A series of glasses with chemical composition (50−x−y) TeO2–30ZnO–10YF3–10NaF–xHo2O3–yYb2O3 (x=0.5 and y=0.5, 1.0, 3.0, 5.0mol%) were prepared by melt-quenching procedure. The absorption spectra, excitation, down conversion emission spectra, up-conversion (UC) emission spectra and decay time measurements were analyzed. In down conversion, the visible emission transition intensity associated with 5F4→5I8 (547nm), 5F5→5I8 (657nm), and 5F4→5I7 (755nm) of Ho3+ ions decreased with Yb3+ concentration due to the energy transfer (ET) process from Ho3+ to Yb3+ ions. In up-conversion, on exciting with 980nm diode laser beam, we observed a strong green (543nm) and red (657nm) UC emissions, that refers to the energy level transitions; 5F4 (5S2)→5I8 and 5F5→5I8 of Ho3+. The influence of excitation power on UC intensities studies revealed that the population at 5F4 (5S2) and 5F5 levels of Ho3+ ion occurs due to two-photon absorption process associated energy transfer from Yb3+ to Ho3+. The influence of Yb3+ doped concentration on UC was studied, and it is observed that both the green and red UC intensities improved significantly on increasing Yb3+ ions concentration.
The lasers operating in the visible region of electromagnetic spectrum have been employed for a number of applications like optical recording, display, medical diagnosis, etc. [1]. Energy-up conversion (UC) property of rare earth (RE) ions are an efficient way to obtain visible lase under IR pumping. Besides the visible laser, material exhibiting UC luminescence have also getting special consideration due to their potential use for IR sensing, in photovoltaics as solar NIR concentrators, and biological classification [2,3]. It is essential to understand the exact mechanism, in order to exploit a competent up-converting material and to enhance their efficiency.
A number of studies were reported on tellurite glasses during the last few years owing to its attractive attributes. It is transparent for a wide range of wavelengths (0.35–5μm) in comparison to silicate glasses (0.2–3μm), and has large index of refraction [4,5]. It also has excellent stability, and lower phonon energy. Among the number of RE ions available, Ho3+ has exceptional UC luminescence due to its apparent transitions which spans in the visible region [6,7]. Even though, Ho3+ is an excellent candidate, it has some draw backs, like one cannot directly excite it with commercially available diode laser (LD), 980nm because of the absence of appropriate absorption levels in it. Fortunately, co-doping with Yb3+ enables sensitization of Ho3+ to realize visible UC transition due to the accessibility of its large absorption cross-section about 980nm [8,9] in addition to its efficient energy transfer ability to Ho3+[9,10]. Ho3+ can be employed into matrixes as activators to initiate interesting luminescent mechanisms. Due to their rich energy level occurrence, the emissions in red, green and many other wavelengths can occur under IR laser excitations for ex. 980nm wavelength. With Ho3+, Yb3+ addition as a sensitizer and a co-dopant can bring improved up-conversion emissions [11,12]. Balaji et al. have analyzed phonon assisted energy transfer process in Ho3+/Yb3+ co-doped telluride glass, and found that in order to achieve a substantial energy transfer the difference in energy between 2F5/2 (Yb3+) and 5I6 (Ho3+) levels can be crossed with the aid of host phonons [13]. It can be noted that because of the non-resonant transitions the Yb3+ emission and Ho3+ absorption has a very poor spectral overlap of about 1500cm−1 energy gap. Since the telluride glasses have a ∼750cm−1 of phonon energy, nearly two or three phonons are needed to cross the transition gap in energy. On the contrary, the host systems that have high phonon energy like phosphate, silicate, and borate can achieve this energy gap bridging with a smaller number of phonons, but non radiative transition from 5I7 level of Ho3+ restricts the 2μm emission probabilities significantly. Their study also estimated the energy transfer microparameters to be reasonably good magnitude, which substantiates that the ET process benefits from host phonons. Thus, the telluride glass is promising host for Ho3+/Yb3+ co-doping for efficient NIR emissions and up-conversions. For the last few years, numerous research works were conducted on Yb3+/Ho3+ doped tellurite glasses for the advance of solid-state UC green emitting lasers, mid-infrared optical fiber amplifiers and laser devices [11–16]. The studies reported by Wang et al., have shown enhanced luminescent properties via tailoring the phonon energy and crystal fields’ environment in Ho3+/Yb3+ based silicate system [17]. Le et al. reported Ho3+/Yb3+ in tellurite glasses with enhanced up-conversion NIR emissions [18]. Suresh et al. [19] studied Ho3+/Yb3+ co-doped zinc tellurite glasses that exhibit blue-green photon into NIR photons transitions and it is found useful for solar conversion and optical amplifier applications. Zhu et al. [20] presented the emission improvement in the 2.0μm band and effective energy transfer process in Ho3+/Yb3+/Er3+ tri-doped tellurite glasses for solid state laser applications. Guangning et al. [21] depicted the mid-infrared 2.0μm and 4.1μm wideband emission in Ho3+/Yb3+ doped tellurite–germanate glasses that are suitable for mid-infrared fiber and laser materials applications. Azam et al. [22] explained the photo luminescence studies with Judd–Ofelt analysis in Ho3+/Ho3+–Yb3+ doped/co-doped lead tellurite glasses for use in optical multi-functional devices. Chao et al. [23] discussed enhancement in UC and 2μm mid-infrared emission in Ho3+/Yb3+ co-dopped tellurite glass for UC and mid-infrared luminescent material applications. Dogan et al. [24] focused on the UC luminescence in Ho3+/Yb3+ co-doped tellurite glasses and their temperature sensing properties which are useful for lighting and optical temperature sensing applications. Although there are a lot of studies reported on the up-conversion luminescence and NIR emission on Ho3+/Yb3+ co-doped material, the research on Ho3+/Yb3+ co-doped glass is still expected to bring interesting luminescent properties in varying crystal environment, heat treatment, phonon energies, dopant concentrations, excitation wavelengths, pump powers, etc.
Based on the above facts, in this work, we have synthesized a new set of Ho3+/Yb3+ co-doped tellurite glasses, comprising the composition TeO2–ZnO–YF3–NaF. The visible and NIR down conversion emission and intense UC emissions observed in the green and red of Ho3+ in the synthesized tellurite glasses with varied amounts of Yb3+ are analyzed. In the interim the energy transfer mechanism between Yb3+ to Ho3+ ions were analyzed.
Experimental detailsGlass preparationA series of glasses with chemical composition (50−x−y) TeO2–30ZnO–10YF3–10NaF–xHo2O3–yYb2O3 (x=0.5 and y=0.5, 1.0, 3.0 and 5.0mol%) were prepared by melt-quenching technique. In an agate mortar, rigorously mixed high purity TeO2 (99.9%), ZnO (99.9%), YF3 (99.9%), NaF (99.99%), Ho2O3 (99.99%) and Yb2O3 (99.99%) raw materials. An electrical furnace at air atmosphere is maintained at 1050°C and 10g batches of the sample were melted for 30min. Then the melts were quenched via pouring them into a preheated brass mould which is maintained at 200°C. For the purpose of avoiding the internal thermal stress-initiated cracking, the glasses were annealed at 300°C for 4h, and then brought back to room temperature via natural cooling.
Sample measurementsThe density of glass samples was measured using distilled water as an immersion liquid which works based on Archimedes principle. The absorption spectra of the samples were recorded using Perkin Elmer spectrophotometer (Lambda 1050) which operates over the wavelength ranges 300–2000nm with a spectral resolution of 1nm. The down conversion emission, excitation, and decay curve analysis were done using Horiba Fluorolog-spectrofluorimeter, that consists of CW and pulsed Xe lamps. The output signals were recorded using a visible photodiode detector (l PPD-850) and by an infrared Hamamatsu photomultiplier. The up-conversion was obtained by Horiba Fluorolog-spectrofluorimeter with laser diode used as excitation source operating at 980nm. All the studies were conducted at room temperature.
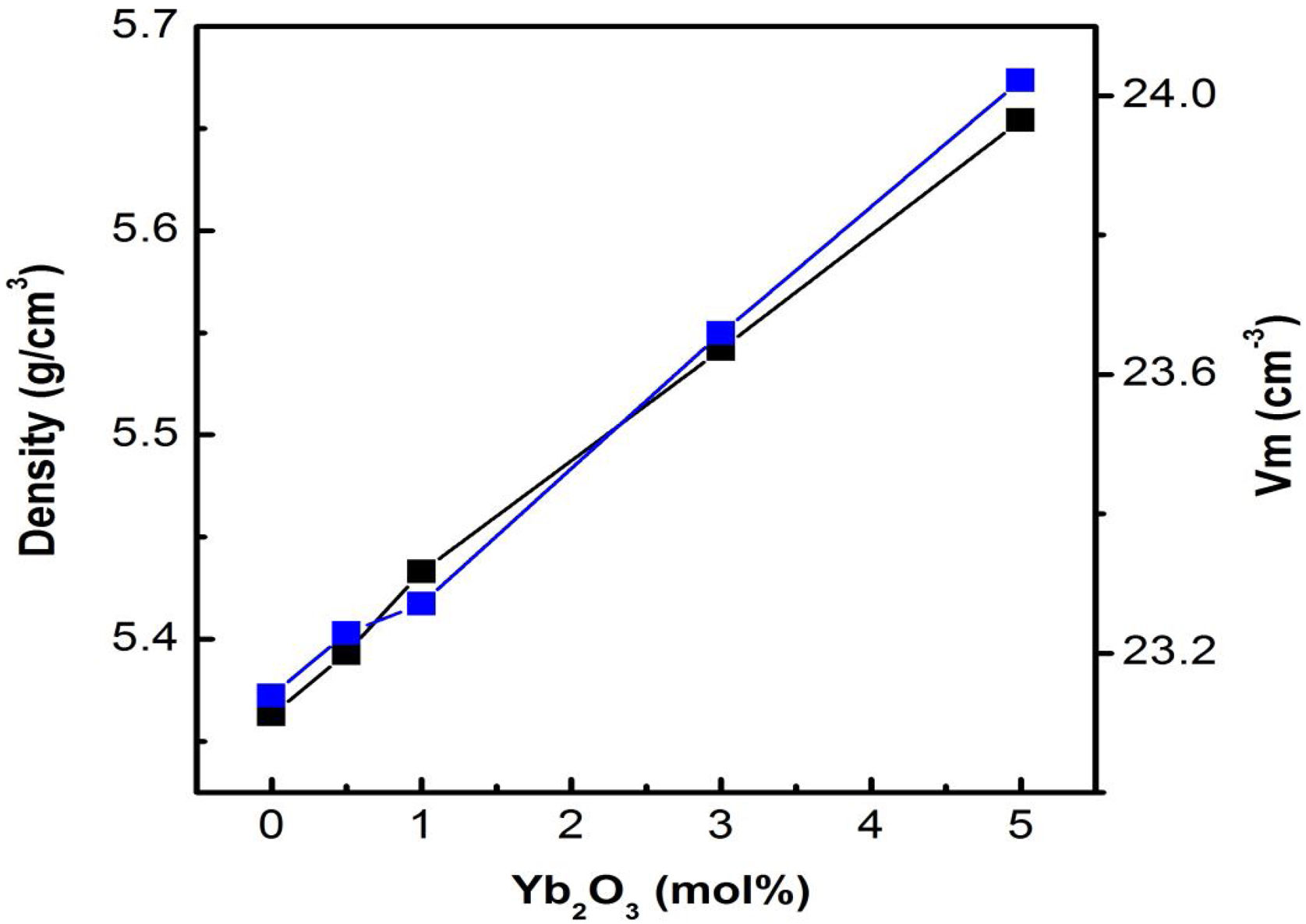
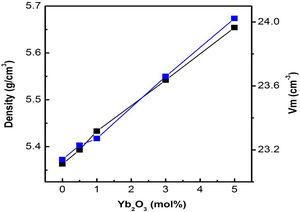
Results and discussionPhysical propertiesThe density of the glass matrix influences the bulk properties of the glass samples, such as linear refractive index, etc. [25]. The variation of density (g/cm3) and molar volume (cm3/mol) with respect to Yb3+ ion concentration in tellurite glasses is studied and is shown in Fig. 1. Both density and molar volume increase with increasing Yb3+ concentration; this could be due to the generation of excess non-bridging oxygen (NBO) bonds, that dictates the structure of the respective glass networks, and the replacement of TeO2 with heavier Yb2O3 in the base glass composition [26].
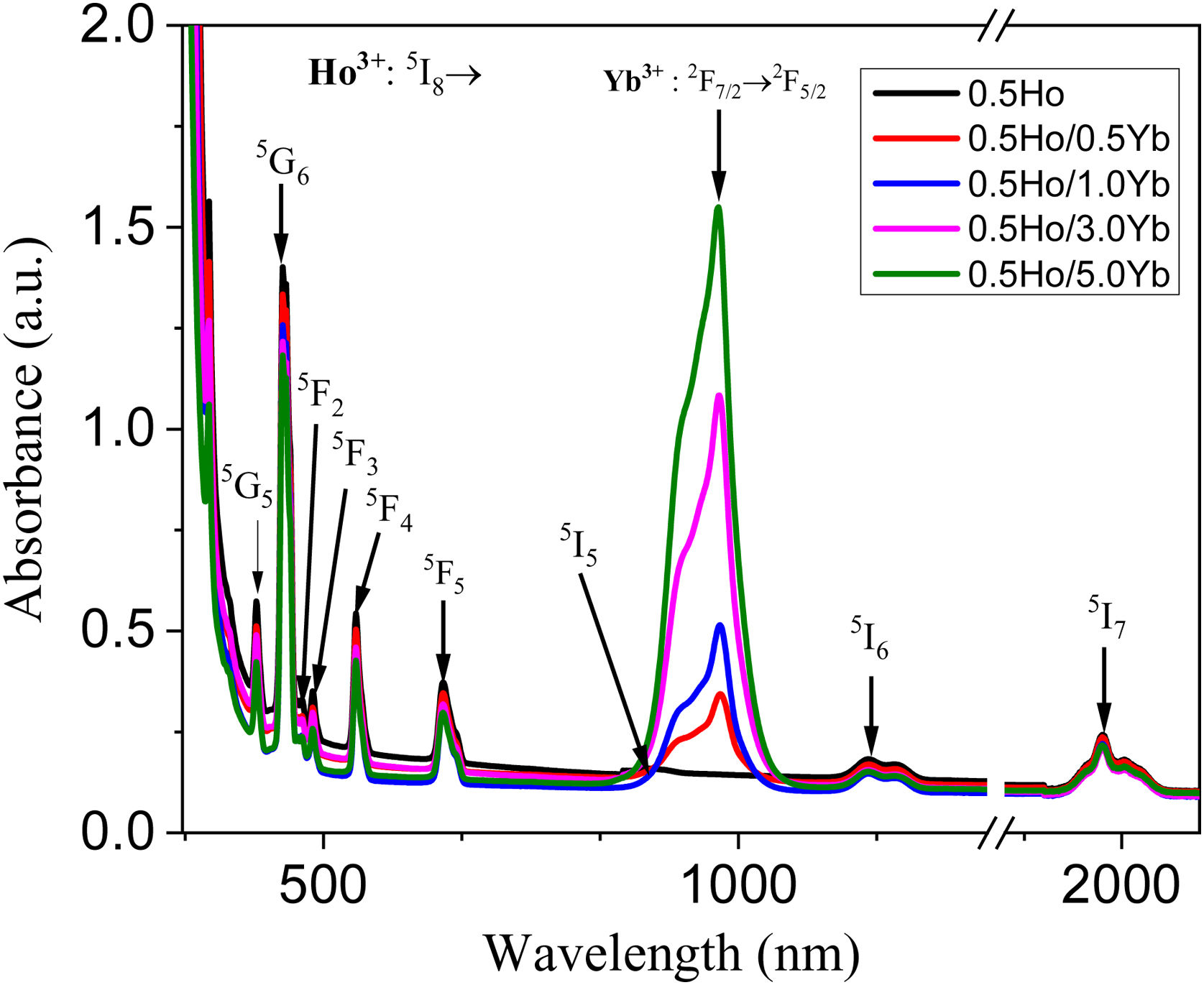
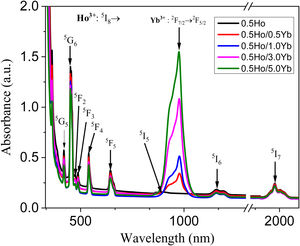
Optical absorption spectraThe optical absorption spectra of Ho3+/Yb3+ co-doped tellurite glasses are illustrated in Fig. 2. Absorption peaks at 420, 450, 474, 489 541, 645, 896, 1160 and 1955nm are referred to the ground level of Ho3+ ions (5I8) to highly excited levels (5G5, 5G6, 5F2, 5F3, 5F4, 5F5, 5I5, 5I6 and 5I7, respectively) transitions. The Yb3+ ions ground level (2F7/2) to the excited level 2F5/2 transitions, are referred to the strong absorption peak observed at 978nm. Therefore, the doping of sensitizer Yb3+ ion provided a channel for mid-infrared emission of Ho3+.
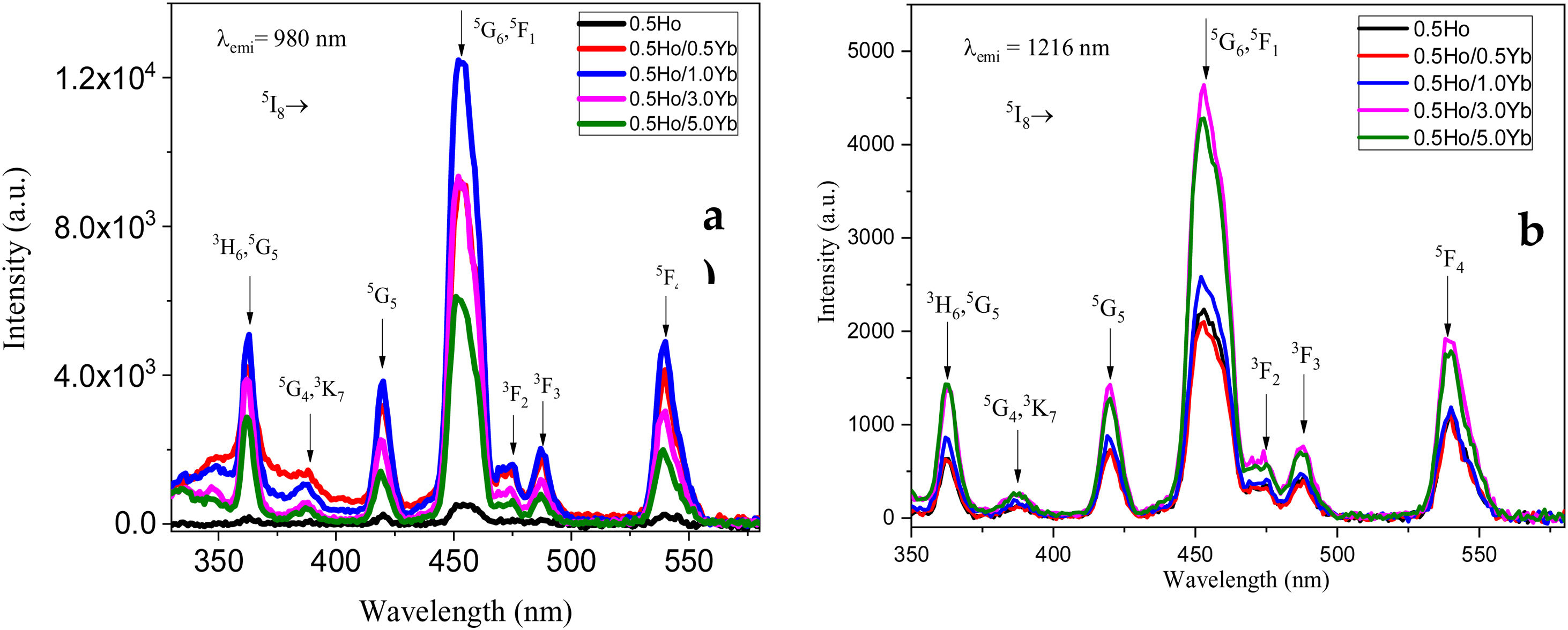
Excitation and down-conversion emissionFig. 3 exhibits the excitation spectra of Ho3+-doped oxyfluoro tellurite glasses. As shown in Fig. 3, the excitation profile for 980 and 1216nm emission closely resembles its absorption spectrum from 350 to 600nm. Hence, some of the excitation bands can be readily assigned to 5I8→3H6 (362nm), 5I8→5G4 (388nm), 5I8→5G5 (420nm), 5I8→5G6, 5F1 (455nm), 5I8→3F2 (475nm), 5I8→3F3 (487nm), and 5I8→3F4 (539nm) transitions of Ho3+ ions, respectively.
The intensity of excitation bands depends on the monitored emission wavelength. For instance, when the spectra were measured at the emission wavelength of 980nm (Yb3+ ion), see Fig. 3(a), the intensity of the bands increased up to 1.0mol% Yb3+ ion contraction. Whereas the intensity of the same bands decreased when monitored at 1216nm (Ho3+ ion), see Fig. 3(b). The energy transfer (ET) from Ho3+ to Yb3+ ions is responsible for this variation in bands intensity. When the Yb3+ ion concentration exceeds 1.0mol%, the opposite effect is observed. This could be attributed to energy back transfer (EBT) from Yb3+ to Ho3+ ions via the cross relaxation channels [28], which will discuss below. The ET between Ho3+ and Yb3+ confirmed by the analysis of down conversion emission spectra, as discussed below.
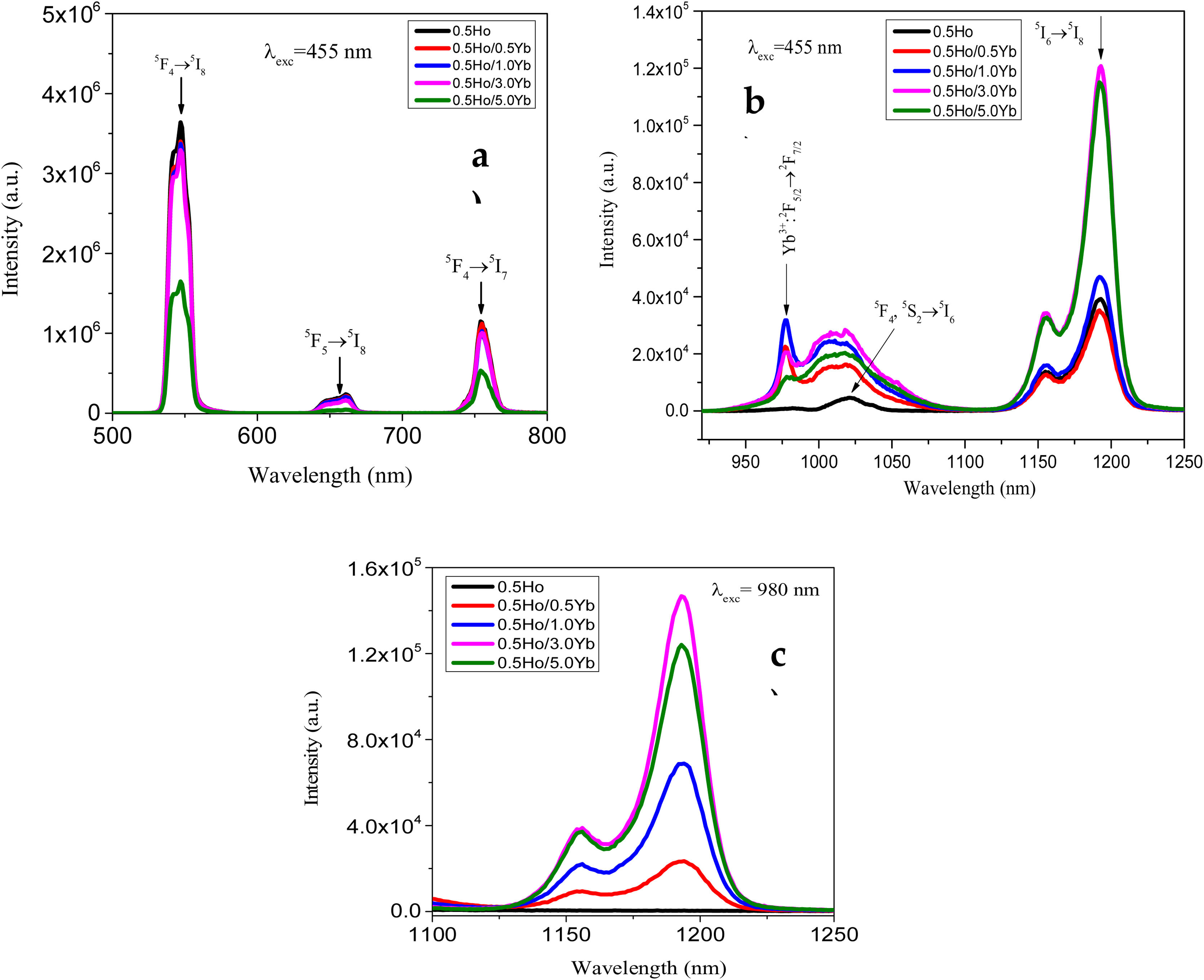
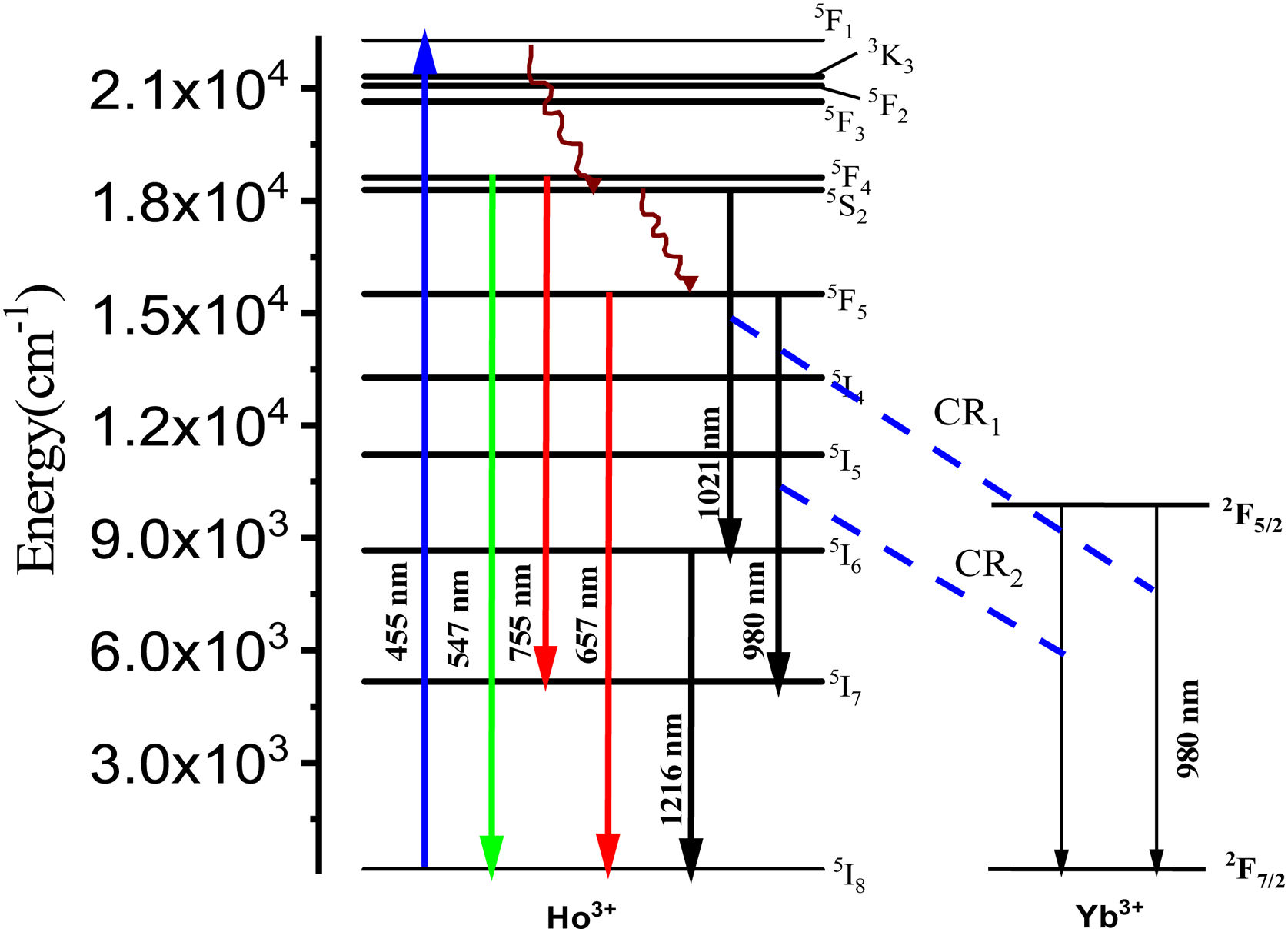
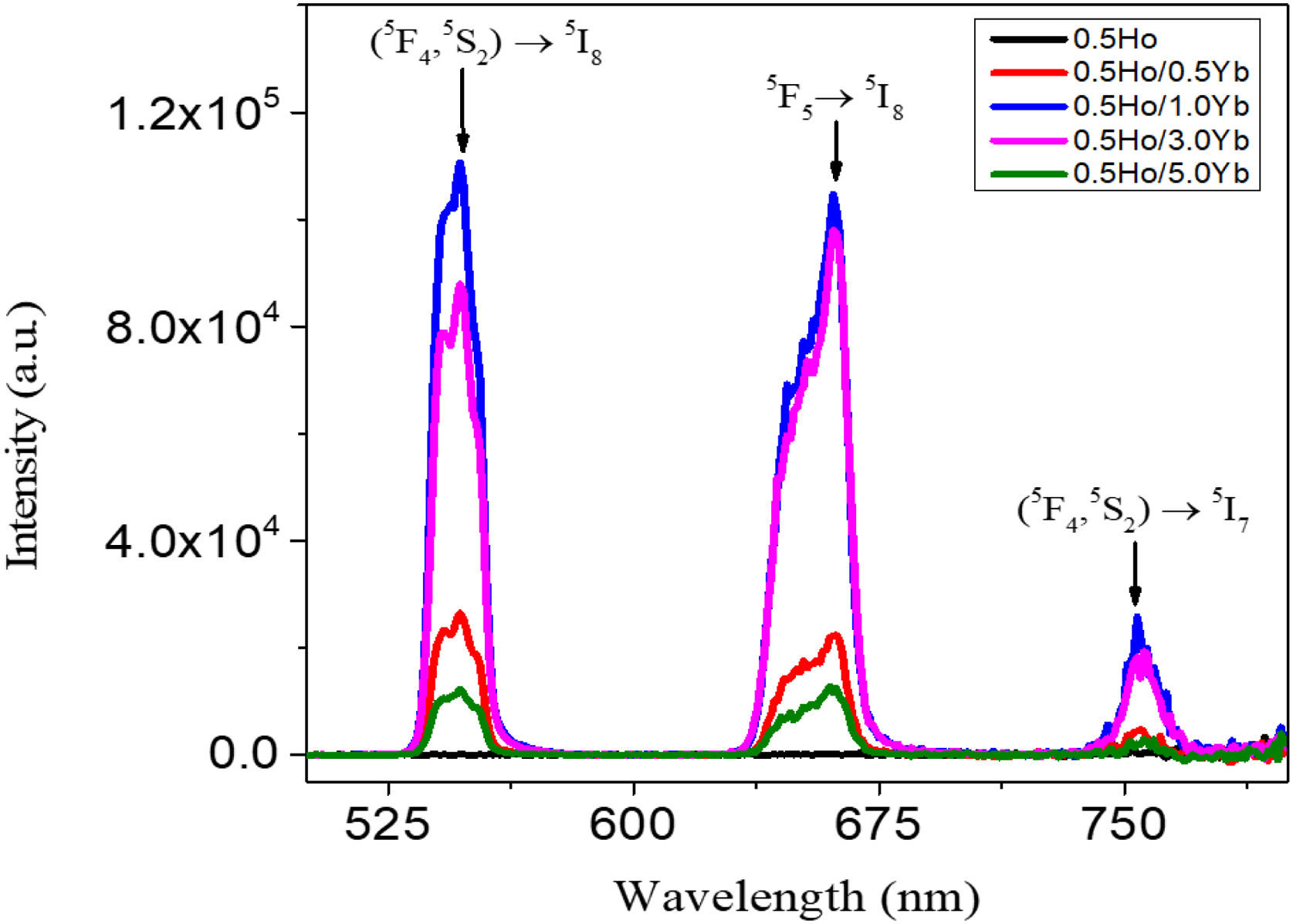
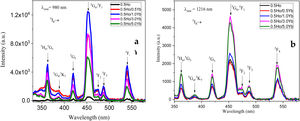
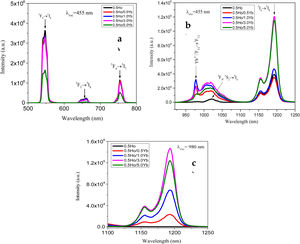
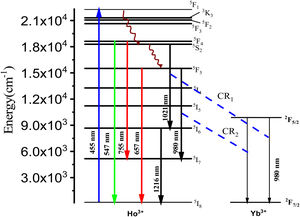
From Fig. 3, the maximum excitation wavelength is located at 455nm. Excitation into the strongest 5I8→5G6, 5F1 transition of Ho3+ at 455nm yields the emission spectrum, as illustrated in Fig. 4(a) in visible (VIS) and Fig. 4(b) in NIR region. The visible spectrum (Fig. 4(a)) consists of the emission bands associated with 5F4→5I8 (547nm), 5F5→5I8 (657nm), and 5F4→5I7 (755nm) of Ho3+ ion's transitions. The intensity of these bands decreases with Yb3+ concentration due to ET process from Ho3+ to Yb3+ ions takes place through cross relaxation channels (5S2, 5F4): Ho3+, 2F7/2: Yb3+→5I6: Ho3+, 2F5/2: Yb3+ (in Fig. 5, marked as CR1) and 5F5: Ho3+, 2F7/2: Yb3+→5I7: Ho3+, 2F5/2: Yb3+ (in Fig. 5, marked as CR2) [28]. Whereas NIR spectra (Fig. 4(b)) consists of emission bands at 980nm, 1021nm and 1216nm, which are referred to the Yb3+: 2F5/2→2F7/2, Ho3+: 5F4, 5S2→5I6, and Ho3+: 5I6→5I8 energy transitions. In contrast to visible emission spectra, although Yb3+ ion lack an absorption band in the visible region, the intensity of the 980nm band increases with Yb3+ concentration up to 1.0mol%, which confirms further that ET takes place from Ho3+ to Yb3+ ions. However, beyond 1.0mol% of Yb3+ concentration, the intensity drops for 980nm band and raises for 1216nm band have been observed. The change in intensity may be due to EBT from Yb3+ to Ho3+ ions. Further, despite of change in intensity of 980nm emission band, change in spectral profile has also been observed with the increase in Yb3+ ion concentration. This could be due the fact that luminescence reabsorption by the Yb3+ neighboring ions [29]. Fig. 4(c) shows the infrared fluorescence spectra under 980nm excitation. The emission bands can be readily assigned to 5I6→5I8 (1201nm) transition of Ho3+ ions.
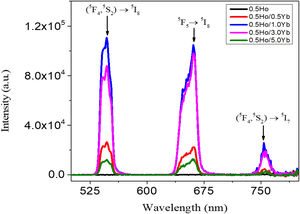
In order to demonstrate the up-conversion emission fluorescence, sensitive fluorescence spectrophotometer adopting at 980nm pumping laser was used and the up-conversion spectra obtained for the investigated glasses are shown in Fig. 6. The emission bands centering around 546, 661 and 775nm wavelengths are assigned to the transitions from the Ho3+ ion's (5F4, 5S2) to 5I8, 5F5 to 5I8, and (5F4, 5S2) to 5I7 levels, respectively. The intensity of these UC emissions gradually raised against Yb3+ content up to 1.0mol% and the emission dropped dramatically with further increase in Yb3+ ion's concentration. The non-radiative energy transfer process overrides and the resulting concentration quenching effect plays a dominant role in the reduction of UC intensities [27]. The earlier studies reported that the upconversion emission intensity of Ho3+/Yb3+ glass centered at 754nm was very weak compared to the emissions bands centered at 546nm and 669nm [22,30–36] and the ratio of peak intensity between 546nm and 659nm UC emissions is less than 1, while it is more than 1 in some other studies [37–39]. The value of UC intensity bands centered at 546nm and 661nm are maximized when the concentration of Yb3+ is 1.0mol%.
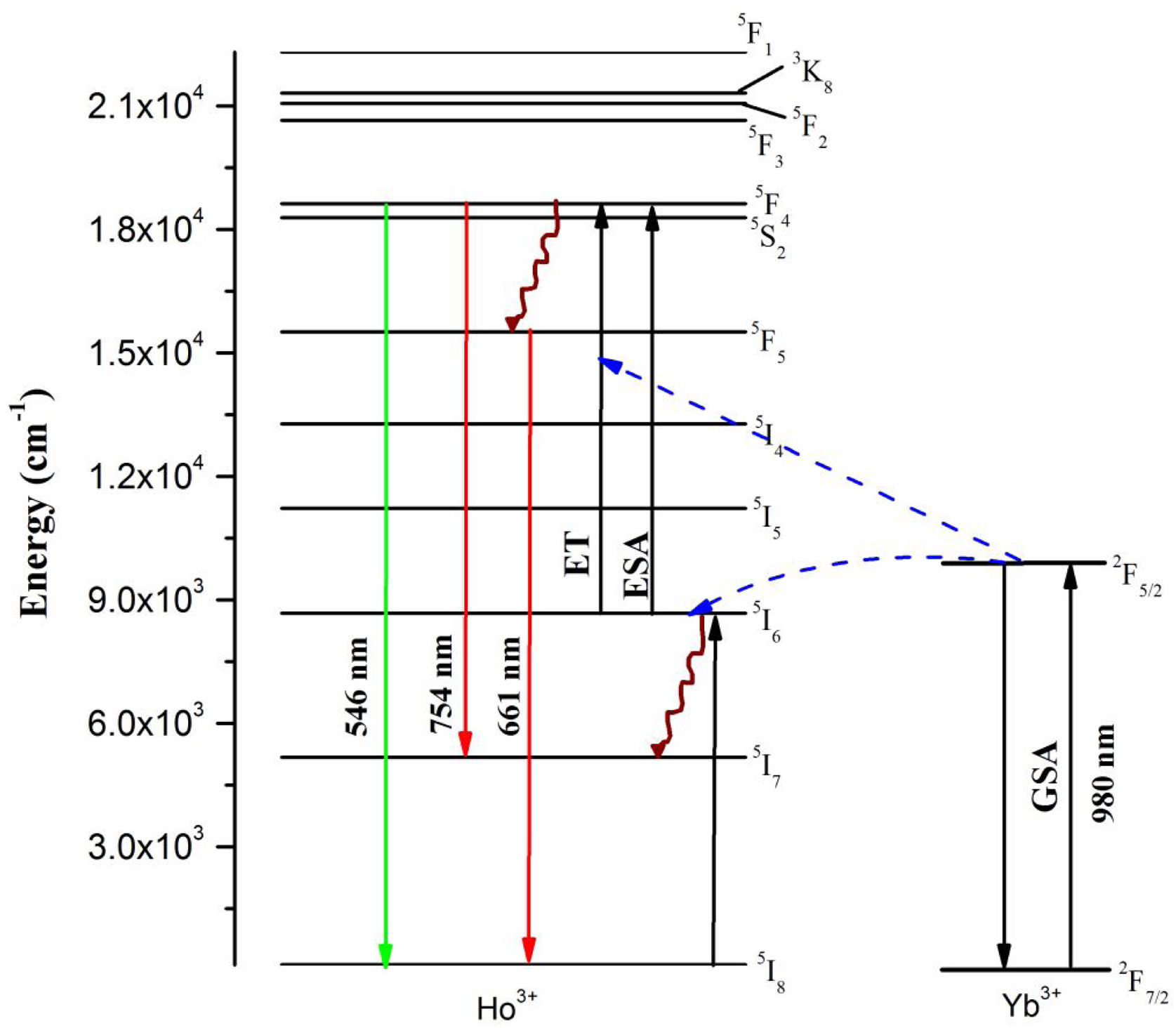
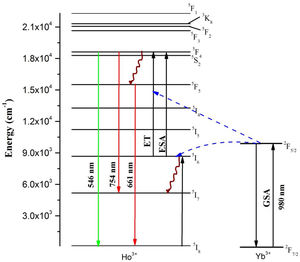
In order to explain the energy transfers from Yb3+ to Ho3+ ions, the energy diagram given in Fig. 7 is utilized. The studies on power dependency of UC emissions revealed that the intense emissions in green and red is two-photon absorption enabled. The Yb3+ ion to Ho3+ ion energy transfer populates the Ho3+ ion's 5S2 and 5F4 levels which leads to the emission in green and red wavelengths. When excited with near infrared (NIR) photons (980nm), at first the ground 2F7/2 level Yb3+ ion gets excited to the 2F5/2 level because of its large absorption cross-section. From the excited level of Yb3+ ion, the energy transfer takes place to the Ho3+ ions which are initially at the ground level, which further gets excited to a 5I6 metastable level through energy transfer (ET). Then, via the excited state absorption (ESA) or the ET, the Ho3+ ions existing at the 5I6 excited level reabsorb the incoming NIR wavelength photons to populate the 5S2 (5F4) energy levels. From there, they return to the ground 5I8 level with the emission of intense radiation in the green region around 546nm, owing to the energy gap. Also, originated from some non-radiative relaxations, the 5S2 (5F4) levels of Ho3+ ions get transferred to the 5F5 level quickly. Moreover, some possible excited state absorption (ESA) or the ET enables the 5F5 level to get populated, which ultimately lead to an intense red-light emission at about 661nm wavelength [40]. Meantime, the 5F4 (5S2) level Ho3+ ions deplete to the 5I7 metastable state thereby emitting a weak NIR emission at about 754nm wavelength [41,42].
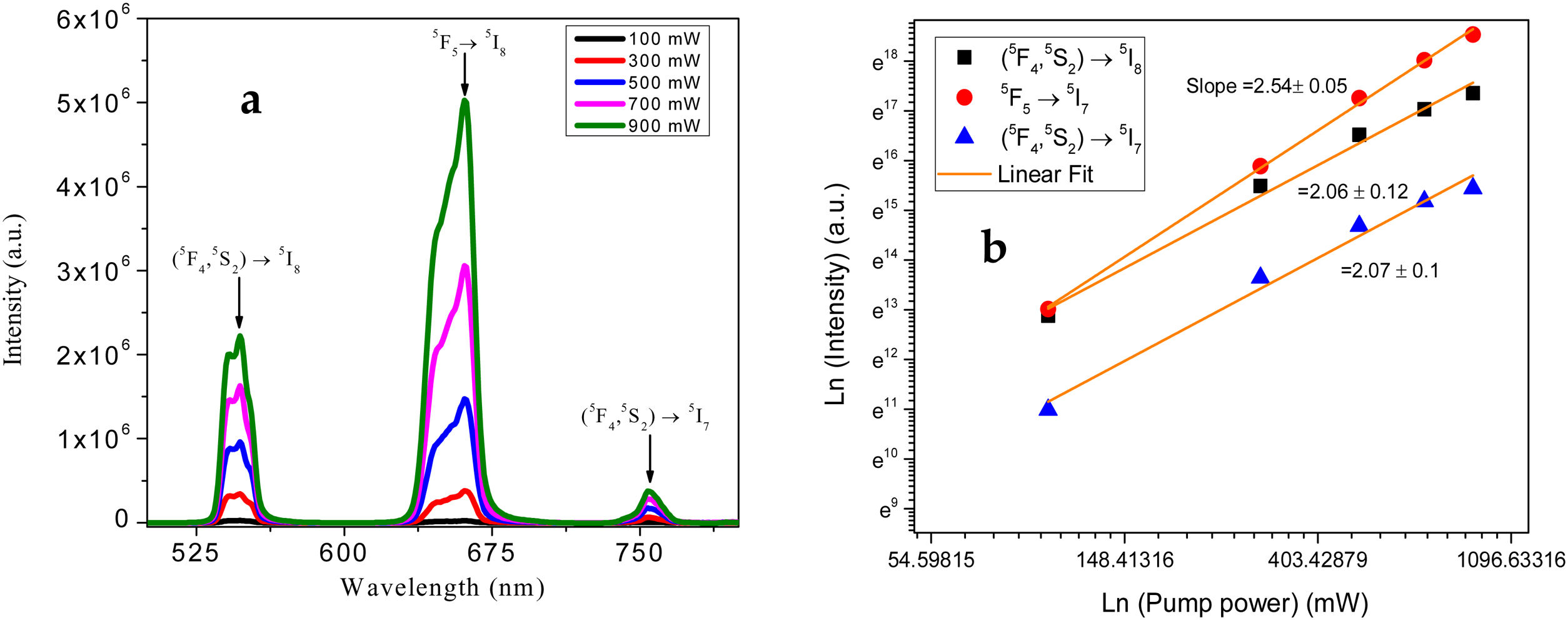
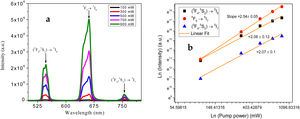
The UC involves nonlinear optical processes, and hence the UC emissions should be excitation laser intensity dependent. In order to analyze that, the UC emission spectra of the sample under varying pump power scheme is studied, and the obtained graph is given in Fig. 8(a). It shows the UC emission intensities’ variation with pump power for all observed transitions. With the increase of pump power, all UC emission intensities shoot up. For these three luminescence peaks, the dependence of integrated up-conversion emission intensities, obtained from Fig. 8(a), is presented in Fig. 8(b). The number of photons (n) take part into the UC process is revealed by linear fit on the intensity-power variation plotted as double logarithmic curve. The slope obtained can give the n value, as it assumed to follow the relation (I∼Pn) [43]. For 0.5mol% Ho3+ and 1.0mol% Yb3+ ions co-doped tellurite glass, the n values of green, red and NIR emissions are 2.07, 2.54 and 2.06, respectively; confirming that three UC emission transitions are populated predominantly by two-photons absorption process. The high n value of red transition (5F5–5I7) is indicative of multiphoton cooperative process, in addition to the two-photon absorption process.
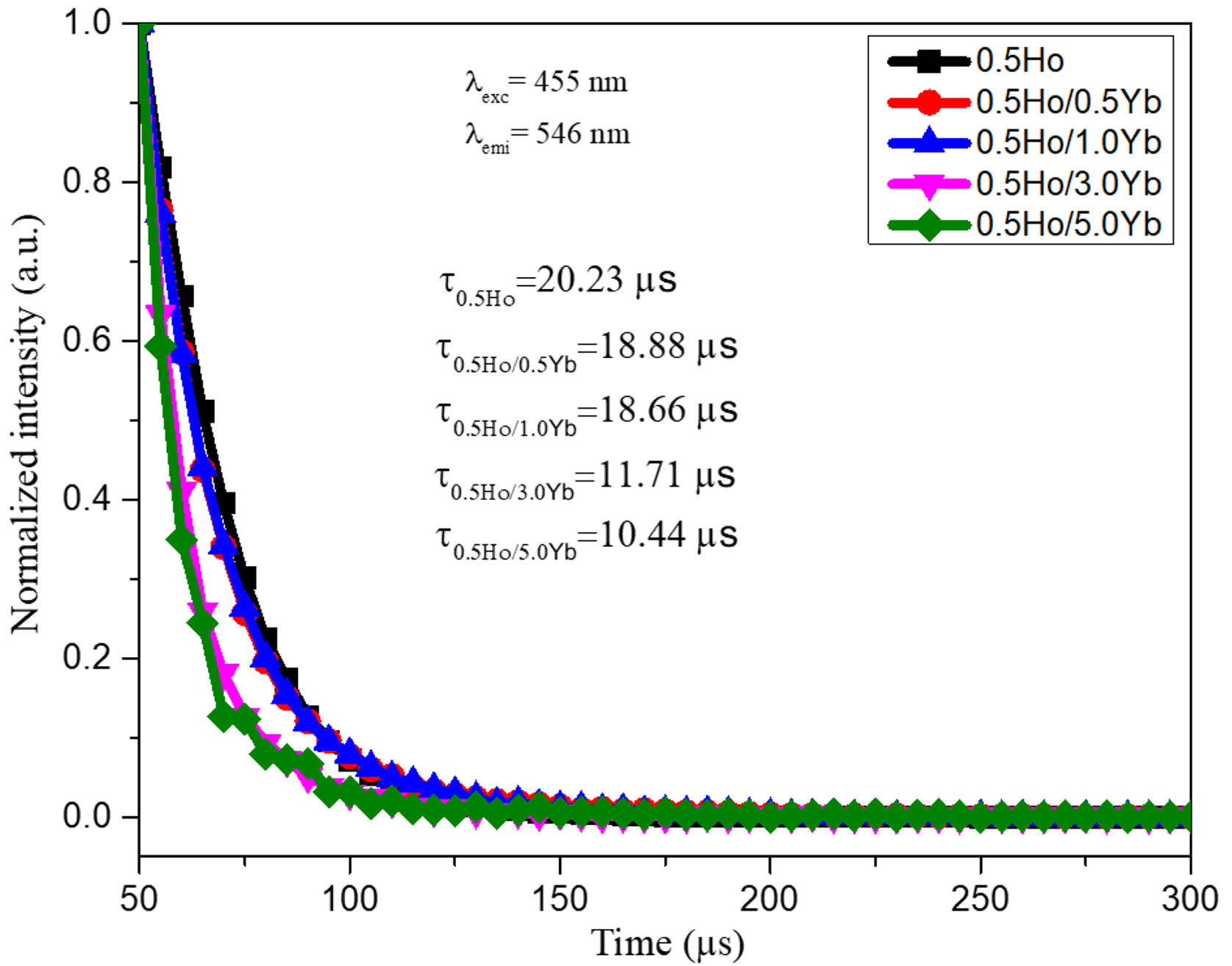
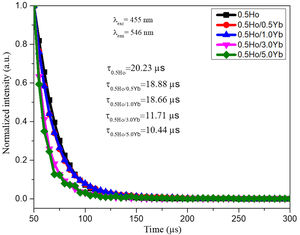
Fluorescence decay analysisIn order to unravel the ET process the lifetime measurement was carried out. The time resolved emission spectra (decay curves) of 546nm (5F4,5S2) emission of Ho3+ ions by exciting at 455nm in tellurite glasses are shown in Fig. 9. All decay curves are well fitted with single exponential function. The obtained decay times found to be decreased from 20.23 to 10.44μs. The reduction in lifetimes with the increase of Yb3+ ions concentration warrants the occurrence of strong energy transfer process from Ho3+ to Yb3+ ions, via cross relaxation channels (5S2, 5F4): Ho3+, 2F7/2: Yb3+→5I6: Ho3+, 2F5/2: Yb3+ and 5F5: Ho3+, 2F7/2: Yb3+→5I7: Ho3+, 2F5/2: Yb3+ (see in Fig. 6) [14].
ConclusionIn this report, a series of glasses with chemical composition (50−x−y) TeO2–30ZnO–10YF3–10NaF–xHo2O3–yYb2O3 (x=0.5 and y=0.5, 1.0, 3.0 and 5.0mol%) is prepared by melt-quenching technique. The absorption spectra, excitation, visible emission, up-conversion emission spectra and decay time measurements were characterized. Under the 980nm laser excitation, the sample exhibited intense green (546nm), red (661nm), and NIR emissions (754nm). The UC emission intensity variation study with respect to excitation pump power indicated that the two-photon absorption mechanism based on the energy transfer from Yb3+ to Ho3+ was the source of the Ho3+ population at 5F4 (5S2) and 5F5 levels. With the increase of Yb3+ ions concentration, the green and red up-conversion emission intensities show significant enhancement. Hence, the present results indicate that the newly synthesized Ho3+/Yb3+-co-doped TeO2–ZnO–YF3–NaF glass with intense up-conversion emissions in the green and red wavelengths could be a promising material that will find applications in the visible-band fiber lasers and photonics.
FundingThis research was funded by Kuwait Foundation for the Advancement of Sciences, Kuwait, grant number PR18-14SP-11.
S.K. acknowledges Kuwait Foundation for the Advancement of Sciences (KFAS) for the research fund (PR18-14SP-11). This work is a part of the activities of the FunGlass project. R.D. acknowledges the funding from the European Union Horizon 2020 research and innovation program under grant agreement No. 739566 and S.E. acknowledges SERB, India for NPDF No. PDF/2020/002494.