
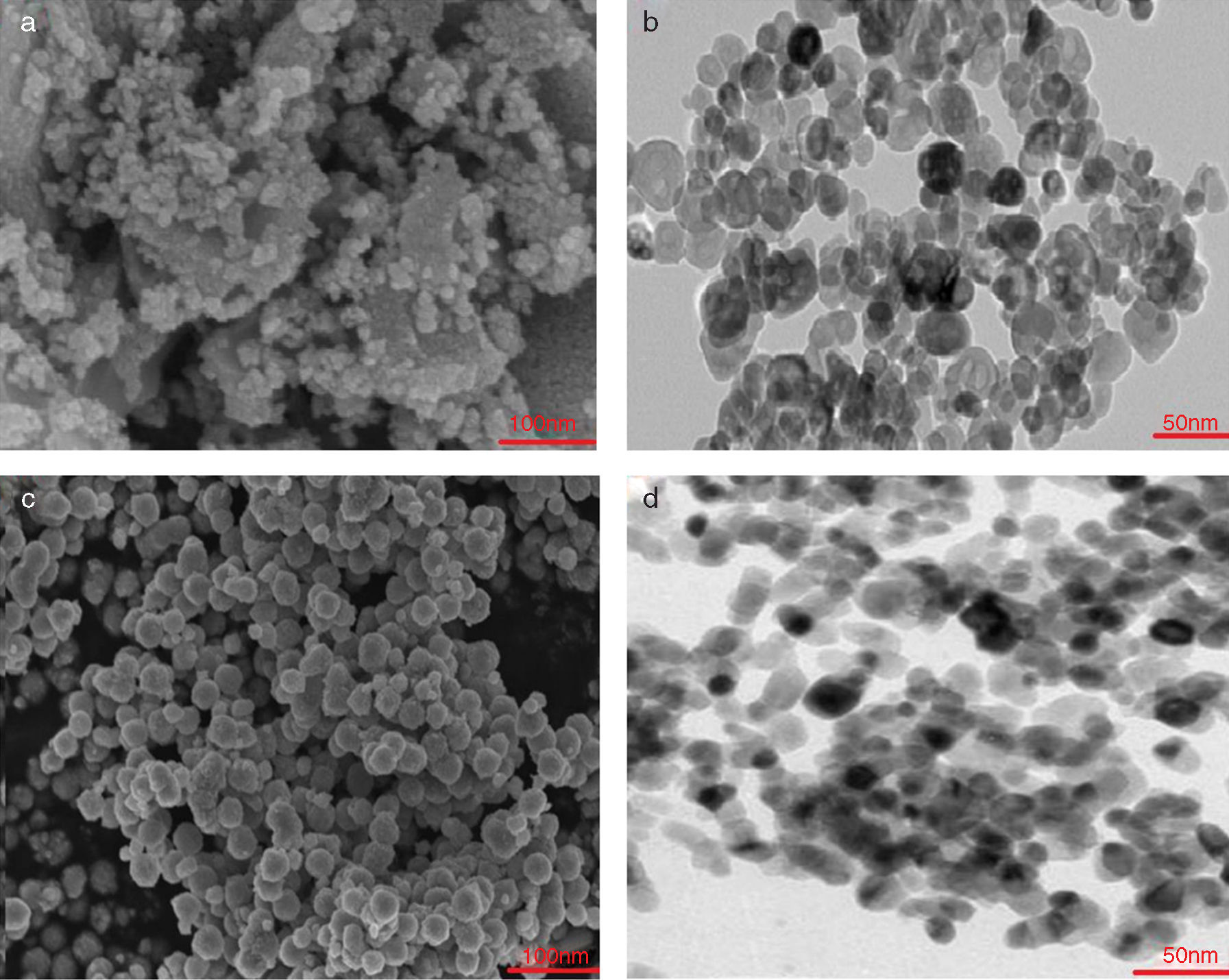
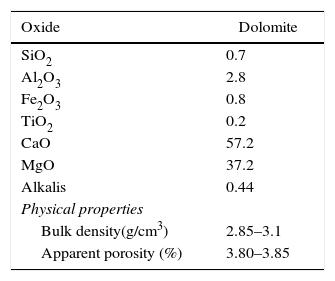
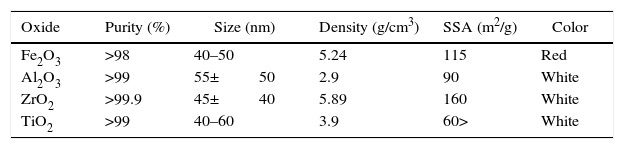
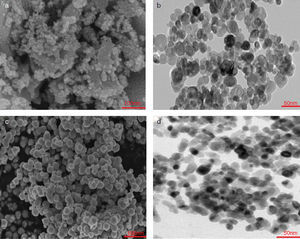
Experimental procedureStarting materials (raw material, binder, and additives)The raw materials used for create the batches composition of the dolomite refractories was dolomite (Table 1). The liquid paraffin was utilized as a binder (Table 2). Also, trivalent and tetravalentn-sized oxides (Supplier: US Research Nanomaterials, Inc., Fig. 1, Table 3) such as iron (Fe2O3), alumina (Al2O3), zirconia (ZrO2), and titania (TiO2) were used as additives.
Firstly, dolomite materials were calcined at 900°C for 1h and after it crushed to micro, 0–1, 1–3, and 3–5mm particles. Composition comprise 0, 2, 4, 6, and 8wt.% nano-sized oxides were made according to Table 4. Micro-sized dolomite is substituted by aforementioned nano-sized oxides (Table 5). Then all specimens pressed at 100MPa. Green briquettes were dried at 110±5°C for 24h and after it fired at 1600°C with 4h soaking at peak temperature.
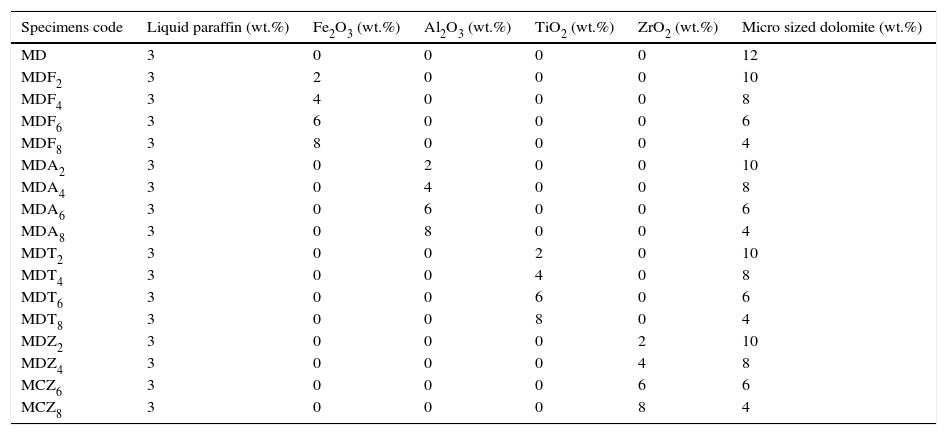
Batch composition with specimen's code.
| Specimens code | Liquid paraffin (wt.%) | Fe2O3 (wt.%) | Al2O3 (wt.%) | TiO2 (wt.%) | ZrO2 (wt.%) | Micro sized dolomite (wt.%) |
|---|---|---|---|---|---|---|
| MD | 3 | 0 | 0 | 0 | 0 | 12 |
| MDF2 | 3 | 2 | 0 | 0 | 0 | 10 |
| MDF4 | 3 | 4 | 0 | 0 | 0 | 8 |
| MDF6 | 3 | 6 | 0 | 0 | 0 | 6 |
| MDF8 | 3 | 8 | 0 | 0 | 0 | 4 |
| MDA2 | 3 | 0 | 2 | 0 | 0 | 10 |
| MDA4 | 3 | 0 | 4 | 0 | 0 | 8 |
| MDA6 | 3 | 0 | 6 | 0 | 0 | 6 |
| MDA8 | 3 | 0 | 8 | 0 | 0 | 4 |
| MDT2 | 3 | 0 | 0 | 2 | 0 | 10 |
| MDT4 | 3 | 0 | 0 | 4 | 0 | 8 |
| MDT6 | 3 | 0 | 0 | 6 | 0 | 6 |
| MDT8 | 3 | 0 | 0 | 8 | 0 | 4 |
| MDZ2 | 3 | 0 | 0 | 0 | 2 | 10 |
| MDZ4 | 3 | 0 | 0 | 0 | 4 | 8 |
| MCZ6 | 3 | 0 | 0 | 0 | 6 | 6 |
| MCZ8 | 3 | 0 | 0 | 0 | 8 | 4 |
The bulk density and apparent porosity were examined by a liquid displacement approach using Archimedes principle in a Kerosene medium (ASTM C-20). Three specimens were tested for each composition.
Hydration resistanceFor determine the hydration resistance of the specimens, each specimen was grinded to gain a particle size finer than sieve no. 40 (425μm) and after weighing, located in Petri dish in a climate room with 25°C and 95% relative moisture. The specimens then were weighed at various times to 72h. The percentage mass gains before and after hydration was the measures of hydration resistance (Eq. (3)).
where M2(g)=weigh gain after hydration test, M1(g)=initial weigh of the samples.Phase and microstructure analysisThe existence of ceramic phases was determined by X-ray diffraction method (XRD) with Cu Kα radiation (λ=1.5406Å) operated at 40kV and 30mA. Also, the microstructure analysis was evaluated using SEM 200 scanning electron microscope equipped with an electron dispersive X-ray spectroscopy (EDX) detector.
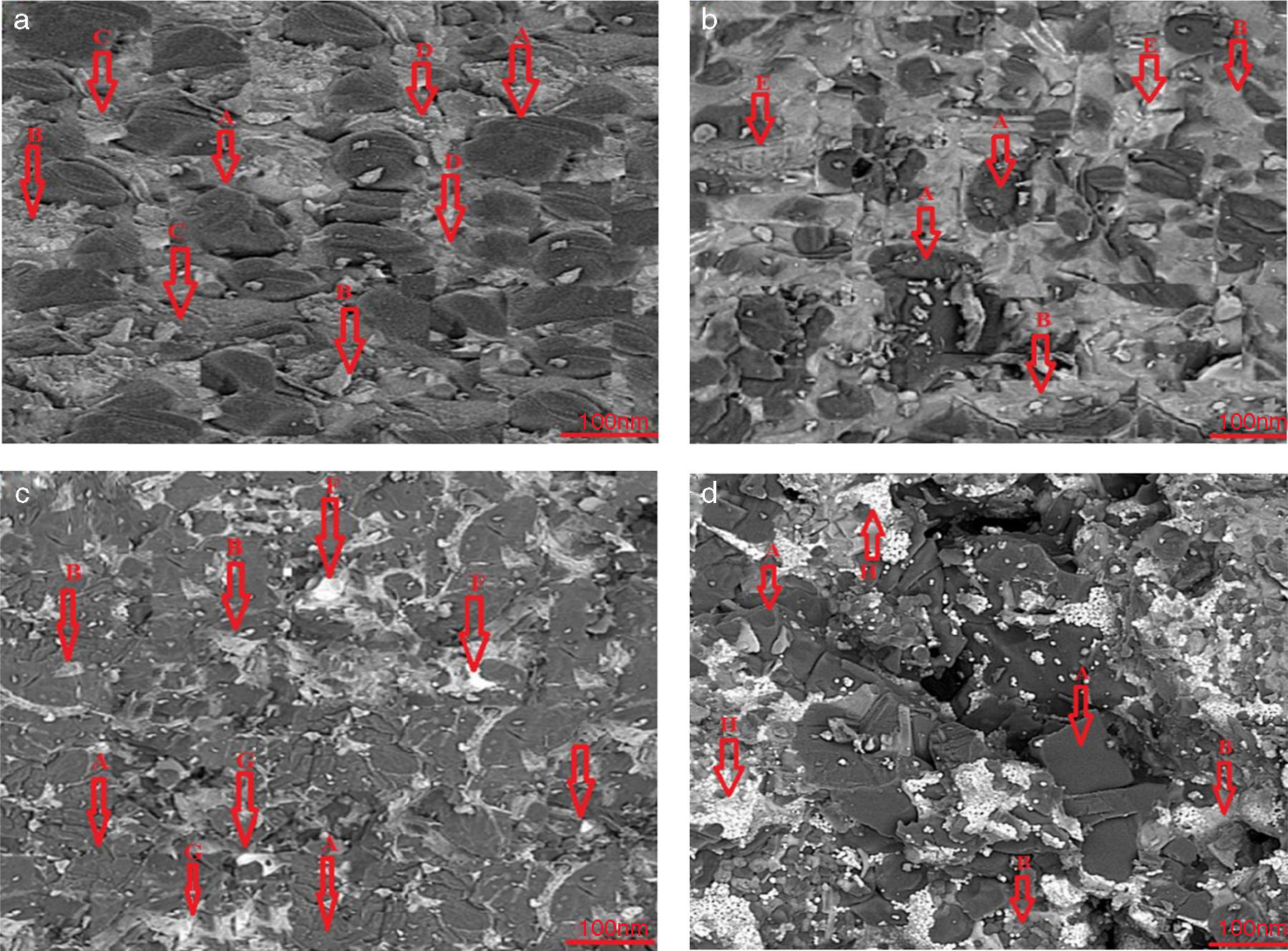
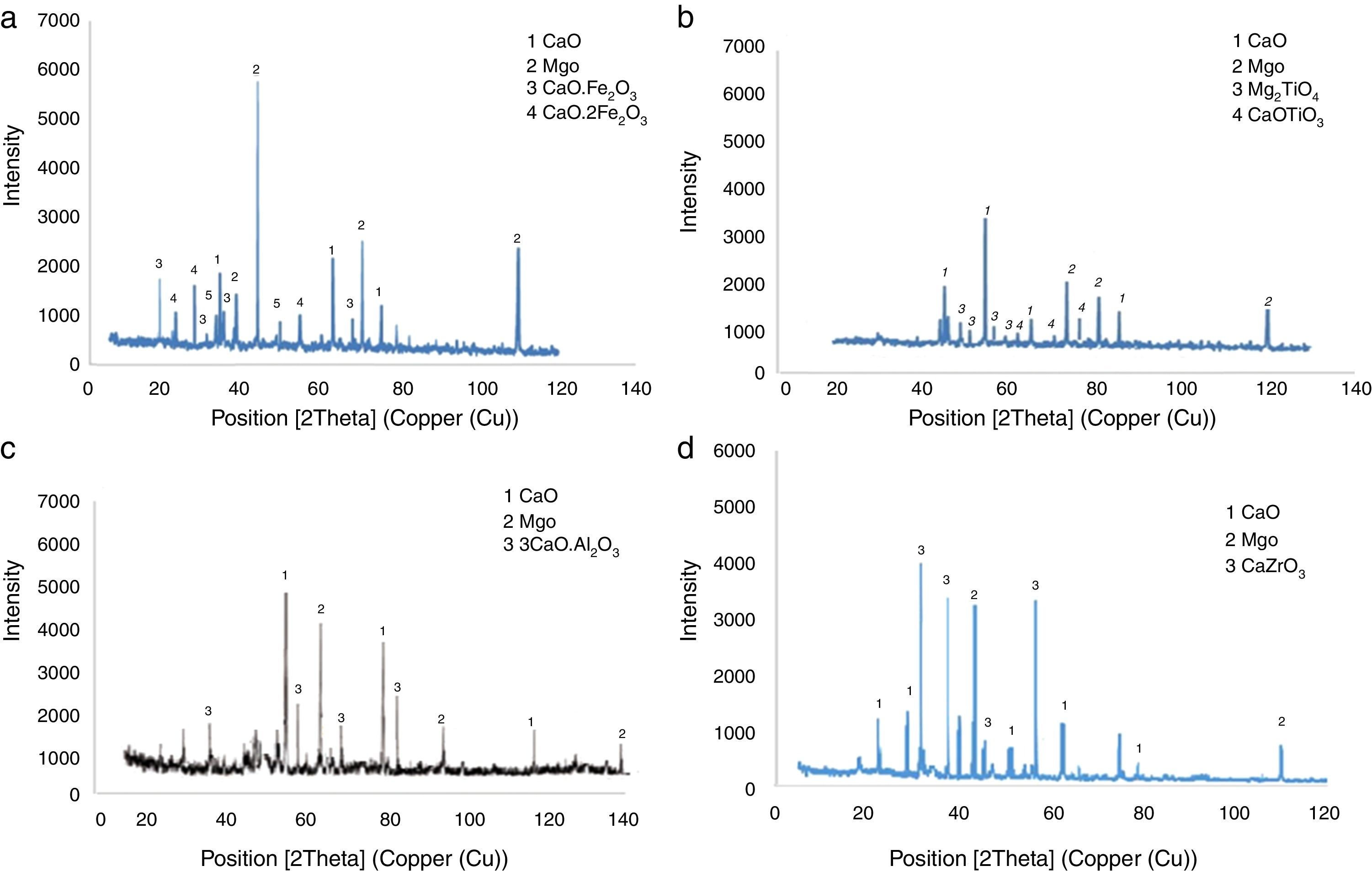
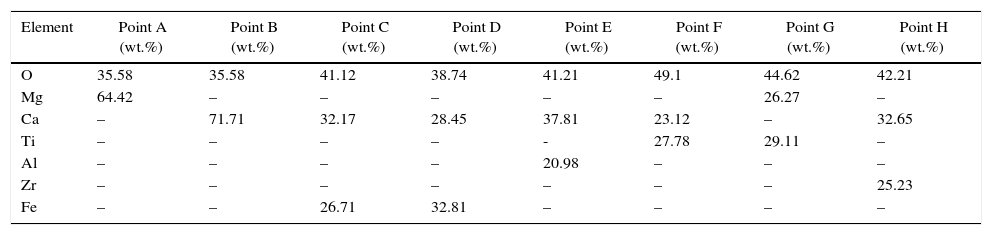
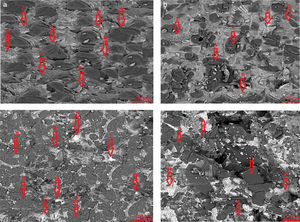
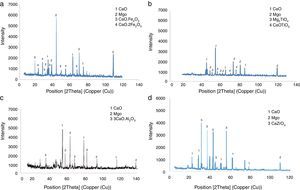
Results and discussionMicrostructure analysisFig. 2 demonstrate the Scanning Electron Microscopy (SEM) images related to the polished fractured surfaces of specimens possesses 8wt.% of trivalent and tetravalent Nano-sized oxides. For specimens own trivalent nano-sized oxides, it was apperceived that direct-bonding among magnesia (MgO) and calcia(CaO) grains was evident, due to generation some low melting point phases such as 3CaO·Al2O3 (1524°C), CaO·Fe2O3 (1449°C, and CaO·2Fe2O3 (<1350°C phases at the grain boundaries and triple points of matrix. For specimens own tetravalent nano-sized oxides more than dark gray and white gray particles recognized as magnesia and calcia phases. Also, there are phases besieged by the MgO and CaO ground mass. From the EDX and XRD analysis (Fig. 3, Table 6), these phases identified as CaTiO3 (1975°C, Mg2TiO4 (1732°C, and CaZrO3 (2345°C. Aforementioned phases have high melting point.
EDX analyses of (A) MgO, (B) CaO, (C) CaO·Al2O3, (D) CaO·2Fe2O3, (E) 3CaO·Al2O3, (F) CaTiO3, (G) Mg2TiO4, and (H) CaZrO3 points.
| Element | Point A (wt.%) | Point B (wt.%) | Point C (wt.%) | Point D (wt.%) | Point E (wt.%) | Point F (wt.%) | Point G (wt.%) | Point H (wt.%) |
|---|---|---|---|---|---|---|---|---|
| O | 35.58 | 35.58 | 41.12 | 38.74 | 41.21 | 49.1 | 44.62 | 42.21 |
| Mg | 64.42 | – | – | – | – | – | 26.27 | – |
| Ca | – | 71.71 | 32.17 | 28.45 | 37.81 | 23.12 | – | 32.65 |
| Ti | – | – | – | – | - | 27.78 | 29.11 | – |
| Al | – | – | – | – | 20.98 | – | – | – |
| Zr | – | – | – | – | – | – | – | 25.23 |
| Fe | – | – | 26.71 | 32.81 | – | – | – | – |
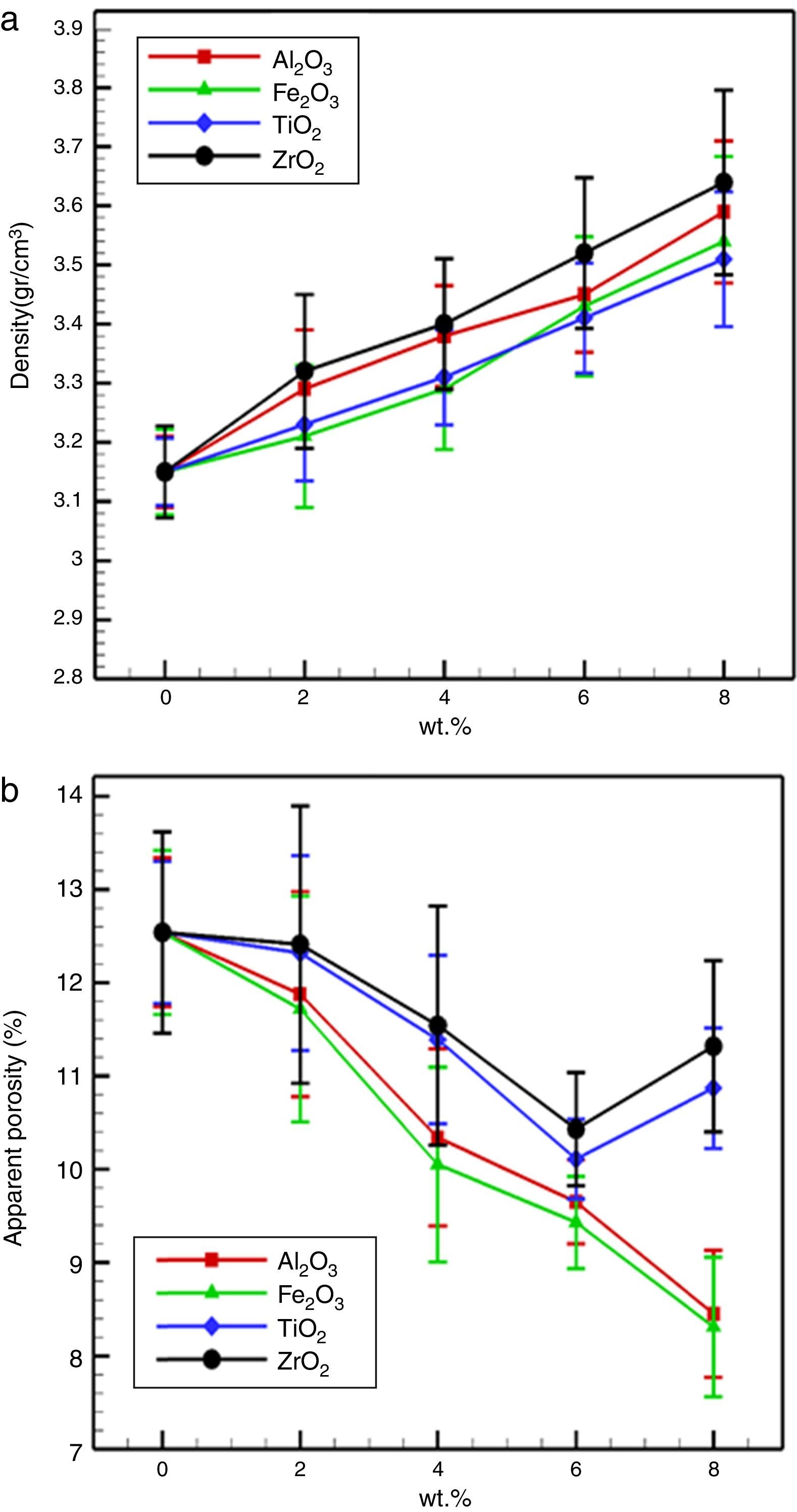
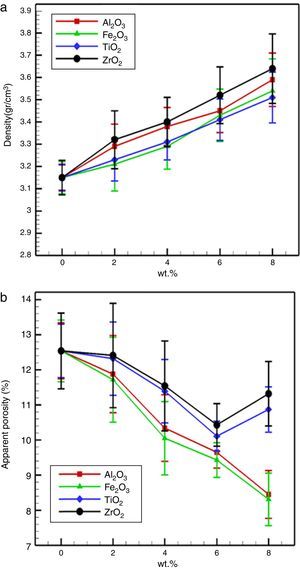
Fig. 4 demonstrate the influence of trivalent and tetravalent nano-sized oxides on the bulk density and apparent porosity of the specimens after firing at 1600 for 4h. It is observed that the bulk density increased and the apparent porosity decreased with additions nano-sized oxides, respectively. Also, it is observed that this trend, for specimens possesses tetravalent nano-sized oxides is higher than specimens containing trivalent nano-sized oxides. Densification improvement of the fired specimens with the incorporation of nano-sized oxides may be associated with the following theories:
- (i)
Further firing procedure of the refractory matrix owning to the attendance of nano-sized particles.
- (ii)
A better compression of the matrix on filling up of the intergranular porosities among magnesia (MgO) and calcia (CaO).
- (iii)
A better mass relocation due to nano-sized oxides can operate as a ceramic bond (abridge) among the MgO and CaO grains.
- (iv)
Generation phases with low melting point such as 3CaO·Al2O3, CaO·Fe2O3 and CaO2·Fe2O3 to diminish the porosity and density is increased.
- (v)
The higher density of Fe2O3 (5.24g/cm3), Al2O3 (3.95g/cm3), TiO2 (4.23g/cm3) and ZrO2 (5.68g/cm3), compared to the dolomite (2.87g/cm3) and magnetite (3g/cm3).
- (vi)
Also, increasing in apparent porosity at high TiO2 and ZrO2 nanoparticles concentrations is due to large diversity in thermal expansion coefficients between MgO (∼13.5×10−6°C−1), CaO (∼13.8×10−6°C−1) CaTiO3 (∼12.2×10−6°C−1), Mg2TiO4 (∼10.1×10−6°C−1), and ZrO2 (7.5×10−6°C−1), it can generate extreme micro-cracks in the microstructure, stimulate a porosity enhancement.
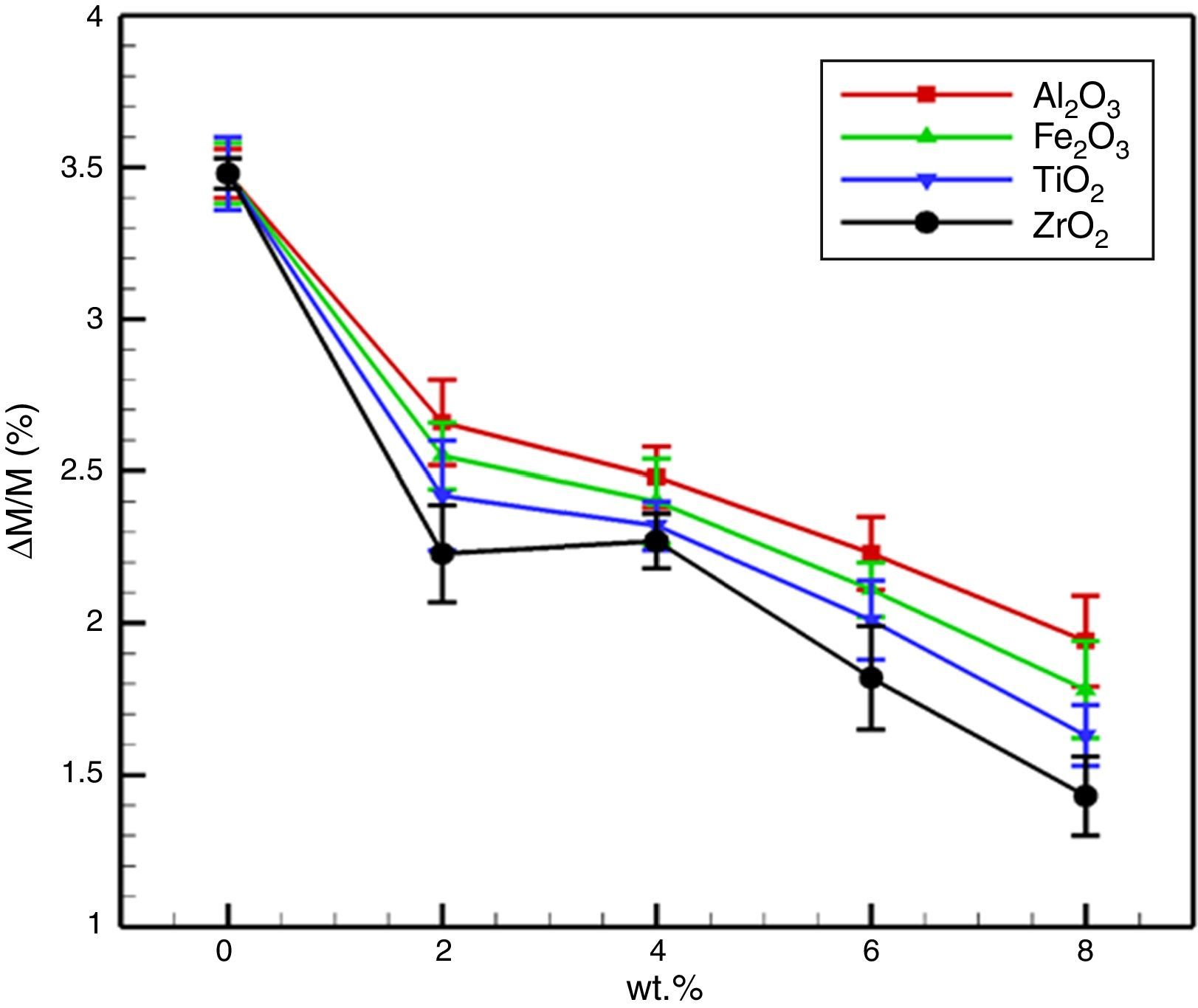
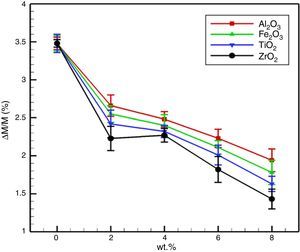
From Fig. 5 it is illustrated that the mass gain of the dolomite specimens reduced extremely with nano-sized oxides addition. For the MD, the mass gain after 72h was 3.49%. For specimens with Fe2O3 and Al2O3 nano-sized, with increasing the amount of additives reduces the weight gain, due to more grain growth and decreased grain boundary and porosity. The surface area was reduces along with grain growth with due to generation low melting phases, which is another contribution for improved hydration resistance. It is reported that the hydration resistance of lime (CaO) systems, strongly relies on the content of free CaO [1,3,4]. When ZrO2 and TiO2 nano-sized were added, the enhancement of the hydration resistance of the specimens is due to the following reasons:
- •
Transition of partial free CaO to high hydration resistance phases such as CaZrO3, Mg2TiO4 and CaTiO3.
- •
The densification enhancement of dolomite bricks by nano-sized oxides can be rationalized on the basis of cation vacancy generation as follows, which increase the hydration resistance of dolomite specimens.
Generally, the use of trivalent nano-sized oxides leading to the generation of some low melting phases that crated a protective layer on free-CaO and MgO surfaces which increases the hydration resistance of the specimens. But the application of tetravalent nano-sized oxides leading to the Zr4+ and Ti4+ cations create a solid solution with CaO, which diminish the Ca2+ concentration and results in the improved hydration resistance of specimens.
Summary- •
The application of trivalent and tetravalent nano-sized oxides lead to improved densification and therewith an increase hydration resistance of dolomite specimens by the liquid phase firing mechanism and solid phase firing mechanism, respectively.
- •
The improvement trend of hydration resistance of specimens possesses various nano-sized oxides is Al2O3<Fe2O3<TiO2<ZrO2.