Geopolymer composite production has become an indispensable product to reduce carbon dioxide emissions, which have become an important problem today, and to provide green sustainability. Concerns about the global climate change problem have also accelerated geopolymer studies. This research investigated the mechanical and durability characteristics of low-calcium fly ash (LCFA) based geopolymer mortars with different curing temperatures and times. Two forms of curing conditions were applied; the first one was standard curing at room temperature (20±3°C and RH 65±10%) and the second one was cured in the hot air at 40°C, 60°C, and 80°C for 24h, 48h, and 72h followed by standard curing. After all curing processes, compressive strength, flexural strength, water absorption, void ratio, resistance to elevated temperatures, and freeze–thaw conditions were determined experimentally. In addition, SEM analysis was performed before and after durability tests for comparison purposes. Also, XRD and TGA analyzes were performed. According to test results, curing specimens at longer times and higher temperatures has been shown to increase compressive strength results. The highest compressive strength value was reached at 80°C after 72h of curing. Geopolymer specimens subjected to elevated temperatures (600°C and 900°C) lost a significant part of their strength value. After the freeze–thaw test, LCFA-based geopolymer specimens showed more than 70% resistance. The freeze–thaw resistance of geopolymer samples was positively affected on long-term curing at high temperatures, but high-temperature resistance was impacted negatively.
La producción de compuestos de geopolímeros se ha convertido en una cuestión indispensable para reducir las emisiones de dióxido de carbono, que constituyen un problema importante actualmente, y para aportar sostenibilidad verde. La preocupación sobre el problema del cambio climático ha acelerado también los estudios sobre geopolímeros. Este trabajo estudió las características mecánicas y de durabilidad de los morteros de geopolímeros con base de cenizas volantes con bajo contenido de calcio (LCFA) a diferentes temperaturas y tiempos de curado. Se aplicaron dos formas de condiciones de curado: la primera, con curado estándar a temperatura ambiente (20±3°C y RH 65±10%), y la segunda con curado mediante aire caliente a 40, 60 y 80°C durante 24, 48 y 72 horas, seguido de curado estándar. Tras la realización de todos los procesos de curado, se determinaron experimentalmente la fuerza compresiva, la resistencia a la flexión, la absorción de agua, el índice de vacío, la resistencia a altas temperaturas y las condiciones de hielo-deshielo. Además, se realizó un análisis por microscopía electrónica de barrido (SEM) antes y después de las pruebas de durabilidad, a fines comparativos. También se realizaron análisis de difracción de rayos X (XRD) y análisis termogravimétrico (TGA). Conforme a los resultados de la prueba, se comprobó que en las muestras de curado con tiempos más prolongados y temperaturas más altas se incrementaban los valores de fuerza compresiva. El valor de fuerza compresiva más alto se logró a 80°C tras 72 horas de curado. Las muestras de geopolímeros sujetas a temperaturas elevadas (600 y 900°C) perdieron una parte significativa de su valor de fuerza. Tras la prueba de hielo-deshielo, las muestras de geopolímeros basadas en LCFA reflejaron más de 70% de resistencia. La resistencia al hielo-deshielo de las muestras de geopolímeros se vieron afectadas positivamente con el curado prolongado a temperaturas altas, aunque la resistencia a altas temperaturas se vio afectado de manera negativa.
The construction industry is one of the fastest-growing industries worldwide. Ordinary Portland cement is the most important building material used in this industry. Cement manufacturing not only consumes natural resources but also releases significant amounts of carbon dioxide into the environment. There are also problems with the disposal of industrial by-products such as fly ash. In addition to the above, a large amount of energy is also essential for cement production; therefore, alternative fuels are needed. Therefore, to overcome such problems, it is needed to produce and use alternative ecological mortars instead of traditional cement mortars. In this context, using LCFA-based geopolymer mortar instead of cement mortar can also reduce the disposal problem caused by fly ash.
Geopolymer is an inorganic polymer formed as a result of the polycondensation reaction of some waste material containing aluminosilicate with alkalis. The name given to this reaction is geopolymerization. Geopolymers are semicrystalline 3D aluminosilicate framework structures formed by the combination of [SiO4]4− and [AlO4]5− tetrahedra. The setting and hardening mechanism of the geopolymers are not fully understood. The geopolymerization process can occur by dissolving aluminosilicate-containing raw materials in alkaline solutions, which leads to the formation of aluminate and silicate monomers. These are then converted into oligomers and then geopolymers. Water is used during dissolution and is released in polymerization [1,2]. The strength development of the geopolymer is affected by many factors including raw materials, activator composition, and curing conditions [3]. The compressive strength development of geopolymers has the most influence on curing conditions. The reaction of geopolymer material is very long drawn out at an ambient temperature and generally exhibits a slow strength development. Heat curing is necessary to produce a rapid geopolymerization process and thus to succeed in admissible strength in a very short time [4–6]. The recommended curing temperature for geopolymers was 60–80°C and it was noted that there was no noteworthy strength gain at temperatures beyond that.
Saludung et al. [7] applied four different curing methods to investigate high-temperature performance in fly ash and slag-based geopolymer mortars: ambient curing, curing with water, heat curing at 70°C for 24hs, and application of heat curing and water curing together. It was observed that curing conditions significantly affected the geopolymer properties before the durability conditions, but the effect on the performance after the high temperature was not significant. On the other hand, all samples formed sufficient compressive strength after exposure to 950°C. Muhammad et al. [8] conducted a study in which the geopolymer concrete sample was cured at room temperature and cured at different temperatures between 60°C and 100°C for 24h. The results determined that the increase in strength was higher at increasing heat curing conditions compared to room conditions. Palomo et al. [9] found that the curing conditions essentially affected some properties of fly ash-based geopolymer mortars. Compressive strength values cured at 85°C were much higher than those cured at 65°C in 24h. Van Jaarsveld et al. [10] compared the development of strength at different curing temperatures. They reported that the compressive strength increased significantly with curing at 70°C, whereas rapid curing at high temperatures could lead to cracks that could affect the geopolymer properties. Fareed et al. [11] also found that the highest compressive strength of fly ash-based geopolymer concrete was achieved at a curing temperature of 70°C, though the compressive strength decreased after 70°C. Furthermore, Temuujin et al. [12] found that curing at 40°C and 80°C temperatures for up to 48h was one of the important factors for geopolymerization. Hardjito et al. [13] stated that the compressive strength values increased with high curing temperatures at later ages but the improvement in the strength may not be important for curing at temperatures higher than 60°C and longer than 48h. In another study, Mustafa Al Bakri et al. [14] cured geopolymer specimens at different temperatures such as 50°C, 60°C, 70°C, and 80°C and they reported that the optimum curing temperature was 60°C. According to some studies, the compressive strength values of geopolymer-based material at higher temperature curing were increased, and also humidity was an important parameter for the strength development of geopolymer materials [15–17]. As a curing period, 24–48h has been suggested and it has been reported that more increase in curing time has not affected geopolymerization [13]. On this issue, Patankar et al. [18] explained that after the 3-day curing process, the compressive strength of geopolymer mortars increased with increasing the degree of heating of the oven. After the 3 days of oven curing, the strength increased less. Rovnanik [19] reported that short-term curing in the oven did not significantly change the strength, but long-term curing of at least 20h resulted in a noticeable rapid reaction rate and early strength.
Research on the properties of geopolymer concrete and mortars is gaining more attention day after day. One of the most important problems of geopolymer concrete and mortars is the need for heat curing for geopolymerization. As a result, the effects of the curing condition on the mechanical and durability properties of geopolymers have not yet been fully elucidated. Class F fly ash-based geopolymers harden slightly when cured at room temperature, but form a lower strength gain at an early age than when cured in an oven. Because of this situation, the curing temperature affects the geopolymerization process. Geopolymerization accelerates with increasing curing temperature (60–80°C), but this can only be used in precast structures. While this limits the wide use of fly ash-based geopolymers, it creates an obstacle for on-site concrete applications, excluding precast concrete. This study reviews the curing methods of geopolymers. In addition, the evaluation of different fly ash as a raw material in the production of geopolymer improves the literature on the use of thermal power plant by-products in geopolymer production. Here, the fly ash obtained from each thermal power plant has different properties. There is no in-depth research on the geopolymer modification of fly ash obtained from the Biga Coal-Fired Power Plant. In this study, the curing conditions of LCFA-based geopolymer mortars were investigated and the properties such as compressive and flexural strengths, water absorption, void ratio, freeze–thaw, and high-temperature resistance of LCFA-based geopolymer mortars cured at various curing temperatures (40°C, 60°C, and 80°C) and curing times (24, 48 and 72h) were searched. Also, SEM, XRD, and TGA analyses were carried out for comparison purposes.
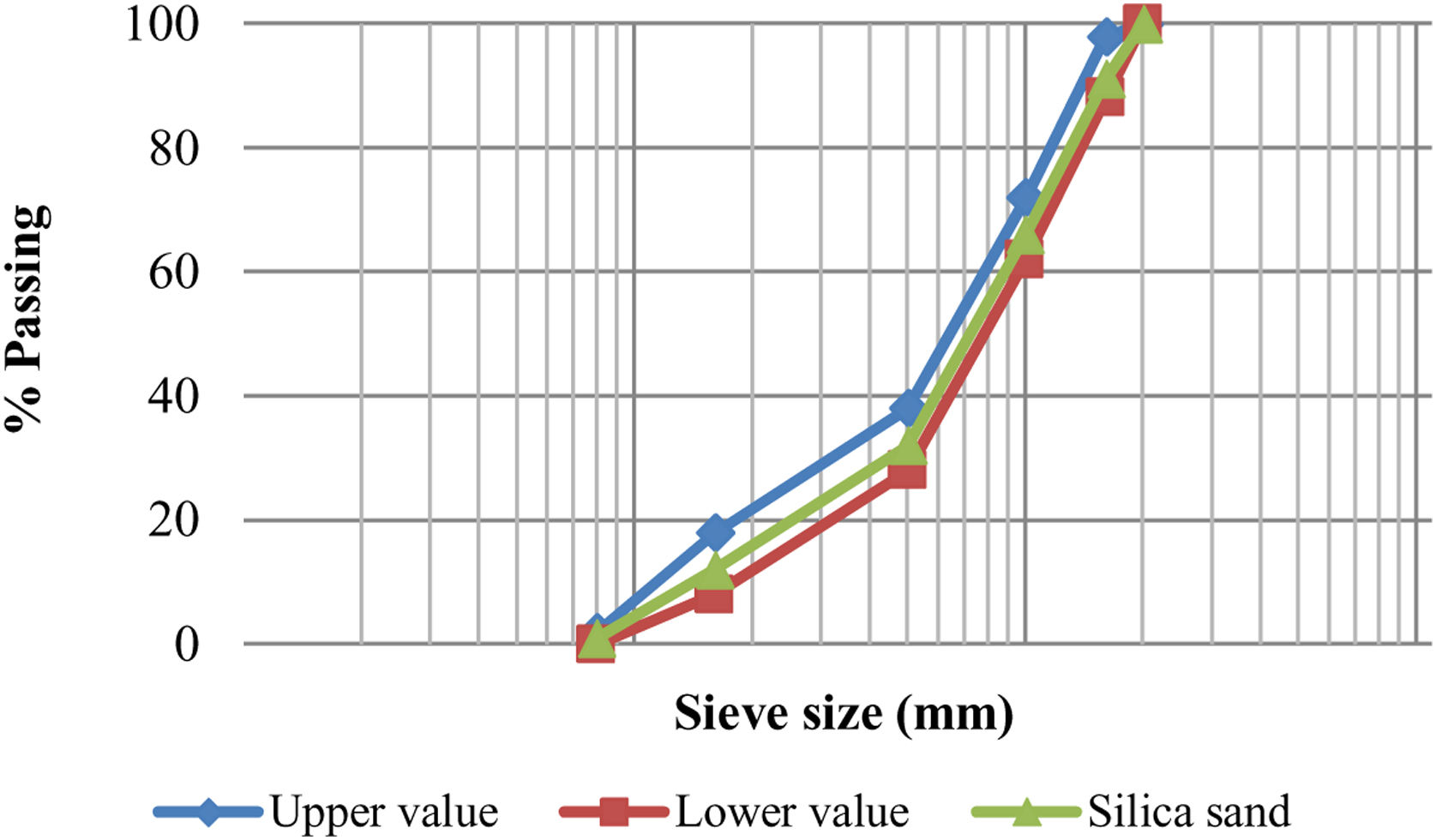
ExperimentalMaterialsLow-calcium fly ash (class F) according to ASTM C618 [20] was selected as the basic material for the preparation of geopolymer mortar mixtures. LCFA materials are more chosen than high calcium fly ash, with the thought that great amounts of calcium (class C fly ash) may affect the geopolymerization process. The fly ash used in this study was supplied from İçdaş Biga Coal-Fired Power Plant. It was stated that the ignition loss (LOI) value and iron oxide content of LCFA should be below 5% and 10%, respectively and the CaO content should be low [21]. The LOI value of the LCFA used in this study was 0.53%, Fe2O3 content was 8.7% and CaO content was 4.75%. Particle size distribution of LCFA ranged from 0.3 to 100μm with a major fraction in the range of 40–90μm. Fly ash is very fine ash particles that move away from the chimney in coal-fired thermal power plants and are dragged up (flying) together with the gases drawn by the chimney due to combustion. The fly ash obtained in this way is used directly without any pre-treatment before use. The specific surface area and specific gravity of LCFA were 2128cm2/g and 2.26, respectively. When the amorphous structure of fly ash was examined, it was seen that it generally contained crystalline phases and a glassy structure. Crystalline phases were determined as quartz (SiO2), calcium oxide (CaO), anhydrite (CaSO4), hematite (Fe2O3), mullite (Al6Si2O13), mayenite (12CaO·7Al2O3) and magnetite (Fe3O4). It was determined that the glassy content was 44.20% in fly ash. The chemical content of LCFA is shown in Table 1. Silica sand was utilized for the manufacturing of mortar mixture. The silica sand used in this study was supplied by the Aydınlar Madencilik Company (Bursa). The silica sand's grain size distribution shown in Fig. 1 was determined according to TS EN196-1 [22].
The grading curve showed that silica sand's grain size allocation was within the standard's limits. Sodium hydroxide and sodium silicate (with 8% Na2O, 27% SiO2, and 65% H2O by mass) were utilized as alkali activators for the mixture preparation of mortar. Sodium hydroxide and sodium silicate were supplied by the Palkim Kimya Company. NaOH and Na2SiO3 solutions were mixed at room temperature of 20±3°C the day before use in the experimental study. Due to the heat generated, it waited for 24h for cooling. The ratio of Na2SiO3 to NaOH was 2 and NaOH concentration was 12M. Three levels of curing time were 24, 48, and 72h followed by several curing temperatures which were 20°C, 40°C, 60°C, and 80°C. The ratio of fly ash to silica sand was 1:3 by weight, and the alkali activator/binder ratio was fixed at 0.5 to produce the geopolymer mortar. Previous studies were also taken into account when making preliminary trial mixtures for the silicate/hydroxide ratio, molarity value, binder material/aggregate, and activator/binding material ratios used in this study [4,5]. The extra water demand of fly ash-based geopolymer mortar was determined according to ASTM C230 [23] with a flow diameter of 110±5mm.
Mixing procedureThe LCFA-based geopolymer mortar mixture was conducted in two stages; firstly the fly ash was mixed up with sand in a dry state in a mixer for 3min. The alkaline activator and extra water were then added to the solid ingredients of the geopolymer mortar and stirring was continued for a further 3min. After measuring the flow diameter of the mixture, the fresh mortar mixture was poured into 50mm×50mm×50mm molds and compacted. After the mixtures were filled into the sample molds by bottling them in two layers, they were placed with the help of a table-type vibrator. All of the prepared mortar mixtures were cured in the curing room under the same conditions (at 20±3°C and 65±10% relative humidity) for 24h after casting. At this stage, the sample was expected to be set. Afterward, different curing temperatures were applied. Geopolymer mortar specimens were taken out from the molds after 24h and placed in the hot air oven at a certain temperature for a certain curing period. It has been reported that the retardation in the initiation of heat curing significantly increased the compressive strength of geopolymer concrete [24]. Although some researchers contradicted this notion and emphasized that the delay time was not important, Bakharev [1] reported that the strength was importantly higher when the specimens were kept at room temperature for 24h before heat treatment. In another study, it was proved that 72h of rest time produced 7 days higher compressive strength than 24h of rest time under an intermittent curing scheme [25].
TestingTwo sets of the mixture were prepared, one set was cured in the oven for 24h, 48h, and 72h at elevated temperatures (40°C, 60°C, and 80°C), while the other set was preserved at room temperature (20°C). Both of the specimens were exposed to compressive strength and flexural strength tests at 2, 7, and 28 days. As a minimum, three samples from each mix have been evaluated and the mean value of strength has been investigated. The compressive strength test on hardened LCFA-based geopolymer mortar was carried out using a flexure/compression testing machine (Utest, UTCM 6710) with a capacity of 15/250kN in accordance with the relevant ASTM C109 [26]. The water absorption and void ratio tests were applied to the samples applied at three different curing temperatures (40, 60, and 80°C). After each curing temperature was applied, the samples should be dried to oven-dry weight to perform the water absorption test. The samples were dried at 80°C for 24h and brought to a constant weight with an accuracy of 0.001g. Three cubes of each mixture were dried in the oven for 24h at 80°C and then cooled to room temperature. Since a higher temperature was thought to disrupt the microstructure of the mortar, a temperature of 80°C was chosen to dry the specimens. The geopolymer specimens weighed after drying for 24h at 80°C were stored in water (at 20±3°C) until they reached a constant weight. After this procedure, the samples were weighed, and their weights were noted down as the final weight. The void ratio and water absorption values were calculated by using the saturated dry surface weight, the weight in water, and the oven-dry weight.
The microstructures of various specimens were determined by using scanning electron microscopy (SEM). The analysis was concentrated on the geopolymer matrix before and after exposure to high temperatures. The Thermo Scientific Apreo 2 S LoVac device was used for scanning electron microscope (SEM). Apreo 2 offers the opportunity to examine a wide spectrum of material types in high resolution. An electron microscope works based on image formation by scanning the surfaces of the samples to be examined with electrons and analyzing the electrons and photons formed as a result of the interaction. In suitable samples, Apreo resolves 0.9nm at 1kV. This value is 8nm or more at 1kV in conventional electron microscopes. Small specimens that will be difficult to polish with the device or to mount to the microscope are molded to make them fit on the automatic polishing disk and to mount it more easily. The sampling process at this stage takes 15min. Non-conductive samples for SEM imaging can be coated with gold/palladium (Au/Pd) at the coating thickness. The Malvern PANalytical X’Pert PRO device was used for X-ray diffraction (XRD). The X-ray diffraction (XRD) method is a technique used to determine the crystallographic structure and chemical composition of a material. XRD provides information about the chemical composition and crystal structure of a material by irradiating the material with incoming X-rays and then measuring the intensities and scattering angles of the X-rays leaving the material. The PerkinElmer, Diamond TG/DTA – Seiko Instruments SII, Exstar 6300 TG/DTA device was used for thermogravimetric analysis (TGA). It is a system for simultaneously measuring the temperature difference and weight change between the sample and the reference.
To determine the high-temperature resistance of the geopolymer mortar, the samples were placed in the hot air oven at elevated temperatures such as 300°C, 600°C, and 900°C with an increase of 11°C per minute from room temperature within 2h. Three samples were used for each elevated temperature (for 300, 600, and 900°C separately) and each sample was exposed to each elevated temperature only once. Before being subjected to elevated temperatures, samples were weighed. After reaching the target test temperature, the oven was kept constant at the elevated test temperature for 1h. The specimens were then cooled to room temperature for 24h and then the weight losses were measured. After this procedure, the geopolymer mortar specimens were preserved at room temperature until a mechanical test was conducted. Freeze–thaw resistance was evaluated at room temperature on 50-mm cube specimens cured for 28 days. In this test, a freeze–thaw cycle was defined as placing the specimens in a chamber (Utest, UTD-1440), freezing at −20°C for 4h, and thawing at 20°C for 4h was conducted after 50 cycles. Instead of a standard method for the freeze–thaw test, the method was followed by considering previous studies [5]. While the freeze–thaw test was in progress, three specimens from different hardening conditions were kept at room temperature. A compressive test was conducted on all samples at the same time. After the freeze–thaw test, the compressive strength test was carried on according to ASTM C109 [26]. Geopolymer specimens were saturated with water before being exposed to the freeze–thaw test and specimens were weighed before and after the test.
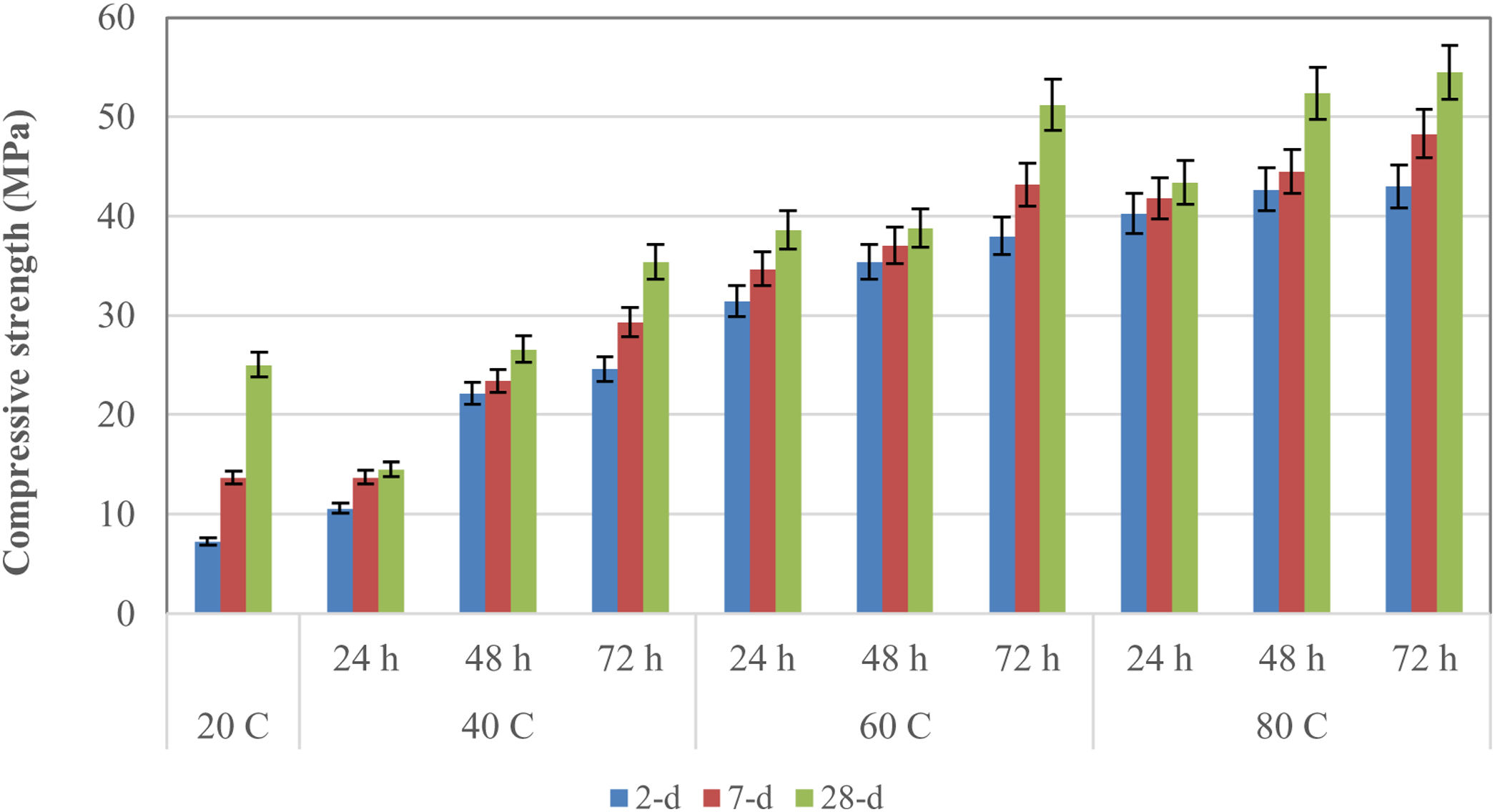
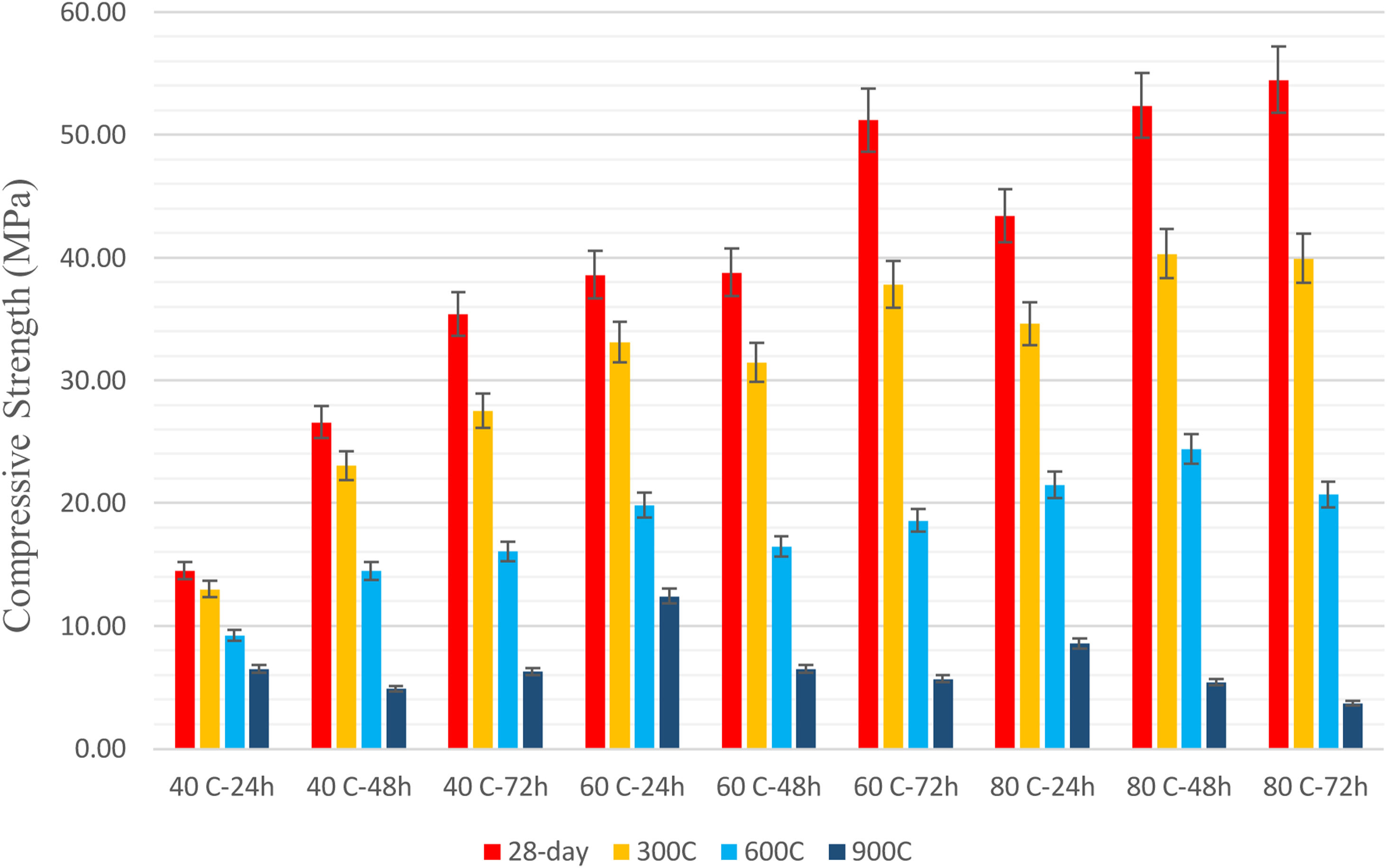
Results and discussionCompressive strength and flexural strengthIn this study, 40°C, 60°C, and 80°C were selected as the curing temperature. As indicated in Fig. 2, the compressive strength values increased with increasing curing temperatures and time. Longer curing allowed a greater reaction product, which ultimately increased the compressive strength. The lowest early-age compressive strength values were obtained in specimens stored at room temperature (20°C). When the curing temperature rose from 20°C to 40°C, the early-age compressive strength values increased depending on the curing times. Considering the curing temperatures it was observed that they contributed to the improvement of the early strength of the specimens, especially the 2-day compressive strength. When the curing temperature increased from 20°C to 40°C, the 2-day compressive strength of the specimens increased more than 3 times. At the same time, increasing the curing temperatures from 20°C to 60°C and 80°C increased the 2-day compressive strength more than 5 times. Considering the 7-day compressive strengths, increasing the curing temperatures from 20°C to 40°C, 60°C, and 80°C increased the strength of the specimens by about 2–4 times. The specimens cured at 40°C had the lowest values according to the 60°C and 80°C. Compressive strength values cured at 40°C for 24h, 48h, and 72h were 14.5MPa, 26.6MPa, and 35.4MPa at 28 days. The greatest compressive strength values were obtained in the geopolymer samples cured at 80°C.
28-Day compressive strengths were 43.4MPa, 52.4MPa, and 54.5MPa for specimens cured at 80°C for 24h, 48h, and 72h, respectively. The compressive strength of the geopolymer specimens was 25.02MPa at 20°C in 28 days. When the curing temperatures rose to 40°C, 60°C, and 80°C, the 28-day compressive strength values reached 35.4MPa, 51.2MPa, and 54.5MPa, respectively, for a curing time of 72h. The 28-day compressive strengths of the mortars cured at 60°C for 72h and 80°C for 48h and 72h have reached 2 times the values obtained at room temperature (20°C). In Turkey, it is obligatory to use concrete with a minimum strength of 30MPa in the cube sample for 28-day results in the carrier system of buildings. According to these results, it was observed that the results obtained at 60 and 80°C curing conditions met the minimum conditions, but did not meet the minimum conditions except for 72h of curing at 40°C. In-room conditions (20°C), it was observed that the required minimum condition was not met. Singh et al. [27] explained that the curing temperature was important for obtaining high strength for alkali-activated fly ash and that the specimens cured at high temperatures had higher mechanical strength. It was found that the curing temperature increase contributed to the development of both early and final compressive strengths of LCFA-based geopolymer mortars, and this contribution was more pronounced, especially in early age strength. In this case, which was formed by high-temperature curing, the dissolution of the binder materials and the acceleration of the reaction product formation played a role. In particular, it has been observed that the heat energy generated by the optimum temperature provided strength development by controlling the geopolymerization [28,29]. Particularly, fly ash having fine particle size and high specific surface area increased its activation. Especially in the case of curing at 80°C, geopolymerization progressed faster due to the unreacted fly ash particles which were the amorphous content of the FA in the LCFA-based geopolymer. A crust of reaction biproducts surrounds the microsphere in the form of unreacted fly ash grain, and the adherence between the crust and sphere seems to depend on the curing temperature for the samples. While this adhesion is weak and brittle in samples subjected to low-temperature cure, the application of high temperatures for curing enables the by-products formed with geopolymerization to form a flexible adhesion in unreacted fly ash. The 80°C curing temperature advances the geopolymerization process with increasing temperature and ensures the breakdown of unreacted fly ash. As unreacted fly ash breaks down, sodium-silicate gel continues to form, forming a cementitious matrix. Thus, the decrease in the high content of unreacted microspheres also increases the strength values [29].
Considering the effect of the curing time, it was observed that the longer curing time was more effective at early age strengths, particularly increasing the 2-compressive strengths of the geopolymer specimens by 2 to 6 times. The 2-day compressive strength values cured at 40°C for 24h, 48h, and 72h were 10.6MPa, 22.14MPa, and 24.6MPa respectively, while these values were 31.4MPa, 35.4MPa, and 38.0MPa for samples cured at 60°C. This value was 7.24MPa for geopolymer specimens stored at room (20°C) temperature. The 2-day compressive strength values of the specimens cured at 80°C for 24h, 48h, and 72h were 40.3MPa, 42.7MPa, and 43.0MPa, respectively. When the effects of curing times on 7-day compressive strengths were examined, it can be said that the strengths increased by 2–4 times. Considering the effects of curing times on 28-day compressive strength, it can be observed that the strength increased up to 2 times. The 28-day compressive strengths of geopolymer mortars kept at 20°C (25.02MPa) were higher (14.5MPa) than those of specimens cured for 24h at 40°C. Nuriddin et al. [30] also stated that long-term curing provided high strength, but the increase in strength was insignificant when the curing time was extended more than 24h. Curing times, as well as curing temperatures, were found to be more effective on the early strength of LCFA-based geopolymer specimens. In this study, it was observed that the curing time increase accelerated the dissolution of the binder materials and contributed to the geopolymerization by creating more reaction products [28,29].
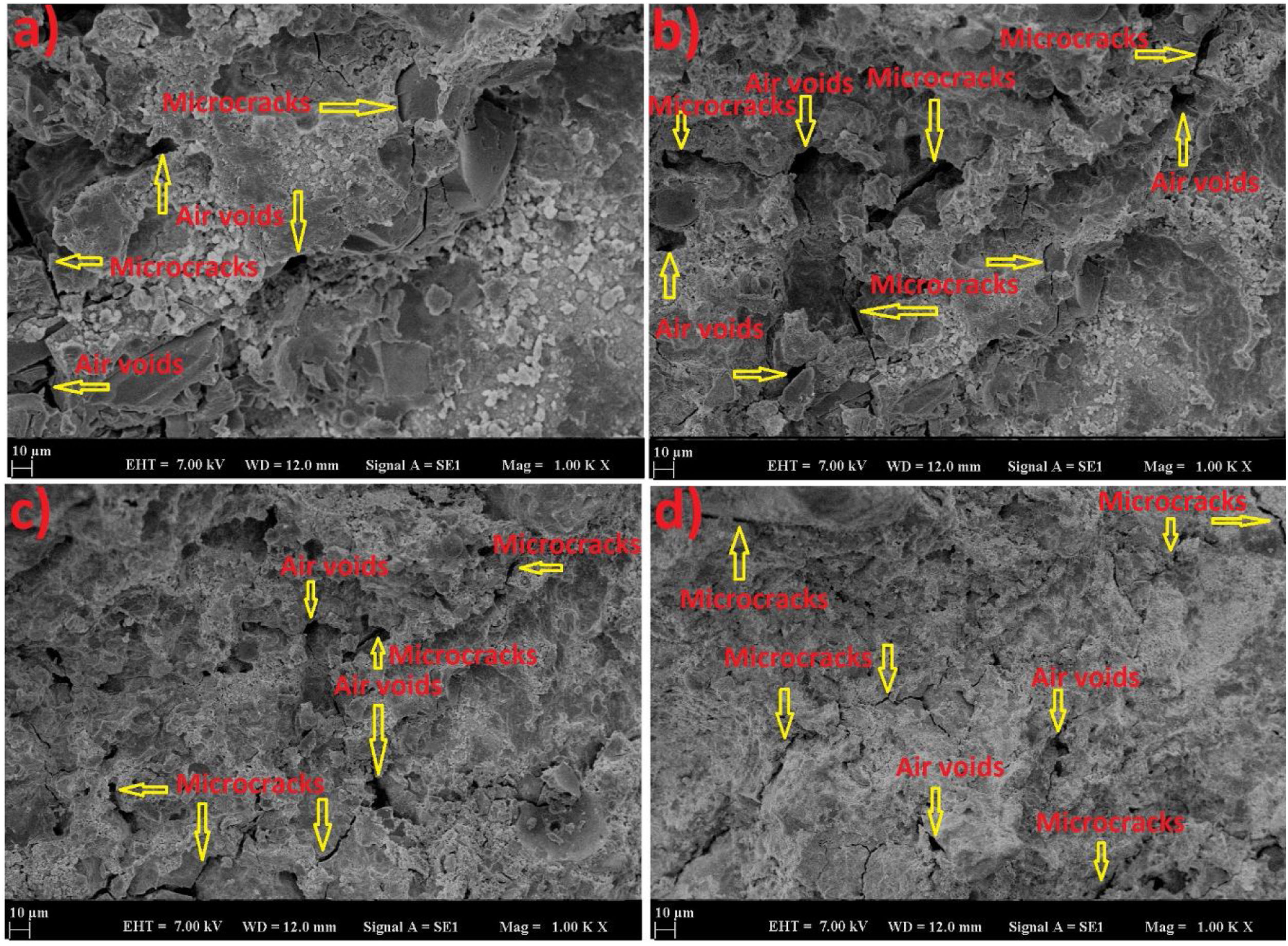
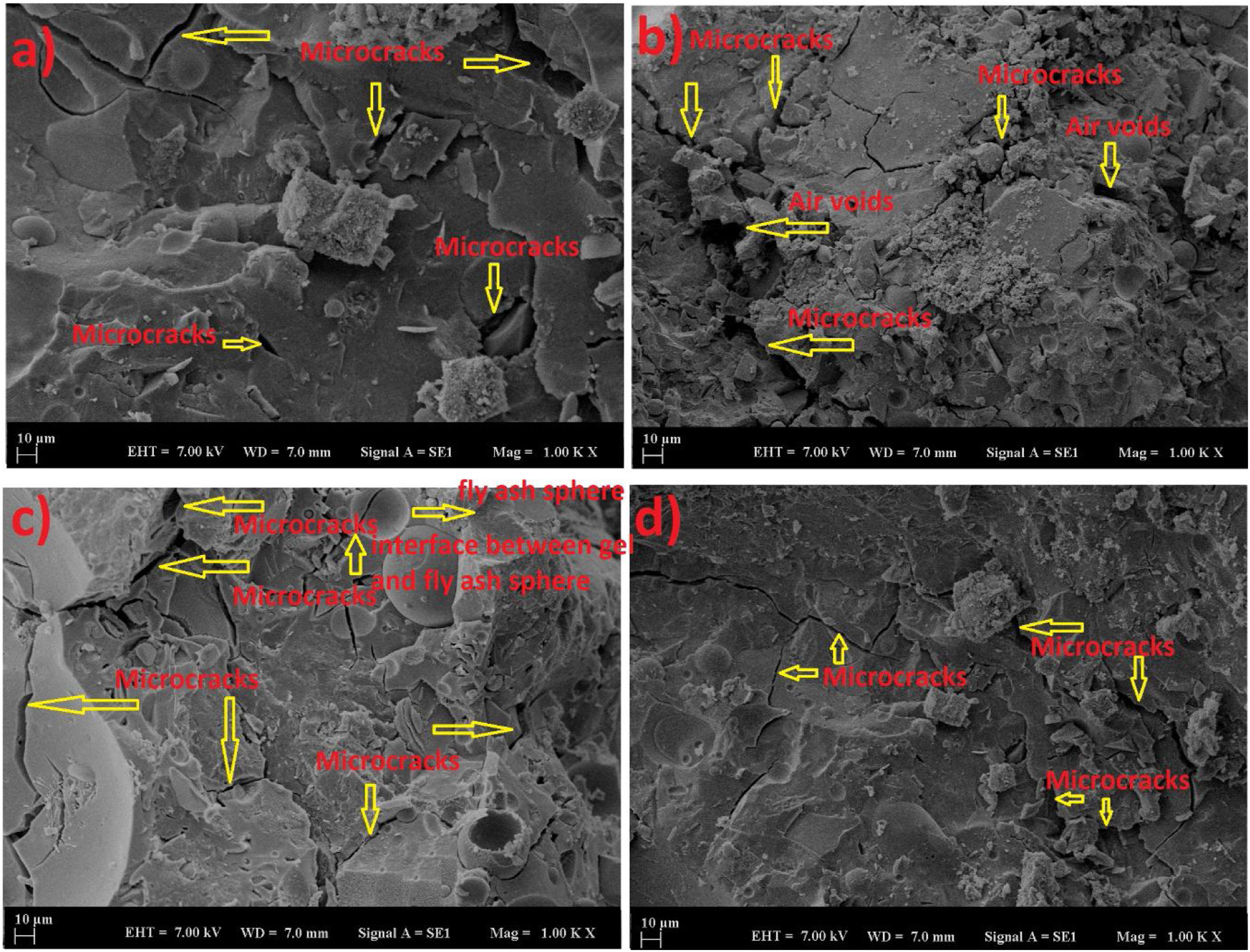
The experimental results have shown that curing temperature played a critical role not only in final compressive strength but also in the early compressive strength gain. It can be stated that the curing conditions were more effective on the early compressive strength of LCFA-based geopolymer specimens than on the final strength. Longer treatment at high temperatures was responsible for accelerating the rate of reaction and increase in early-age compressive strength. The geopolymerization process on the LCFA-based geopolymer mortar was accelerated under heat curing but compressive strength was generally unchanged with the ages. Here, the acceleration of the reaction together with the temperature curing affected the reaction products and the microstructure. As the homogeneity of the microstructure was affected by the reactive intensive treatment, a denser heterogeneous structure was formed. This structure caused a delay in the reactions with elapsed time and led to a decrease in the effect of the heat curing. Due to this situation, it was observed that heat curing was more effective on early strength. This was in contrast to the well-known behavior of Portland cement mortar which was strengthened by the hydration process. Since water curing was applied in Portland cement at room conditions, a slower increase occurred. Here, however, there was a faster reaction. Although heat curing had such a negative effect on geopolymer samples, it has been seen that this situation provided an advantage when the higher strength was taken into consideration. The compact gel structure of the geopolymer samples was effective in this situation [28]. In the SEM analysis (Fig. 3), the acceleration of the reaction with the increase of the curing temperature affected the reaction products and microstructure. As a result of this situation, a denser structure was formed. This was evidenced by the fact that a more homogeneous structure with fewer cracks and air voids was observed at 60 and 80°C.
Unlike Mustafa Al Bakri et al. [14], in this study, the curing temperature above 60°C did not decrease the compressive strength values of the specimens. It was reported that the optimum curing temperature was 60°C and elevated temperature-cured geopolymer specimens did not have sufficient moisture to develop better strength. In this study, maximum compressive strength was achieved in the samples cured at 80°C. In their study comparing different curing methods, Narayanan and Shanmugasundaram [31] stated that mortars cured at 80°C for 6h exhibited the highest strength. Although the maximum compressive strength value (54.5MPa) was achieved after 72h curing at 80°C in this research, the strength value (51.2MPa) obtained after curing at 60°C for 72h and the value obtained after curing at 80°C for 48h (52.4MPa) were quite close to each other.
SEM analysis was carried out to support the obtained results with analyses. SEM images of samples cured at 60 and 80°C for 24 and 72h are shown in Fig. 3. It was observed that the analysis results were parallel to the compressive strength results. The increase in heat curing and time increased the homogeneity by providing continuity in the geopolymer matrix. The contribution of the temperature to the geopolymerization provided the density of the matrix structure and increased the strength. The high specific surface area and fine particle size of fly ash increased its reactivity. In this way, the reaction of the unreacted particles in the amorphous structure of the fly ash with high temperature contributed to the strengthening of the structure.
While the adhesion between the crust consisting of reaction biproducts and the unreacted fly ash microsphere was weak and brittle at low temperatures, it provided flexible adhesion with increasing curing temperature. In addition, increasing the curing temperature ensured the breakdown of unreacted ash. The reduction of unreacted microspheres continued the formation of a sodium-silicate gel with a structure similar to the cementitious matrix [29]. This has contributed to the advancement of geopolymerization. Thus, silica and alumina content provided more geopolymer gel formation. In addition, the strong structure reduced the microcracks by providing a greater bond with the aggregate. Microcracks and air voids in the analysis images confirmed these conditions. The weak bond led to the formation of more microcracks [28,29].
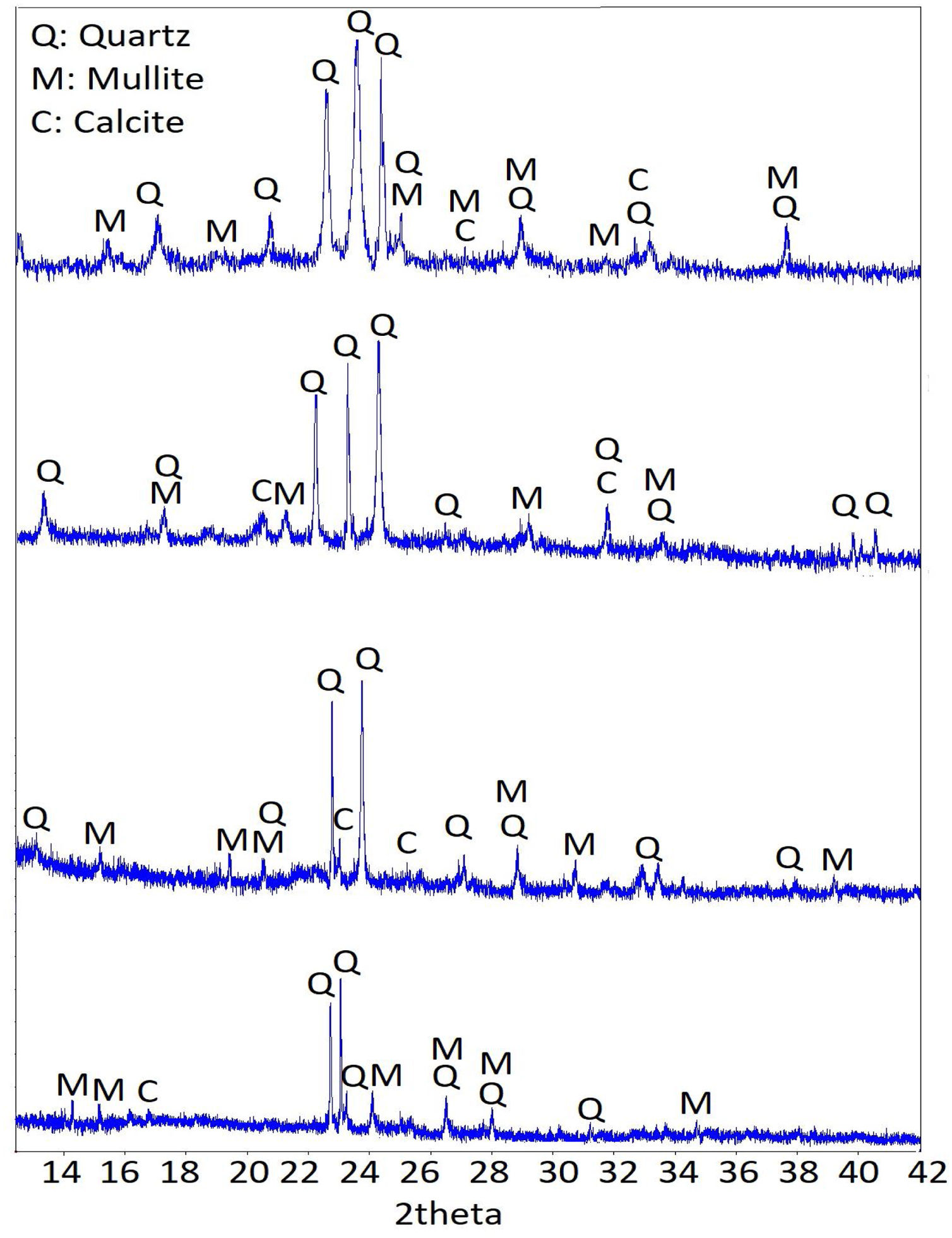
XRD analysis was also carried out to see the condition of the samples under the effect of curing temperatures and times. The compositions of the products formed as a result of the matrix reactions were investigated using the XRD technique. XRD patterns of samples cured at 60 and 80°C for 24 and 72h are shown in Fig. 4. As a result of geopolymerization, calcite, and mullite components were formed together with the main quartz component. While the patterns corresponding to the amorphous phase were detected, quartz peaks were formed at 20–40° 2-theta phase intervals. When the variation of the peaks was examined, it was observed that higher peak values were formed as a result of the increase in thermal curing and time increasing the homogeneity and the degree of geopolymeric reaction by providing continuity in the geopolymer matrix, as in the results of the SEM analysis.
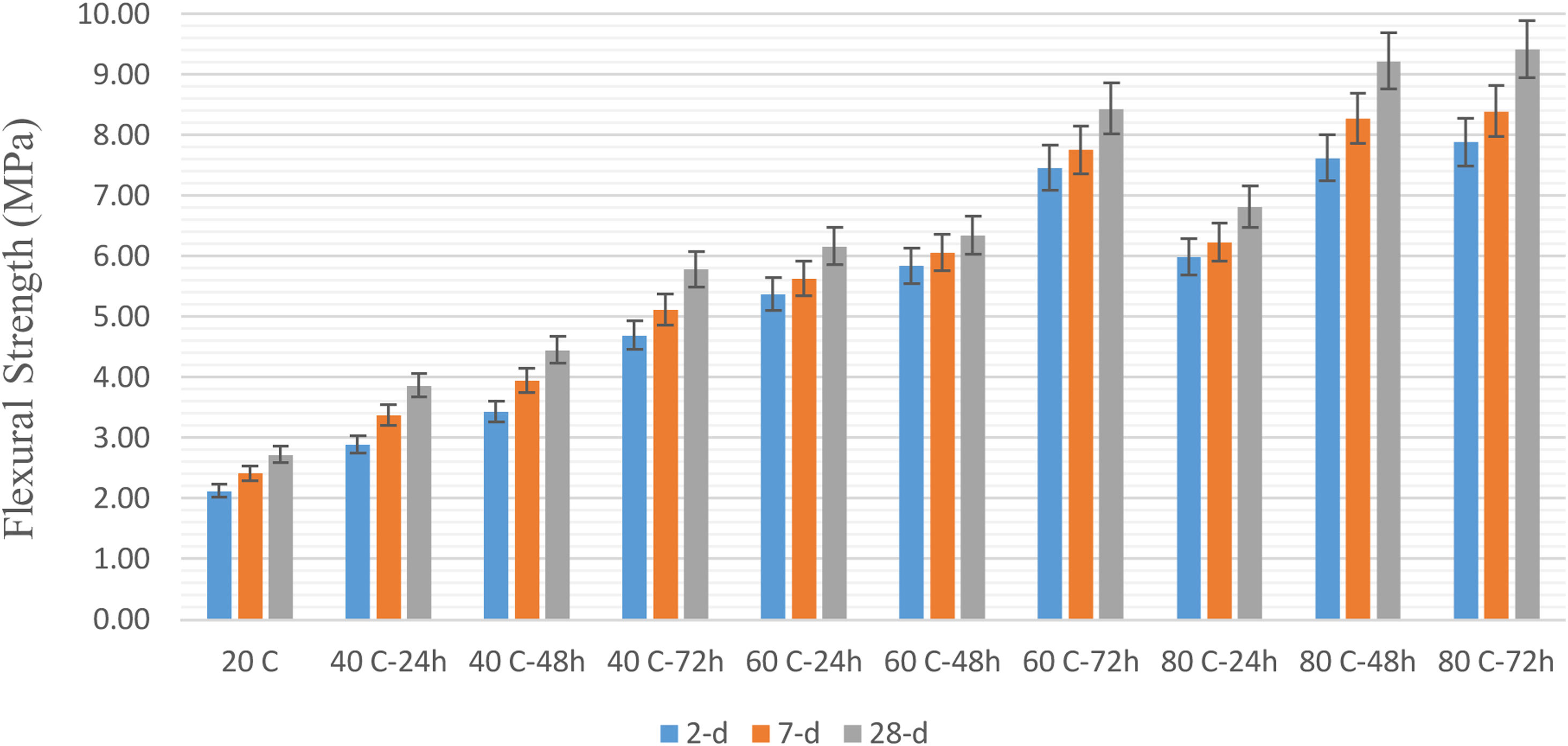
The flexural strengths of 2, 7, and 28 days examined in geopolymer mortars are compared in Fig. 5. When the flexural strengths were examined, it was seen that the results were parallel to the compressive strength results. Curing time and temperature increased the flexural strength results by promoting the progress of geopolymerization [28,29]. In addition, it was determined that the flexural strengths increased with increasing age. When the 28-day results were examined, the highest result was observed in the sample cured at 9.41MPa at 80°C for 72h, while the lowest results were in the sample cured at 2.72MPa at 20°C.
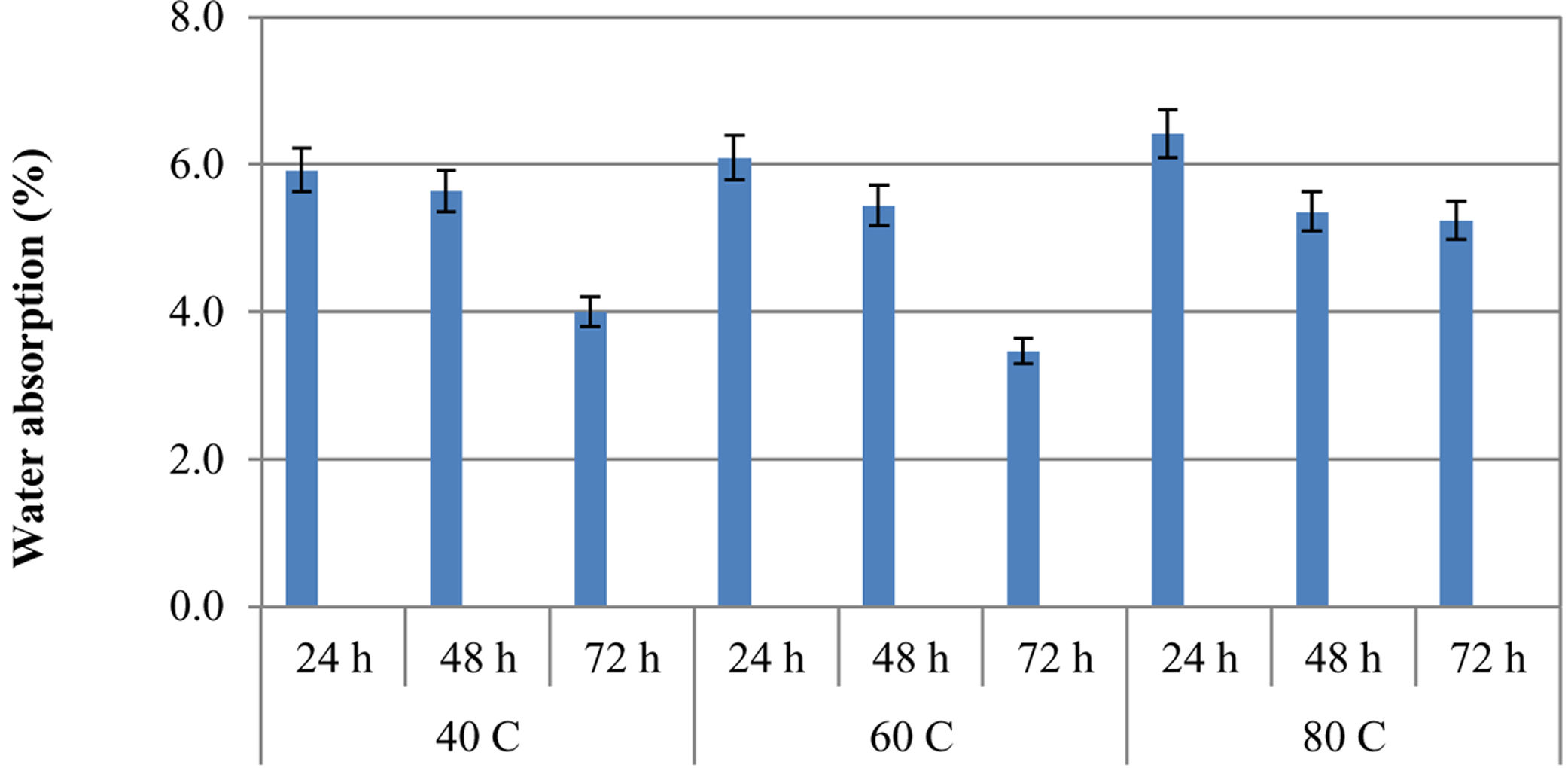
Water absorption and air void ratiosThe effect of curing conditions on the water absorption values of specimens can be noticed in Fig. 6. When the curing temperatures increased, the water absorption values of the geopolymer specimens increased. The water absorption values decreased as the curing times increased. Maximum water absorption values were found in samples cured at 80°C.
The results of specimens cured at 80°C for 24h, 48h, and 72h were 6.42%, 5.36%, and 5.24%, respectively. Considering the curing times, the minimum water absorption values were obtained in the specimens cured for 72h for all curing temperatures. The minimum water absorption value (3.47%) was examined in the specimens cured at 60°C for 72h. The water absorption values of the specimens cured for 24h, 48h, and 72h at 60°C were 6.09%, 5.44%, and 3.47%, respectively. These values for specimens cured at 40°C for 24h, 48h, and 72h were 5.92%, 5.64%, and 4%, respectively. Water absorption values for all samples were less than 7%. The water absorption percentage for concrete is expected to be around 10% at most in Turkey. Accordingly, the water absorption results obtained under all curing conditions were found to be suitable. It was found that curing times were more effective than curing temperatures in terms of water absorption values. Water absorption values decreased as the curing times increased. According to the study of Gorhan and Kurklu [32] water absorption values of specimens cured thermally at 80°C were reduced with the increasing curing time.
In another study, it was stated that the specimens cured at ambient temperature (27°C) had lower water absorption values than the specimens cured at oven temperature (60°C) [33]. Moreover, Djobo et al. [34] also declared that the water absorption of the volcanic ash-based geopolymer mortar cured at 80°C was greater than the cured specimen at 27°C. In this study, water absorption results increased with their curing temperatures, whereas these values decreased as the curing times increased. It has been observed that the water absorption rate was directly related to the porosity according to the resulting situations. The increased curing temperature increased the porosity ratio and led to an increase in the amount of water absorbed. Increasing the curing time, on the other hand, increased the geopolymerization and reduced the void ratio in the matrix structure, resulting in less water absorption. Due to this situation, the water absorption rate decreased with the curing time increase.
Samples cured at 80°C for 72h exhibited a higher compressive strength (54.5MPa) than specimens cured at 40°C (35.4MPa) and 60°C (51.2MPa). While the water absorption value of the specimens cured at 80°C for 72h was 5.24%, for the specimens cured at 40°C and 60°C for 72h, the water absorption values were 4% and 3.47%, respectively. Increasing the curing temperature accelerated the dissolution of the solid material. This has accelerated the formation of basic geopolymer bonds, namely reaction products such as Si–O and Al–O bonds, which were required in the production process of geopolymers. This increased the geopolymerization reaction and thus the strength. However, the rapidity of reactions that developed with heat energy in this way disrupted the homogeneity of the matrix structure and created a heterogeneous structure. In addition, the decrease in the diffusion coefficient with heat curing also led to this situation. Thus, heat curing not only provided rapid strength but also caused other losses in the future. Thus, the water absorption rate can be higher in heterogeneous samples with curing at 80°C [28]. Water absorption was found to be significantly affected by curing temperatures and curing times. It was also observed that specimens with high compressive strength generally had low water absorption values.
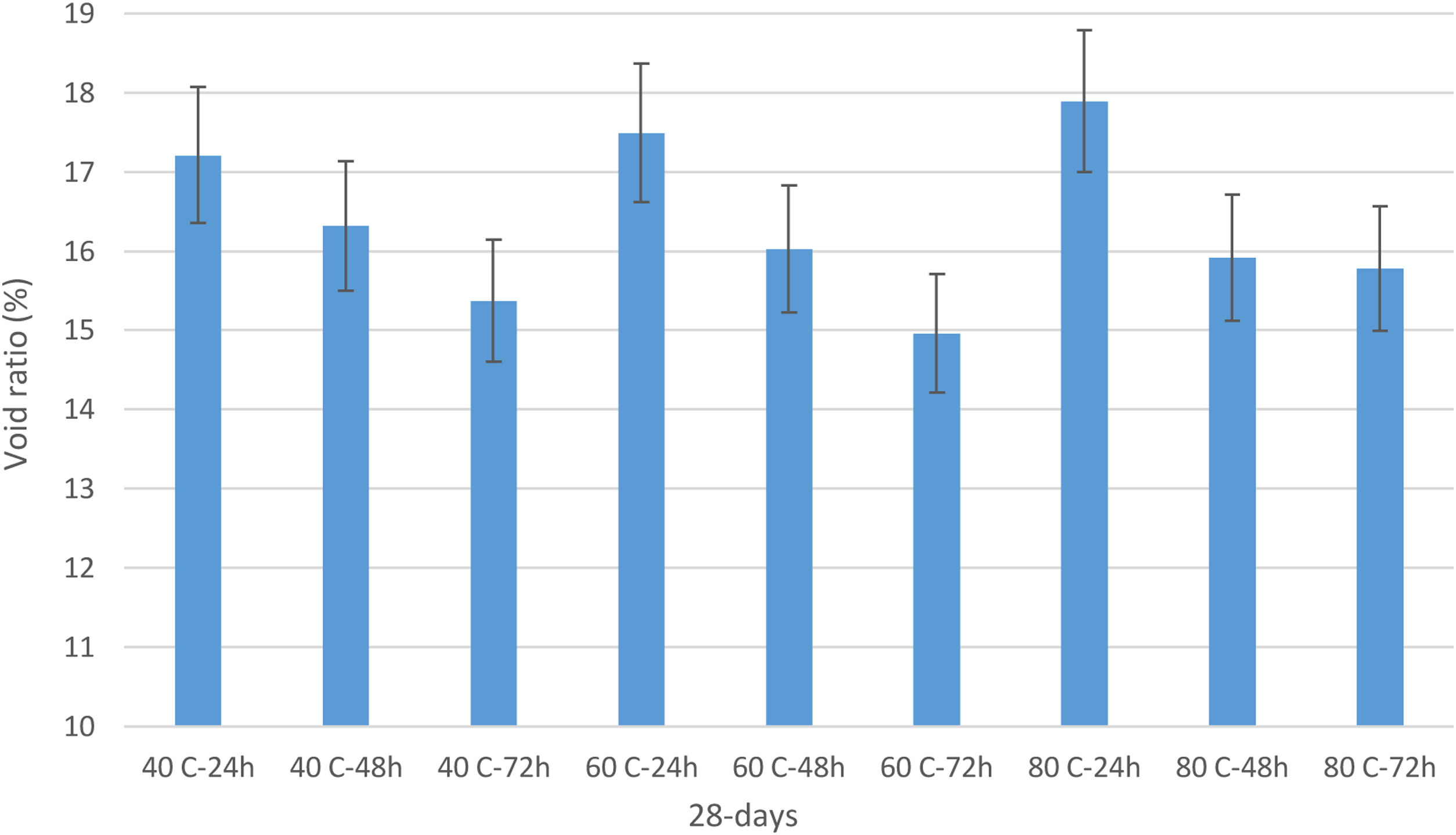
In addition, void ratio changes under the effect of curing conditions were calculated for 50mm cube samples (Fig. 7). With the increase in curing temperatures, the void ratio rates increased in parallel with the water absorption rate. Also, increasing the curing time decreased the void ratio value. The increase in void ratio under the influence of temperature was due to the transformation of the homogeneous structure in the matrix into a heterogeneous structure with rapid reactions. While rapid reactions increased the strength, the heterogeneous structure negatively affected the void ratio. Thus, the highest void ratio value was in the samples cured at 80°C [28].
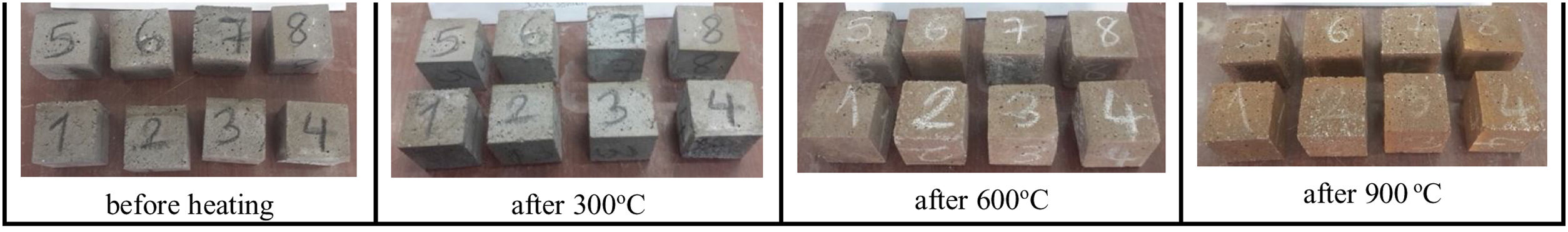
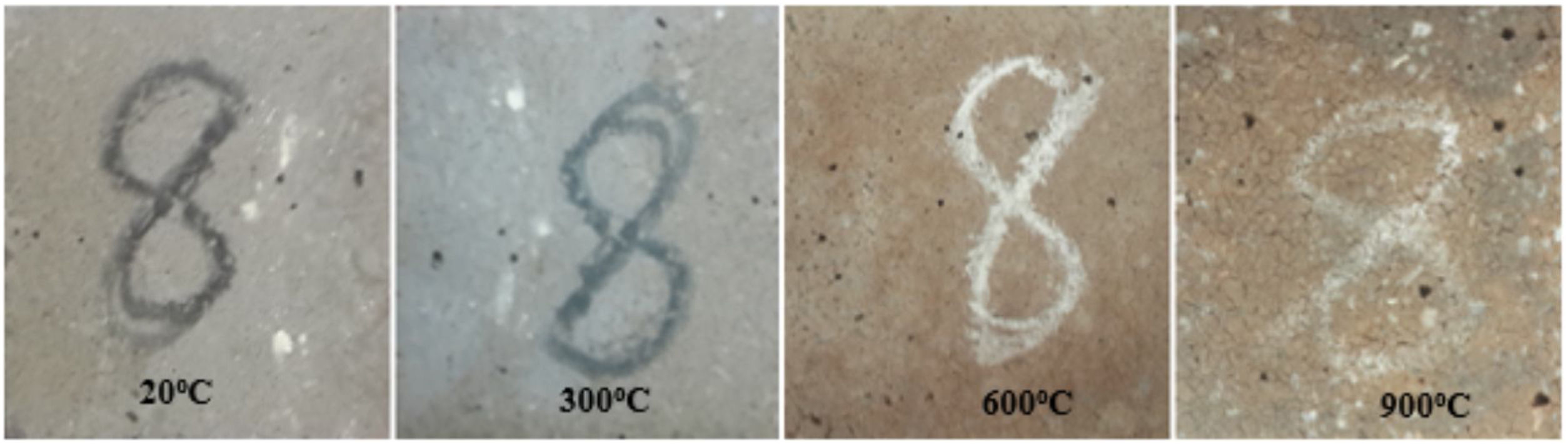
High-temperature resistanceLCFA-based geopolymer samples were subjected to elevated temperatures varying from 300°C to 900°C to determine the effect of curing conditions on the high-temperature resistance of geopolymer mortars. The visual appearance of the geopolymer samples is exhibited in Fig. 8.
It was found that the visual appearance of the samples subjected to elevated temperatures did not change and could remain structurally intact compared to the condition before exposure. Additionally, there was no visible cracking on the surface and spalling on the fly ash-based geopolymer specimens after exposure at 300°C. The specimens exposed to 300°C did not exhibit any noticeable color change. However, there was a change in color in the LCFA-based geopolymer mortar specimens subjected to 600°C and 900°C. Small cracks and discoloration were discovered on the surface of the geopolymer specimens subjected to 600°C and 900°C, but the specimens retained their shape. The specimens’ colors were found to turn reddish-brown, especially after being subjected to 900°C. This color change in geopolymer mortar can be based on the iron oxide and oxidation of iron present in fly ash at elevated temperatures [6]. As the curing temperature increased from 300°C to 600°C, small cracks started to occur on the surface of the specimens but there was not any sign of mortar spalling. Fig. 9 shows that the specimens began to crack at 600°C and the cracks became very obvious at 900°C. However, all specimens have maintained their dimensional stability. Many researchers have reported that dimensional stability and resistance to elevated temperatures depended on the binder composition, alkali percentage, and liquid-to-solid ratio [35,36]. It was concluded that geopolymer mortar specimens showed good behavior against elevated temperature according to surface visual.
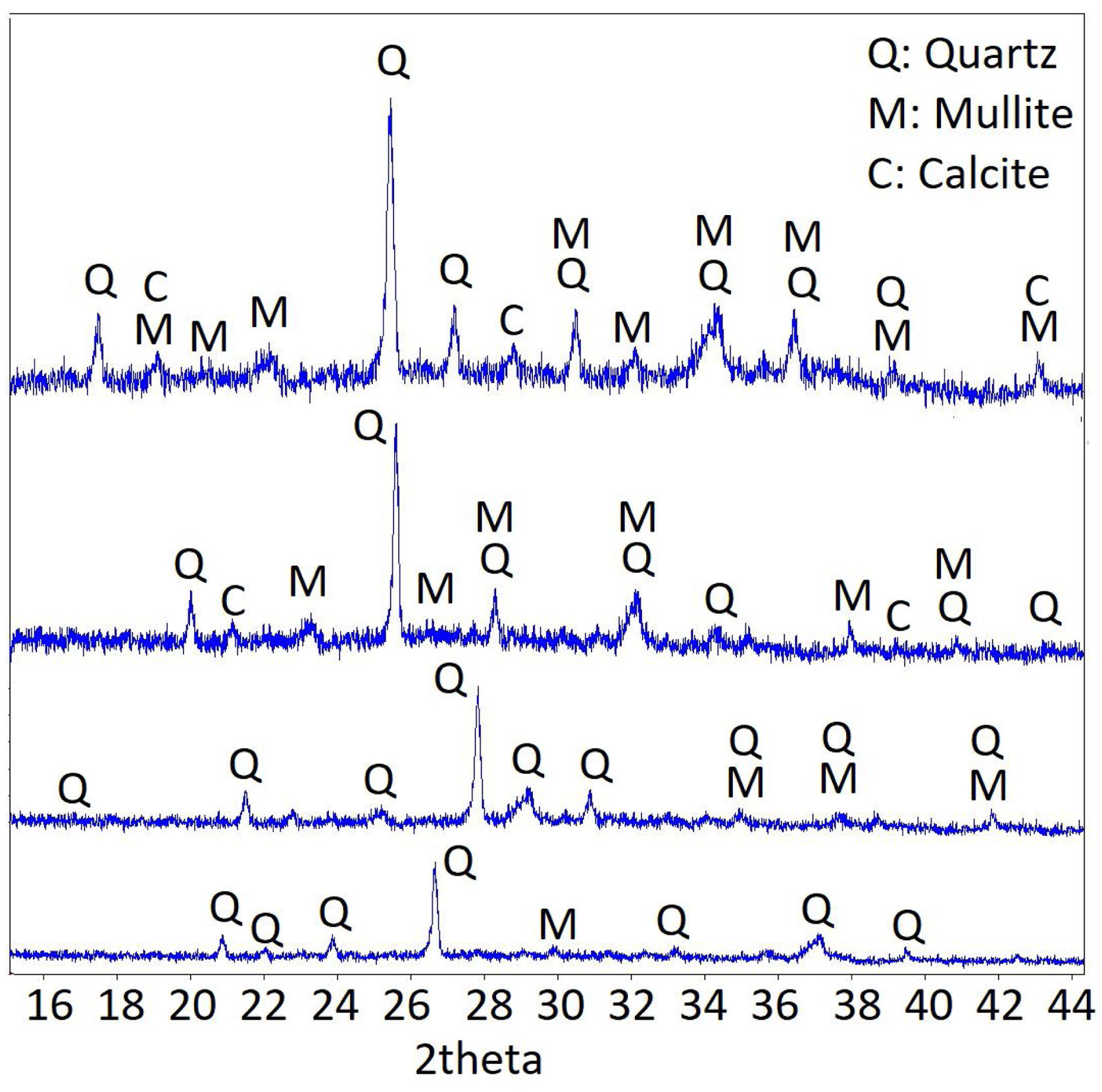
The XRD technique was used to examine the transformations of samples before and after high temperatures. The composition of the products formed as a result of matrix reactions was investigated. The XRD patterns of LCFA-based samples after 20°C, 300°C, 600°C, and 900°C are shown in Fig. 10. As a result of geopolymerization, quartz was formed as the main component together with calcite and mullite as a component. While patterns corresponding to the amorphous phase were observed, quartz peaks were observed in the 20–40° 2-theta phase ranges. When the change of the peaks after the high temperature was examined, the changes were parallel to the strength drops. When the phases were examined, it was observed that the peaks shifted to lower angles with increasing temperature. Despite this situation, it was observed that the structural stability was preserved. The obtained results also confirm the color change and visual status results [36].
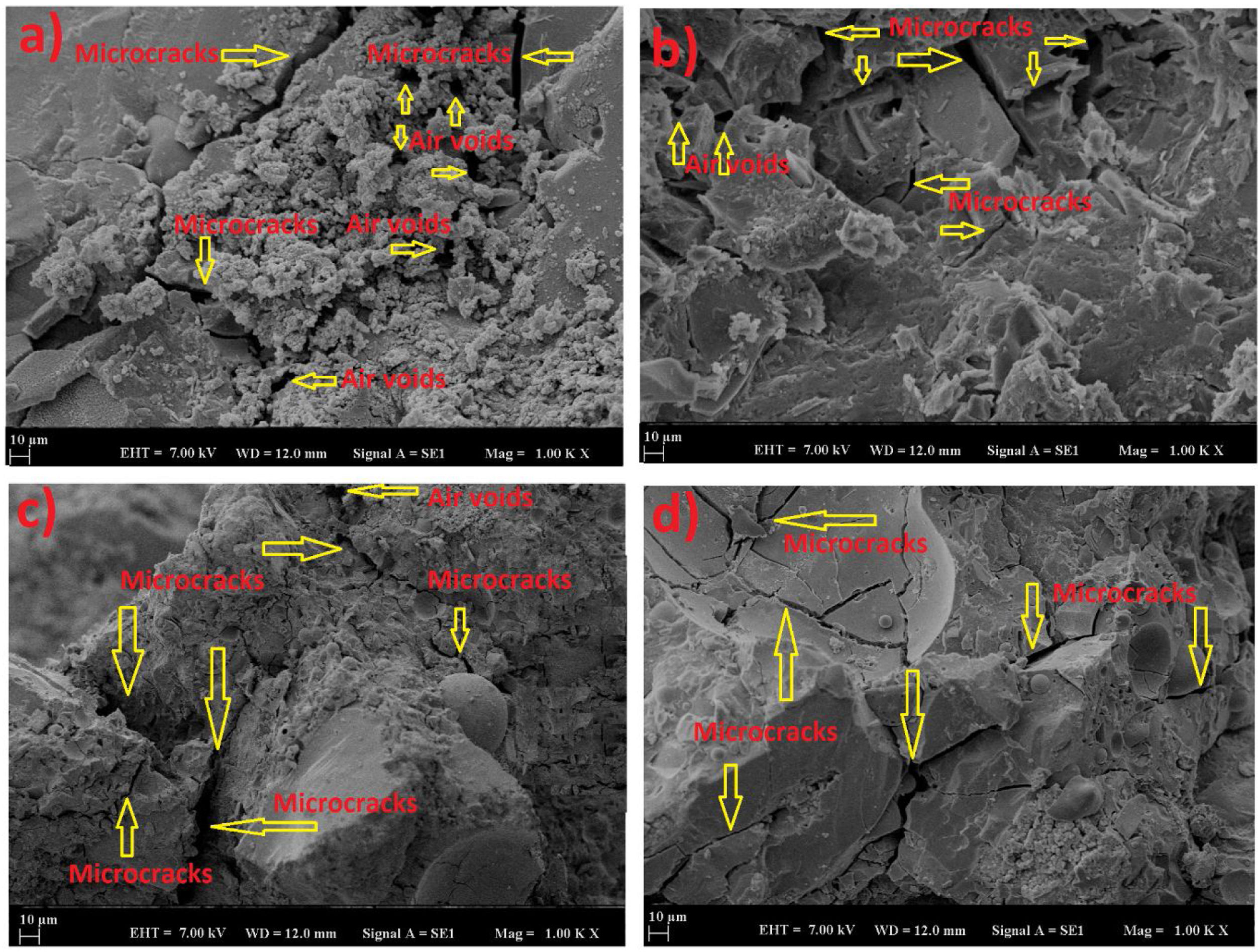
SEM images of geopolymer mortars cured at 60 and 80°C for 24 and 72h after being subjected to 600°C are given in Fig. 11. It was realized that the number of voids and cracks increased with the increasing temperature. It was observed that the microcracks progressed compared to the initial conditions. However, it was observed that the microcracks formed remained at a limited level. Geopolymer mortar exposure to 600°C had more cracks as shown in SEM images. These cracks occurred due to the decomposition of the geopolymer matrix, weight losses of samples, and increasing pressure due to the dehydration of the matrix. These conditions intensified the formation of structural defects. As the structure started to become more porous, it was observed that the overall stable structure was protected against the effects. The influence of elevated temperatures (up to 1000°C) on the geopolymer mortar was investigated at the micro level by Kuri et al. [37]. The findings obtained in the study [37] were compatible with this study. Kuri et al. [37] showed that with increasing temperature, more voids and cracks occurred in all geopolymer samples. However, despite this situation, it has been observed that geopolymer samples maintained their thermal stability due to their compact microstructure. When the images of the samples were examined, it was observed that there was less microcrack formation in the sample cured at 80°C for 72h, similar to the situation before the high-temperature test. This showed that with the advancement of geopolymerization, the sample with a stronger matrix structure exhibited more resistance to high-temperature tests [28,29].
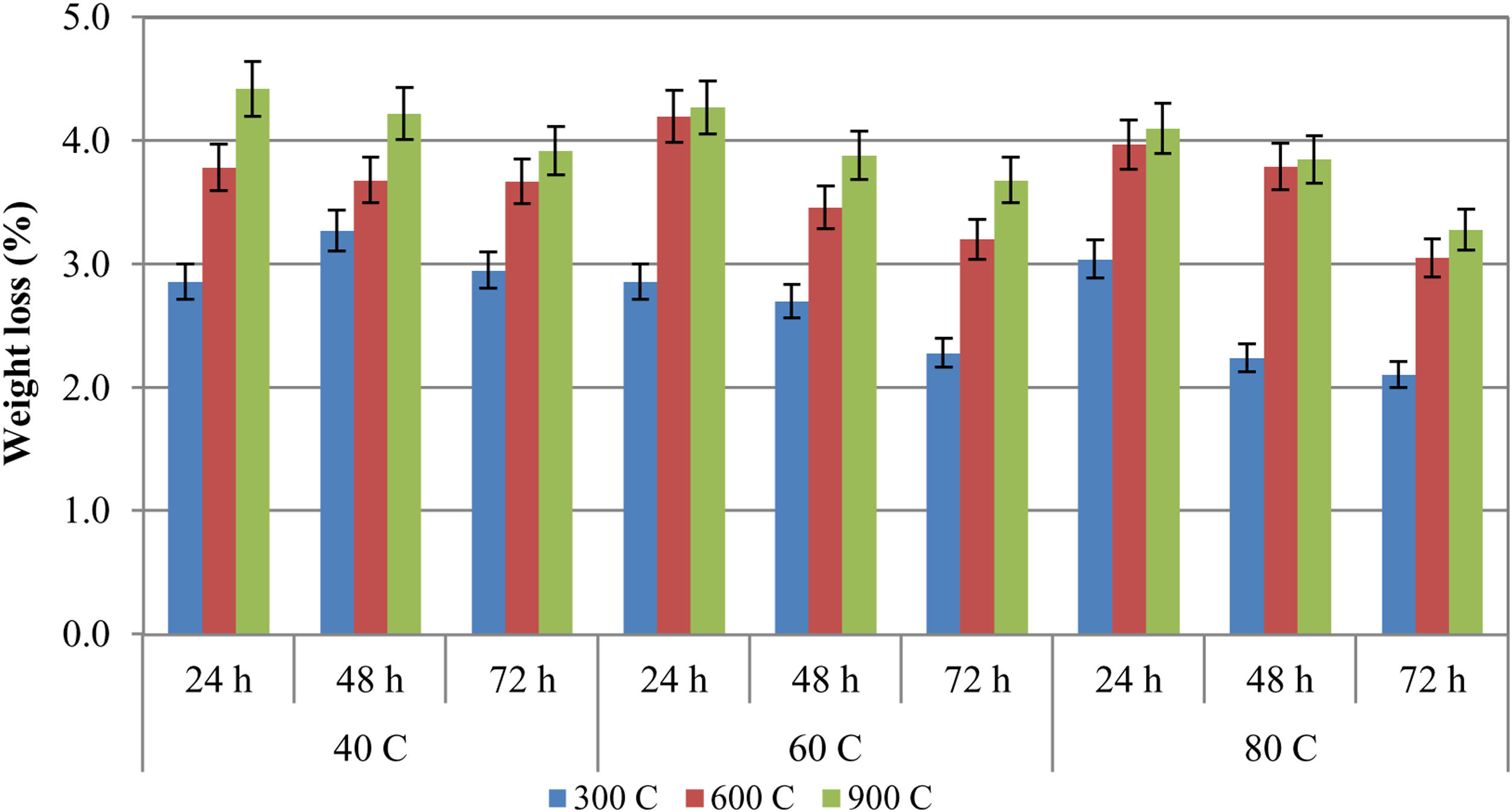
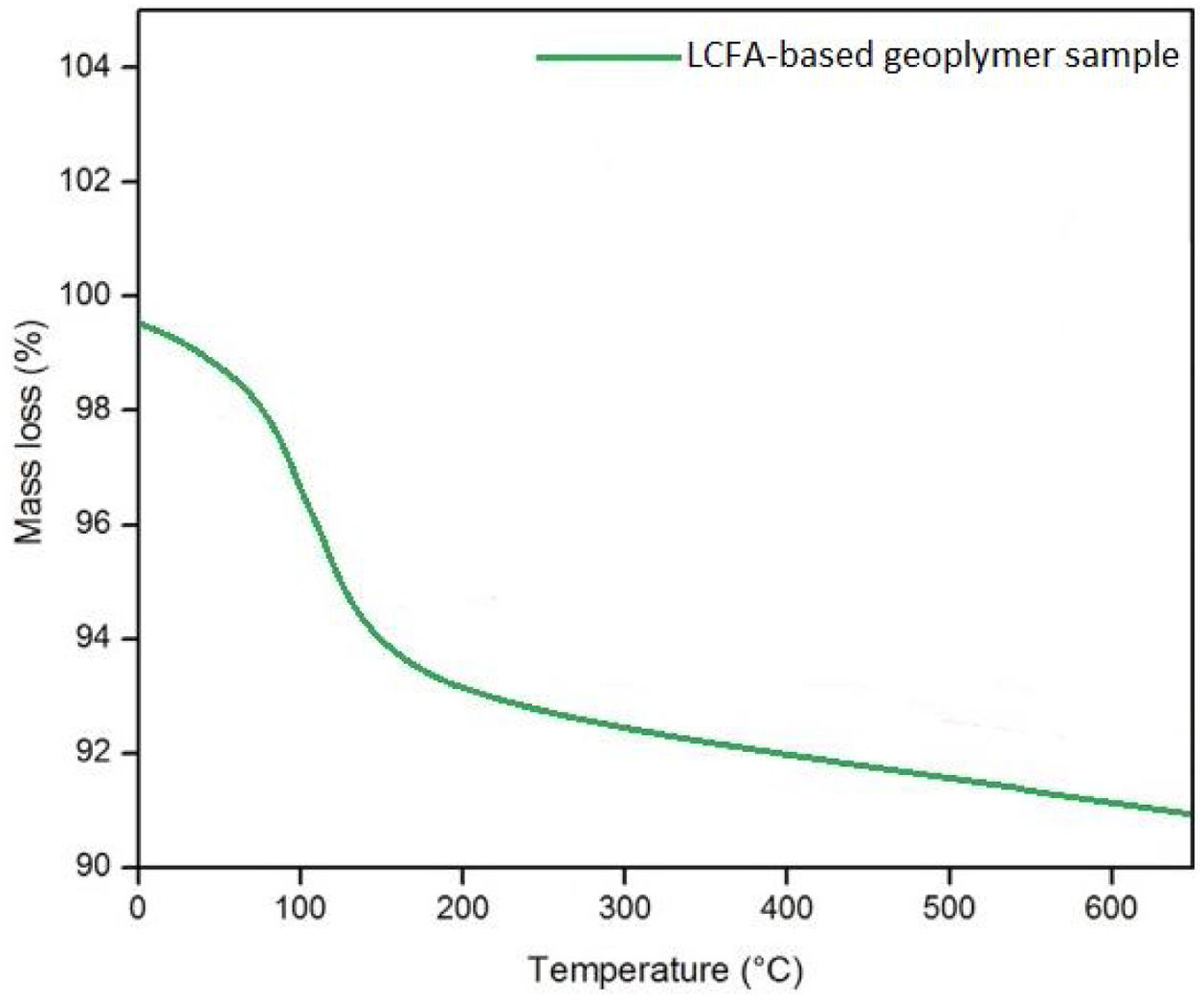
The weight losses of LCFA geopolymer mortars subjected to elevated temperatures are given in Fig. 12. It can be concluded that the percentage of weight loss increased with an increase in elevated temperatures. The lowest weight loss was examined in specimens subjected to temperatures of 300°C, and the highest weight loss was examined in specimens subjected to temperatures of 900°C. This weight loss of specimens subjected to elevated temperatures can be assigned to the dehydration process of the structure [6]. The high temperature accelerated the dehydration mechanism and provided the movement of moisture in the matrix structure to the outer surface of the sample. Thus, weight loss occurred in the internal structure. If weight loss was examined in three stages; physical water loss occurred between 30 and 210°C, deterioration of the polymer side chains occurred between 210 and 400°C, and deterioration of the main polymer chains occurred between 400 and 500°C. With these stages, weight loss increased continuously. In addition, disruption of the aggregate-matrix interface also caused weight loss [28]. To confirm these results, the TGA curves of the LCFA-based geopolymer sample in Fig. 13 are examined. When the weight losses after high temperatures were examined, it was seen that the losses were limited and more intense between 0 and 400°C. The evaporation of free and bound water in the matrix structure was the main reason for this situation. The linear decrease in weight losses continued after 500°C due to chemically bound waters of hydroxyl groups. Curing specimens at high temperatures for a long time had a notable effect on the weight losses of geopolymer mortars. It was examined that the specimens cured at high temperatures had less weight loss than the weight losses of the samples cured at lower temperatures under the effects of elevated temperatures.
Less weight loss was noted in specimens cured at high temperatures for a long time under the effects of elevated temperatures. Geopolymer specimens cured at 40°C for 24h had a higher weight loss under the influence of 900°C, while specimens cured at 80°C for 72h had less weight loss under the same temperature effect. Curing geopolymer mortar specimens at high temperatures for a long time affected the reduction of weight loss. In another study, it was stated that the weight losses of low temperature-cured geopolymers were higher than those of cement-free specimens cured at high temperatures [38]. The weight loss in LCFA-based geopolymer mortar for all temperatures was less than 5%. As a result of this test, it was determined that the weight loss of the samples varied between 2% and 5%. In another study, for fly ash-based geopolymer, an average weight reduction of 11% was recorded after 800°C of temperature exposures [39]. This weight reduction was thought to have resulted from evaporation of water loss.
Shrinkage occurred in geopolymer specimens due to weight loss when exposed to elevated temperatures. The thermal reaction mechanism and the free water evaporation in the matrix were also effective in this case. This vapor effect concept can be used to explain the strength loss in detail. After 100°C, the internal pressure increased continuously. With increasing temperature, the internal water evaporated and expanded, creating pore vapor pressure. But this situation did not disperse quickly. In addition, while the water on the surface evaporated, the water vapor in the mortar flowed into the medium with high porosity with the effect of the temperature gradient. With the increase in temperature, the inward diffusion of water vapor increased and the exchange layer between the different temperature parts in the structure became saturated with the vapor pressure. This prevented any gas flow from advancing, creating a moisture blockage in the medium. With the increase in temperature, thermal stress (created by temperature gradients with external constraint conditions) and pore pressure interacted. In this case, cracks occurred when the internal pore vapor pressure exceeded the tensile strength of the mortar. The tensile strength of the mortar showed the vapor pressure maximum limit. After this situation, the matrix structure became permeable. Thus, the resistance to thermal effects was reduced. In addition, the weakening of the aggregate-paste interface also increased thermal incompatibility. The aluminosilicate gel structure has also undergone crystallization. Exceeding the crystallization stress also disrupted the thermochemical structure of the crystal lattices. This heterogeneous situation also increased thermal incompatibility and led to the loss of strength. Despite these conditions, the compact geopolymer structure increased heat dissipation and reduced thermal cracks and fragmentation [28]. Fig. 14 gives the residual compressive strengths of specimens exposed to elevated temperatures. The compressive strength values decreased with elevated temperatures and the minimum strength loss was seen at 300°C. The compressive strength of specimens decreased significantly at 600°C and 900°C as a result of dehydration of the geopolymer matrix [40]. When the temperature rose from 300°C to 900°C, the pressure increased due to the dehydration of the binder and this pressure caused shrinkage and cracks, thereby reducing the strength. A rapid decrease in the weight of all specimens was observed after 300°C.
While the most compressive strength loss was seen at 80°C curing, the least compressive strength loss was seen at 40°C curing. The least loss of compressive strength value was observed as 10.34% in specimens (cured at 40°C for 24h) exposed to a temperature of 300°C. The maximum loss of strength of 93.2% was observed in specimens (cured at 80°C for 72h) exposed to a temperature of 900°C. The decrease in compressive strength values for all specimens was found to be less than 30% at 300°C and less than 70% at 600°C. At 900°C, geopolymer mortar specimens had a loss of strength of over 90%. According to Kaya et al. [38], specimens cured at low temperatures completed the activation that they could not complete in the previous stage when exposed to high temperatures, resulting in increased strength at elevated temperatures. Therefore, specimens produced by curing at low temperatures showed more resistance to high temperatures than specimens produced by curing at high-temperature curing. Kong et al. [39] stated that there was a 53% decrease in geopolymer paste strength and 65% in geopolymer mortar strength. They also stated that aggregates expanded by 1.5–2.5% after 800°C, causing strength loss. It can be concluded that the low-calcium fly ash geopolymer mortar can be considered a suitable material under an environment temperature below 300°C. Concerning specimens cured for a long time at high temperatures, it can be said that their compressive strength decreased when exposed to elevated temperatures. Long-term curing at high temperatures increased the contraction of reaction products as a result of excess shrinkage. This can develop shrinkage cracks in the specimens, which led to reduced strength [41]. High-strength geopolymer specimens exhibited less resistance to elevated temperatures, whereas the relatively low initial-strength geopolymer specimens exhibited more resistance. The LCFA-based geopolymer samples (cured at 40°C for 72h) showed 82.3% strength loss with a compressive strength of 35.4MPa for 28 days, while specimens (cured at 80°C for 72h) showed 93.2% strength loss with a compressive strength of 54.5MPa.
Freeze–thaw resistanceLCFA-based geopolymer mortars were exposed to 50 cycles of freezing and thawing. After the freezing–thawing test, weight and compressive strength loss were investigated, and the visual appearance of the specimens was observed to determine the effect of curing conditions.
The visual appearance of LCFA-based geopolymer samples after being exposed to 50 cycles of freezing–thawing is shown in Fig. 15. After the test, the specimens’ appearance did not change depending on the situation before exposure. Geopolymer mortar specimens manufactured by activation of low-calcium fly ash did not display any change in shape and remained structurally intact excluding visible cracks. As can be seen in Fig. 16, a slight whiteness was examined on the surfaces of the specimens cured at 40°C and 60°C for 24h after exposure to 50 cycles of freezing and thawing.
SEM analysis was performed after 50 cycles of freeze–thaw test of the samples cured for 24 and 72h at 60 and 80 °C, respectively. SEM analysis of the samples is shown in Fig. 17. The weakening of the interface between the gel structure and the fly ash spheres by the freeze–thaw effect triggered the formation of defective zones by spreading the microcracks (Fig. 17(c)). The resulting defects caused low damage due to its compact geopolymer structure. The compact structure slowed down microcracks and this has also been effective. Slow propagation of microcracks reduced the decrease in compressive strength. When the SEM analysis results were examined, it was determined that microcracks were less common in the sample cured at 80°C for 72h, similar to the situation before the freeze–thaw test. Progressive geopolymerization formed a strong matrix structure and increased the freezing resistance of the sample [28,29,42].
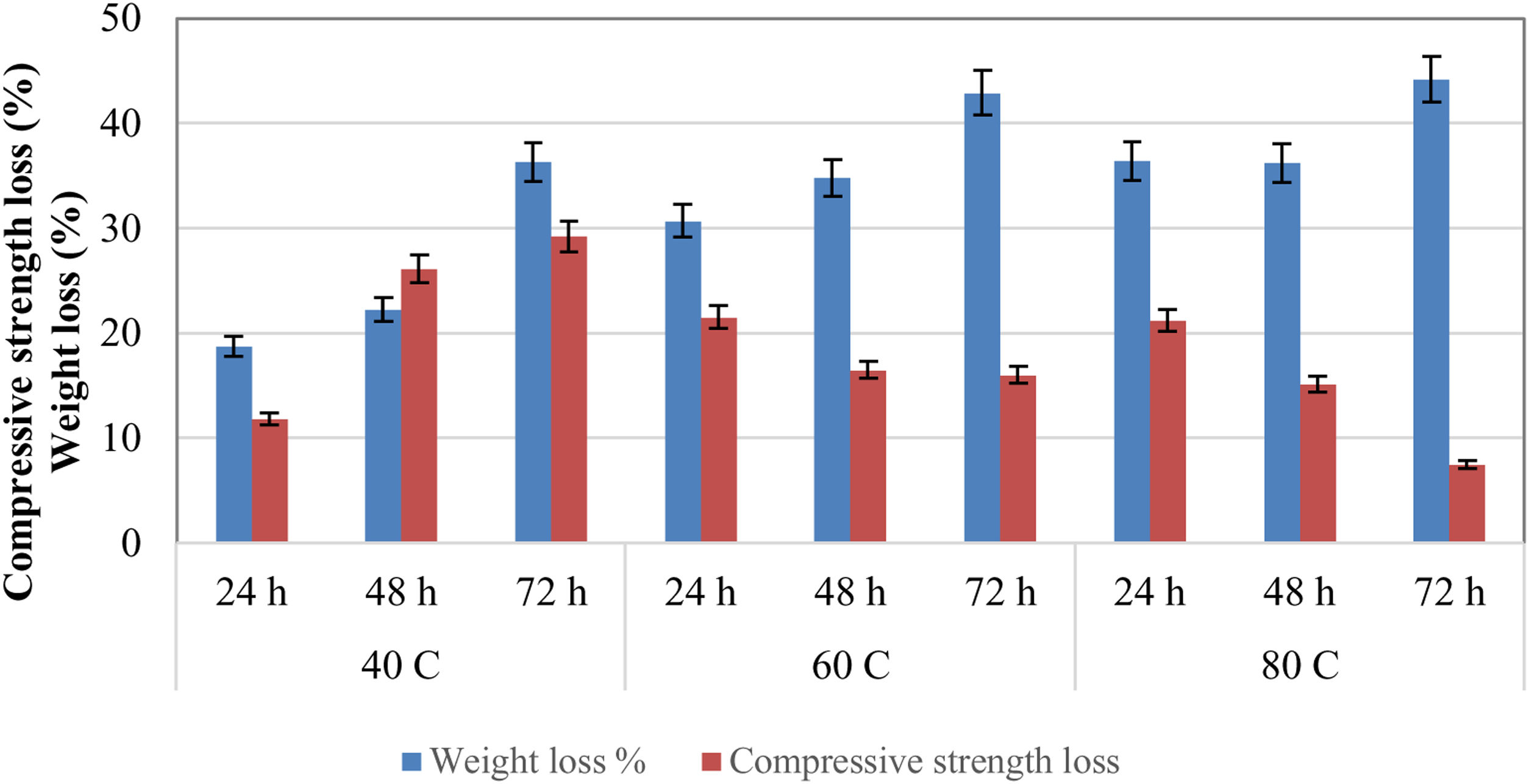
The reduction in compressive strength and weight of geopolymer specimens after 50 freeze–thaw repetitions are shown in Fig. 18. As a result of 50 cycles, the weight loss of the cured specimens at 40°C was minimal. The highest weight loss was seen in the geopolymer specimens cured at 80°C after 50 cycles of the freeze–thaw test. Weight loss was 36.3% for specimens cured at 40°C and 44.2% for specimens cured at 80°C for 72h. After the freeze–thaw test, the weight losses of the specimens cured at 80°C for 24, 48, and 72h were 36.4%, 36.2%, and 44.2%, respectively. The weight loss of geopolymer specimens cured for a long time at high temperatures was greater under the effect of freeze–thaw. Here, the increase in curing temperature caused the geopolymerization to progress by dissolving the solid materials. Due to this situation, the geopolymer matrix structure turned into a heterogeneous structure and lost its regular structure feature. This also negatively affected the porosity. These conditions increased the weight loss against the freeze–thaw effect. Due to this heterogeneous structure, a fluctuating situation was created in the correlation between weight losses and compressive strength losses especially at high curing temperatures.
Compressive strength reduction was increased at 40°C curing temperature with time. Moreover, the strength reduction cured at 60°C and 80°C was decreased. The minimum strength loss was obtained in the specimens cured at 80°C (7.5%), and the maximum strength loss was obtained in the specimens cured at 40°C (29.2%) for 72h. The specimens cured at 40°C illustrated a relatively weak durability performance against freeze–thaw cycles compared to specimens cured at 80°C. The LCFA-based geopolymer specimens showed more than 70% resistance after 50 cycles of freezing and thawing. The effect of hydraulic pressure theory was important in the strength losses that occurred after the freeze–thaw effect in geopolymer samples. Concrete or mortar samples contained pores ranging in size from nanometers to millimeters. Water with different ions partially filled these pores. When the mortar sample was exposed to freezing temperatures, ice formation began to appear and damage began to occur. Internal frost damage caused damage to the mechanism. While there was a 9% increase in the volume of water with the freezing effect, the expansion also occurred. After the volume increased, the pore water was expelled from the frozen pores. The growth rate of ice, together with the cooling level, created hydraulic pressure according to the matrix pore structure. When the resulting pressures exceeded the tensile stress of the sample, freezing damage occurred and a decrease in the compressive strength occurred [42].
In general, it was seen that geopolymer mortar specimens cured for a long time at high temperatures were able to withstand the freeze–thaw test. Geopolymer specimens can withstand freeze–thaw effects since they had high initial compressive strength. Increasing the curing temperature accelerated the dissolution of solid materials and accelerated the formation of reaction products such as Si–O and Al–O bonds, which were the main bonds in geopolymerization. This has provided an increase in strength. On the other hand, the heat energy caused the homogeneity of the matrix to deteriorate while developing the reactions. The heterogeneous structure formed in the matrix adversely affected the water absorption and porosity. However, the high initial strength also increased the resistance after the durability tests. Research on the freeze–thaw resistance of geopolymers was limited, and most of these studies were directed to determine the mechanical properties of geopolymers. Among these limited studies, the study carried out by Cai et al. [43] investigated alkali-active slag-based concrete with freeze–thaw resistance. They explained that specimens indicated excellent resistance at the end of the test and the freeze–thaw resistance coefficient was about 90%. Moreover, Degirmenci [44] studied the freezing–thawing resistance of fly ash-based geopolymer mortars to be formed in different Na2SiO3/NaOH ratios under 25 cycles. The residual compressive strength with a Na2SiO3/NaOH ratio of 2 was found as 85.47%. Geopolymer mortars can be expected to have high freeze–thaw resistance due to the compact structure of the matrix.
ConclusionIn this study, curing times, as well as curing temperatures, were found to be more effective on both early and final compressive strength, especially in the early age strength of geopolymer mortar specimens. The highest compressive strength values were examined in the geopolymer specimens cured at 80°C. When the SEM analysis results were examined, it was seen that the results were parallel to the compressive strength results.
After 900°C, a significant color change of the specimens was recorded but all geopolymer specimens remained stable under the influence of elevated temperature. Curing temperature and time were effective in reducing the weight loss of geopolymer mortars when exposed to elevated temperatures. Geopolymer mortars subjected to elevated temperatures lost a significant part of their strength values. High-temperature cured specimens showed less resistance to elevated temperatures while relatively low-cured specimens showed more resistance.
After 50 cycles of the freeze–thaw test, there was no change in the visual appearance of geopolymer mortar samples. As curing temperatures and curing times increased, the weight loss of samples subjected to the freeze–thaw test was also enhanced. The compressive strength losses decreased as the curing temperature and curing time increased after freeze–thaw. The low-calcium fly ash-based geopolymer specimens showed more than 70% resistance after 50 cycles.
Significant results were obtained in the durability conditions with the heat curing used here. While high temperature gave early strength with fast reaction (80°C), better results were obtained in durability conditions with 60°C. However, in all curing conditions, geopolymer samples remained stable under high temperatures and freeze–thaw conditions.
The application of heat curing in geopolymer production has limits in large-scale production in terms of real-world application. The most important limits created by the application of heat curing are the economic effects and the difficulty of application. Also, the activator used is an expensive product. Due to these conditions, it is mostly used in precast applications today. On the other hand, reducing CO2 emissions and using waste materials bring significant advantages. Due to these situations, it is important to increase the prevalence of curing studies in terms of solving the disadvantages. In future studies, it will be an important alternative to investigate the optimum conditions by comparing different curing conditions (electric curing, steam curing, etc.).
Conflict of interestThe authors state that there is no conflict of interest.
This work has been supported by the Balikesir University Scientific Research Projects Coordination Department [BAP. 2018/120].