The continuous research of new functional materials combining both advanced properties and increased sustainability has dramatically risen up in the last decades. Instead of searching for new solutions, composites (formed by a combination of already present materials) are subject of different studies due to their capability of merging the advantages of components. Hence, chitosan, a biowaste-derived biopolymer, has been thermally-converted into chars by pyrolysis treatment. Subsequently, both chitosan and its char are introduced into cementitious matrix forming cement-based composites. The analysis of the mechanical properties of these materials evidenced that char-containing composites show an incipient fracture toughness capability, very appealing for possible structural applications.
La investigación continua de nuevos materiales funcionales que combinan propiedades avanzadas y una mayor sostenibilidad ha aumentado dramáticamente en las últimas décadas. En lugar de buscar nuevas soluciones, los compuestos (formados por una combinación de materiales ya presentes) están sujetos a diferentes estudios debido a su capacidad de fusionar las ventajas de los dos componentes. El quitosano, un biopolímero derivado de residuos biológicos, se ha convertido térmicamente en carbón mediante tratamiento de pirólisis. Posteriormente, tanto el quitosano como su carbón se introducen en una matriz cementosa que forma compuestos a base de cemento. El análisis de las propiedades mecánicas de estos materiales puso de manifiesto que los compuestos que contienen carbón muestran una incipiente capacidad de resistencia a la fractura, muy atractiva para posibles aplicaciones estructurales.
In recent years, several efforts were realized by researchers for finding new materials with advanced peculiar properties and reduced costs [1–5]. In this context, a great attention has been dedicated to the development of composite materials made by combinations of different materials (acting as either matrix or reinforcing agents/fillers) and merging the best technical characteristics of both components [6–10]. In materials science, numerous studies are devoted to the improvement of the intrinsic properties of materials (i.e., mechanical, thermal, electrical, magnetic, optical, properties and so on) or their workability by introducing one (or more) fillers/additives with specific characteristics [11–14].
The possibility of improving the mechanical performance of cement and concrete (in particular the fracture toughness) by means of nanoscopic and/or microscopic fillers is an interesting research field with promising application in the built of earthquake-resistant and monitoring-enabling cementitious materials [15–17]. Among the different types of fillers, carbonaceous ones are widely investigated due to the possibility of introducing novel advanced properties (e.g., the introduction of graphene in cement favored the development of enhanced electrical conductivity) [18]. Moreover, the introduction into cement of bio-based renewable materials and/or natural fibers derived from animal, vegetal and mineral sources attests itself as a real sustainable alternative choice encourage by the building construction industry [19–22].
To date, chitosan (a biopolymer derived from the processing of shellfish biowaste) [23] is a very promising material due to its chemical structure and physicochemical properties [24]. In detail, chitosan is widely exploited in several scientific fields, such as: wastewater treatments [25–28], agriculture and food processing [29,30], cosmetic industry [31], sustainable packaging (e.g., bioplastics) [32–34], and biomedicine (mostly as drug-delivery system since it is bioresorbable) [35–38]. From the chemical viewpoint, chitosan is an amino-polysaccharide and when thermally-treated under inert/reducing atmosphere (i.e., pyrolysis), it can be easily converted into a N-containing carbonaceous materials (i.e., biochar) [39]. According to previous studies [40–42], several natural biopolymers with analogous structure (i.e., polysaccharides) were successfully converted into chars with residual functionalities trough controlled pyrolysis treatments, and exploited mostly for environmental and energetic uses (e.g., adsorbents for the removal of contaminants from wastewater, CO2 sequestrating agents from gas phase, cathodes in Li-S batteries).
Therefore, in this study chitosan was converted into valuable chars via controlled pyrolysis. Subsequently, both bio-based materials (namely, chitosan and its char) were used for producing reinforced cementitious composites for structural application. Mechanical tests evidenced promising results and an incipient fracture toughness capability, encouraging future studies devoted to the development of such materials, for instance by using a high performance cement as matrix or adding further carbon fibers as reinforcements in order to promote the mechanical response.
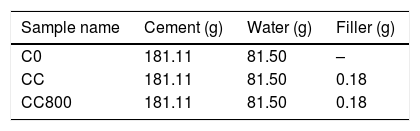
ExperimentalPyrolysis treatmentPyrolysis of chitosan (CAS 9012-76-4, high molecular weight 310–375kDa, DD=75–85%, Aldrich) was performed in a quartz tube reactor (Carbolite MTF 12/38/400) under Ar atmosphere with the following thermal program: ramp from room temperature (RT) to target temperature (either 440°C or 800°C, rate: 5°Cmin−1), isothermal step 1h. After the pyrolysis treatment, samples were washed twice with deionized water to remove all soluble residues, and dried overnight at 80°C in an oven. Chars produced were coded as follows: C440 refers to char obtained after heating at 440°C, whereas C800 refers to the one heated at 800°C. Samples preparation is reported in Table 1.
Preparation of the cement-base compositesAn American Petroleum Institute (API) oil-well cement Class G (Lafarge North America) was used in this work.
Either chitosan or its char (only C800) were dispersed in deionized water (0.1% by weight of cement, or BWOC as in [17]) with an ultrasonic tip (Vibra-cell™) for 15min at 100W. The suspensions were mechanical stirred while cement powder was added into the aqueous medium. The water-to-cement (W/C) ratio selected was 0.45. In this work, dispersant or surfactant agents or other additives were not used. The slurry, composed only by cement and chitosan/char, was then poured into molds. The curing for this category of cement is 24h at 85°C and at 100% relative humidity. Prismatic molds of size 20×20×80mm were used for building the cement-based composites. Composites were coded as follows: CC refers to the cement-chitosan composite, whereas CC800 refers to the cement composite with char C800. Additionally, for the sake of comparison, also bare cement samples were prepared and identified with the acronym C0.
CharacterizationThermo-gravimetric analysis was conducted in a TGA instrument Mettler Toledo 1600, under Ar atmosphere. The analysis was performed on pristine precursor (chitosan) with the following thermal program: heating ramp from 25°C to 1000°C (10°Cmin−1). The Ar was supplied with a constant flow rate (50mLmin−1).
Field emission scanning electron microscopy (FESEM) micrographs were collected by means of a FESEM Zeiss Merlin. Various micrographs were taken in different areas of the samples analyzed in order to observe the average morphology.
Elemental analysis of precursor and char was carried out in a Thermo FlashEA 1112 CHNS-O analyzer. Average values of three replicas were provided for each measurement.
FTIR spectra were recorded in attenuated total reflectance mode (ATR, equipped with diamond cell for single reflection) by means of a Vector 22 spectrometer (Bruker) equipped with Globar source, DTGS detector and working with 128 scans at 4cm−1 of resolution in the 4000–400cm−1 range. Spectra were recorded directly on powdery samples.
Mechanical properties were measured with a three-point bending test using the procedure prescribed by JCI-S-001-2003. The specimens were tested with a single-column Zwick-Line z050 flexural testing machine with a load cell having a maximum capacity of 1kN. As described in the literature [43], the tests were performed on notched specimens (notch width: 2mm, depth: 5mm, i.e., equal to one fourth of the width of specimen) by controlling the crack mouth opening displacement (CMOD). CMOD was controlled at a fixed displacement rate of 0.005mmmin−1 by placing an extensometer on the two sides of the notch. All tests were performed at least on three specimens, and average values were provided.
The flexural strength was calculated by using the standard formula for un-notched bending tests, using the thickness in the notched area. The toughness was calculated by integrating the load-displacement curve as in [43]. Compressive strength tests were conducted on at least three cubic specimens for each sample (size: 20×20×20mm) and controlled at a fixed displacement rate of 0.01mm min−1.
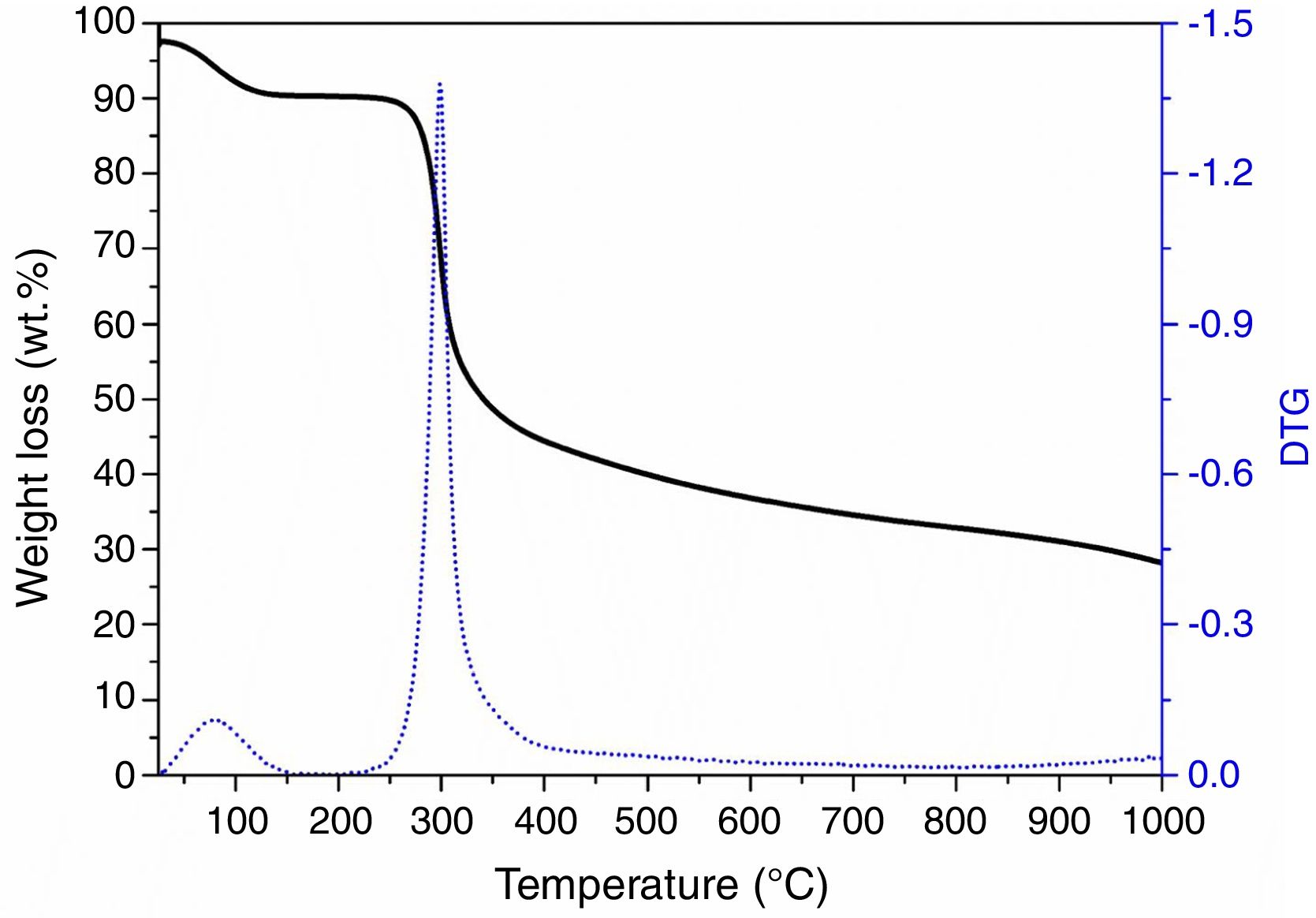
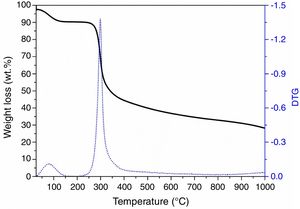
Results and discussionChitosan-to-char conversion: a chemical analysisTo evaluate the pyrolysis induced effect on chitosan structure, TGA analysis was performed on chitosan under inert/reducing atmosphere. Fig. 1 reports the TG profile, which presents two main weight losses: the first one at ca. 100°C due to the loss of physisorbed water molecules (7wt.%, i.e., chitosan is a hygroscopic material) and the second one in the 250–400°C range due to the degradation of the polysaccharidic structure with formation of volatiles products [44]. According to the literature [39], pyrolysis of chitosan leads to the formation of H2O, CO2, CO, NH3, CH3COOH and N-containing heteroaromatic compounds (mostly pyrazines, pyridines and pyrroles). The curve profile evidences also that at higher temperature (>450°C) the degradation still continues very slowly, with release of CH4 and consequent formation of a carbonaceous residue (ca. 27wt.% at 1000°C) via dehydrogenation mechanism [39].
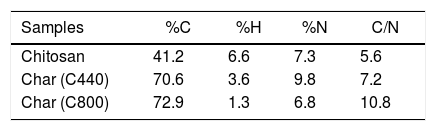
For a more detailed comprehension of the chitosan-to-char conversion mechanisms, elemental analysis was performed on either bare chitosan or its chars, sampling at two different target temperatures: namely 440°C (before) and 800°C (after the formation of methane documented by the literature for analogous systems) [39]. The analysis revealed a progressive increment of the content of C (from 41.2% to 72.9%) and a reduction of H (from 6.6% to 1.3%). Interestingly, the amount of N increases from pristine chitosan (7.3%) to C440 char (9.8%), while it subsequently decreases to 6.8% for C800. The explanation of this trend is assumable to the fate of N during chitosan pyrolysis. In fact, it has been demonstrated that the amino groups of chitosan can evolve toward either volatile ammonia or being integrated into aromatic structures. Such structures can evolve into a solid N-containing residue (N-doped char) as well as into volatiles N-containing heteroaromatic compounds [39,45]. Therefore, one can easily assume that at high temperatures (800°C), the loss of residual functionalities is remarkably higher than at low temperature (440°C). This trend is in agreement with previous study devoted to the pyrolysis of chitin [40] (Table 2).
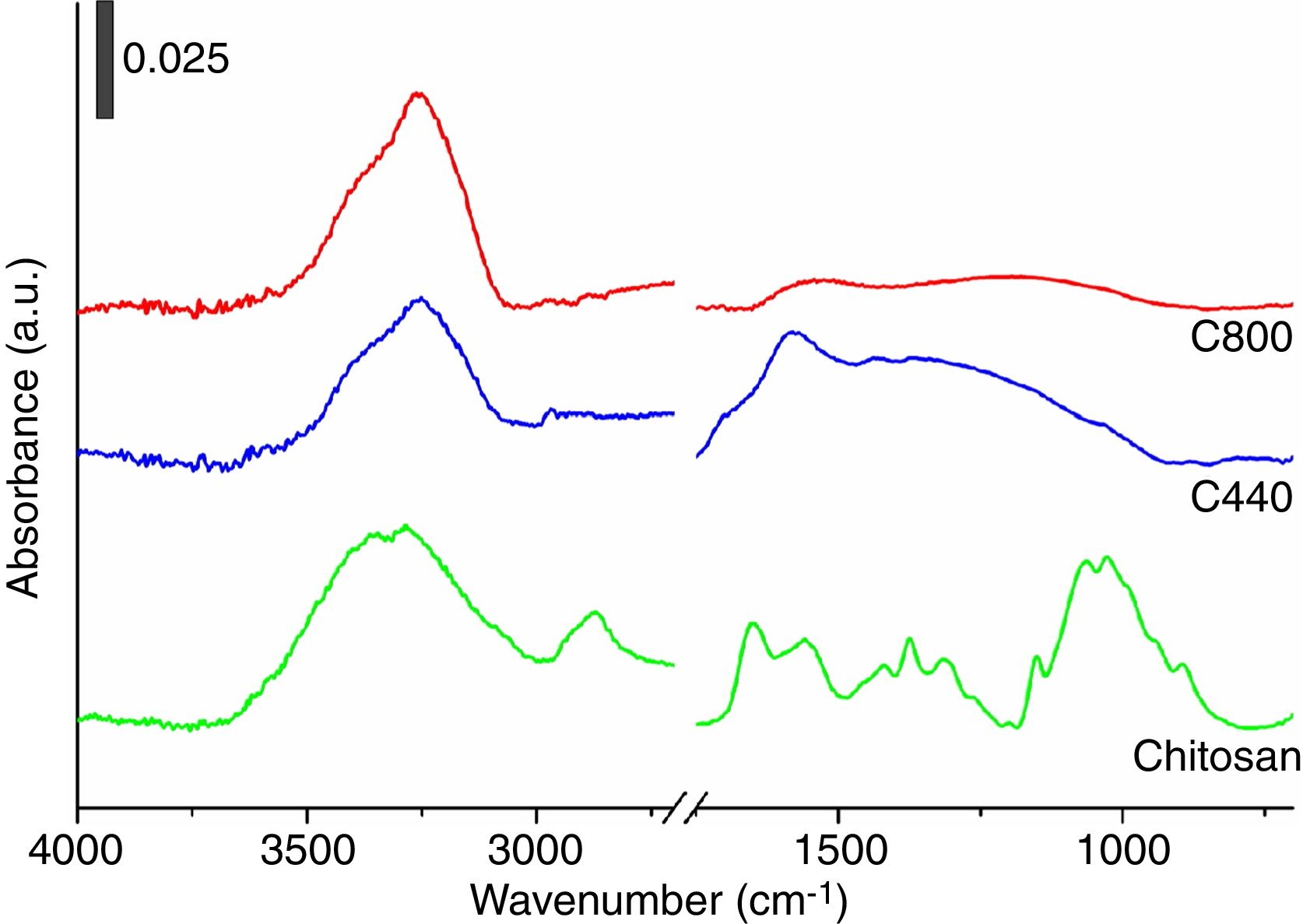
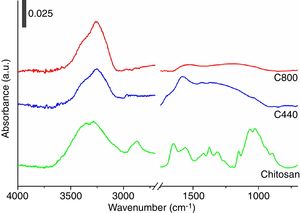
To deepen the conversion mechanism, ATR–FTIR spectra on chitosan and its chars were collected and results shown in Fig. 2. As evidenced in the figure, the IR spectrum of pristine chitosan revealed the presence of an intense and broad band centered at ca. 3440cm−1 attributable to the stretching mode of O–H and N–H functionalities, followed by a small signal in the 2850–2880cm−1 due to stretching mode of aliphatic C-H, the two characteristic signals at ca. 1655cm−1 due to the axial stretching vibration mode of acetamido group (amide I) and the one at ca. 1580cm−1 due to the angular deformation of amino groups, the signals in the 1300–1500cm−1 range commonly assigned to C–N stretching and N–H deformation as well as CH3 deformation modes, and lastly a broad signal centered at 1000cm−1 due to the skeletal C–O and C–O–C stretching modes. Since chitosan is hydroscopic, the signals at ca. 3440cm−1 and at ca. 1655cm−1 could be associated also to the presence of physisorbed water molecules (O–H stretching and bending modes, respectively). Pyrolysis carried out at high temperatures (both 440°C and 800°C) caused the loss of the characteristic glycosidic functional groups with growth of a broad signal in the 1600–1000cm−1 attributable to the formation of carbonaceous CC and C–C stretching modes, with presence of an IR signal at ca. 3200–3400cm−1 attributable to N–H residual polar functionalities (or still sorbed water molecules) [40]. Additionally, it should be notice that the aliphatic C–H stretching modes at 2850–2880cm−1 are still present in traces in C440, whereas they are absent in the C800 (in agreement with the elemental analysis).
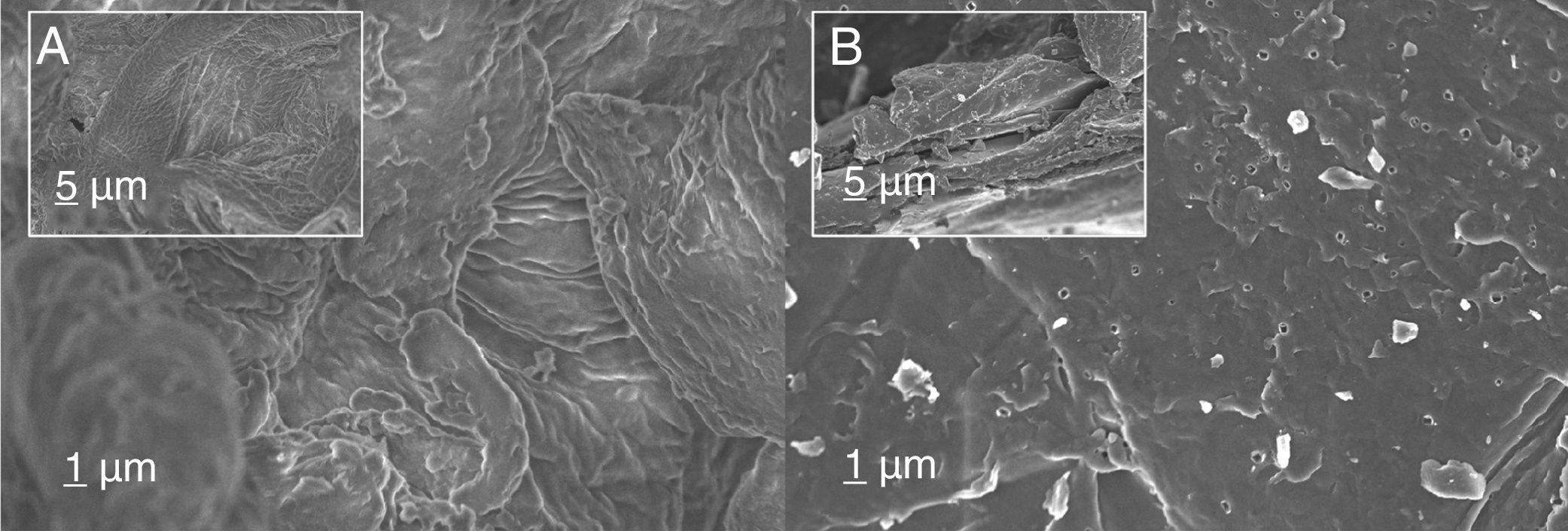
Fig. 3 reports the main morphological differences between precursor prior to perform pyrolysis (Fig. 3A) and at the end of the pyrolysis process (C800, Fig. 3B). Images revealed a rather dense corrugate surface in the case of bare chitosan. On the contrary, pyrolysis treatment favored the formation of surfaces characterized by the presence of debris and defects. The analysis of the cross-section evidenced also the formation of cracks and delamination sites.
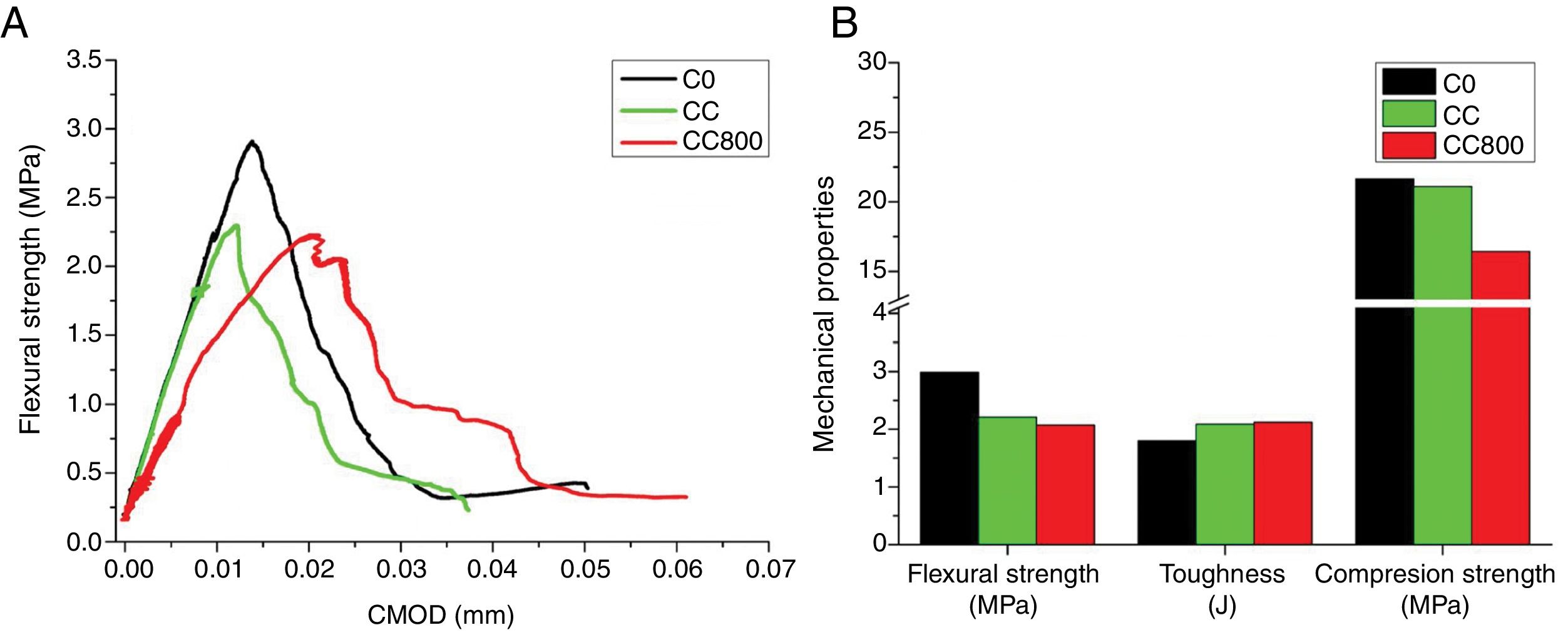
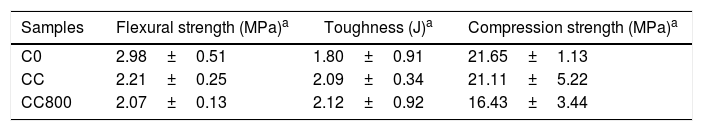
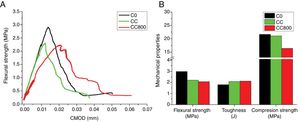
Composites production and mechanical testsFig. 4 and Table 3 report the results of mechanical tests performed on bare cement (C0) and on composites containing either chitosan (CC) or chitosan-derived char (CC800). Results demonstrate that composites with chitosan possess lower flexural strength compared to reference cement, whereas both toughness and compression strength are not-significantly different between each other. C800-containing materials, instead, show a slight reduction of both flexural strength and compression strength, whereas the relative toughness value is slightly higher than both bare cement and chitosan-containing composites. The loss in terms of flexural strength registered in both cases could be associated to a certain inhomogeneity of samples caused by a difficult interaction between chitosan and its char with the hydrophilic cement paste. On the other hand, the dispersion of the fillers in the cement paste assumes a key role. A bad dispersion of the filler can create defect in the cement matrix composite with a worsening in the mechanical resistance. The presence of functional groups in bare chitosan should favor its integration within the cement matrix, although it must be notice that chitosan is hygroscopic and might compete with the hydration processes involved during the setting step. Moreover, interfacial phenomena are surely the main concern for char-containing composites since char is chemically reduced (and hydrophobic). However, all these issues could be overcome by introducing in the formulation dispersants and compatibilizers (e.g., amphiphilic molecules) or further additives, as well as the chemical functionalization of the char surface.
In general, the analysis of these data (even if not rousing) are still promising since highlights the possibility of successfully introduce chitosan or its char without causing a drastic depletion of the mechanical performances in cement. Furthermore, it must be notice that the experimental value for the toughness in char containing composites is very encouraging. It can be note that the improvement of the toughness response is attributable to the bridging effect of the particles of char dispersed in the cement matrix. In analogy with graphene (see for instance [46,47]), this effect allows a delay in the propagation of micro-cracks if the composite is subjected to an applied stress. The load vs. displacement profile for CC800 confirmed this behavior, demonstrating an incipient fracture toughness capability. Hence, even if these results are only preliminary (and deserve a significant improvement), experiments revealed being an interesting starting point for the development of sustainable toughened cementitious composites.
ConclusionsIn this study, chitosan (a biopolymer derived from shellfish industry's biowaste) has been converted into valuable char via pyrolysis. Elemental analysis performed at different pyrolysis conditions revealed a progressive increment of the content of C together with a reduction of H by increasing the process temperature, thus evidencing the progressive chitosan-to-char conversion. The fate of N, instead, results being much more complex, with formation of a N-doping in the residual char, even after pyrolysis at high temperature condition (namely, 800°C). These results are in agreement with the analysis of the ATR–FTIR spectra of bare chitosan and its chars. Furthermore, from the morphological viewpoint, micrographs revealed the formation of debris and defects at the char's surface, with presence of cracks and delamination sites along the materials’ cross-section.
Afterwards, both chitosan and its char (obtained after pyrolysis at 800°C) were dispersed into a cementious matrix to produce cement-based composites. Mechanical tests revealed that composites enriched with chitosan possess lower flexural strength compared to reference bare cement, whereas the variation of both toughness and compression strength is negligible. Interestingly, by introducing the chitosan-derived char, a slight reduction of both flexural strength and compression strength is registered. However, such side effect is encouragingly balanced by a higher toughness value (even higher than bare cement).
The loss in terms of flexural strength registered in both composites is attributable to a certain inhomogeneity caused by interfacial phenomena occurring between fillers (chitosan/char) and the cement matrix. On the contrary, the improvement of the toughness response is attributable to the bridging effect of the filler's particles dispersed in the cement matrix, which caused a delay in the propagation of micro-cracks when the composite is subjected to an applied stress.
In general, even if the results here discussed are only preliminary, experiments revealed that composite containing the chitosan-derived char evidenced an incipient fracture toughness capability, which is an interesting starting point for further studies on toughness materials.