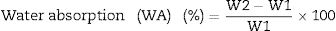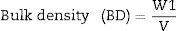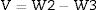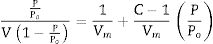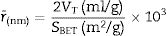This research presents the effect on the color of the green ceramic pigment CoCr2O4 when Co2+ was replaced by Mg2+. The objective is to reduce the concentration of cobalt in the pigments, which is considered an expensive and toxic raw material. The pigments were synthesized by solution combustion in one-step. The X-ray Diffraction (XRD) and Fourier Transform Infrared Spectroscopy (FTIR) of the as-prepared powders showed that the spinel structure was obtained during the combustion reaction. The microstructure of the pigments was observed by Scanning Electron Microscopy (SEM) and the powders are porous due to the gases formed during the reaction. Finally, the color change of the powders was evidenced by UV–vis–NIR Diffuse Reflectance Spectroscopy (DRS) and calculation of CIEL*a*b* chromatic coordinates. The largest color change ΔE*ab of 9.8 was between CoCr2O4 and MgCr2O4 as a result of the absence of electronic transitions in Mg2+. The results of thermal stability of the green pigments using a commercial frit showed that they could be used in ceramic decoration at 1050°C.
Esta investigación presenta el efecto en el color del pigmento cerámico verde CoCr2O4 cuando el Co2+ se sustituyó por Mg2+. El objetivo es disminuir la concentración del cobalto en los pigmentos, el cual se considera una materia prima costosa y tóxica. Los pigmentos se sintetizaron en una etapa por combustión en solución. Los patrones de difracción de rayos X (DRX) y la espectroscopía de infrarrojo (IR) de los polvos mostraron la obtención de la estructura espinela durante la reacción de combustión. La microestructura de los pigmentos se observó por microscopía electrónica de barrido (MEB) y los polvos son porosos debido a los gases formados durante la reacción. Finalmente, el cambio de color se evidenció por espectroscopía de reflectancia difusa UV-Vis y cálculo de las coordenadas cromáticas CIEL*a*b*. El mayor cambio de color ΔE*ab de 9.8 fue entre CoCr2O4 y MgCr2O4 como consecuencia de la ausencia de transiciones electrónicas en el Mg2+. Los resultados de estabilidad térmica de los pigmentos verdes usando una frita comercial mostraron que podrían ser usados en decoración cerámica a 1050°C.
Ceramic pigments are inorganic compounds that have high thermal and chemical stability to attack of molten glass and are used in ceramic decoration [1,2], classified in category A by Color Pigments Manufactures Association (CPMA) [3]. Spinels with general formula AB2O4 are a large family of inorganic compounds that have been used as magnetic materials [4], ceramic pigments [5], anode materials for lithium-ion batteries [6], catalytic materials [7]. Chromites are members of this family which have been studied in detail where CoCr2O4 and MgCr2O4 are two compositions of great interest due to their thermal stability. Cobalt chromite, CoCr2O4, has the normal spinel structure where Co2+(3d7) divalent cations are in tetrahedral oxygen coordination, while Cr3+(3d5) trivalent cations occupy the octahedral sites [4] with interest as ceramic pigment [8] and catalyst [9]. In recent years, the ceramic industry has been interested in using pigments with low concentration of cobalt [10], but the new compositions have good colorimetric properties among these as is the color change when a structure is diluted with a cation considered non-toxic and cheap as is the magnesium. Moreover, Magnesium chromite, MgCr2O4, also has the normal spinel structure with the Mg2+(2p6) divalent cations occupy the tetrahedral sites and Cr3+(3d5) trivalent cations occupy the octahedral sites [11], which is very used as a refractory material in the cement and steel industries. Therefore, it is interesting to explore the formation of a substitutional solid solution between these spinel structures to study the effect on the color when there is a substitution between Co2+ and Mg2+.
CoCr2O4 has been synthesized by various methods such as hydrothermal synthesis [12], coprecipitation technique [13], flame spray pyrolysis [8] and solution combustion [14], among others. On the other hand, MgCr2O4 has been synthesized by polyvinyl alcohol solution using microwave [15], sol–gel route [16] and solution combustion [11], among others. These different routes of synthesis show the importance of producing these spinels.
The process of solution combustion synthesis (SCS) was discovered in India when aluminum nitrate and urea were heated up to their ignition temperature obtaining alumina in a single step [17], from then until now, the method has been used to obtain simple and complex oxides [18–21] with different properties, such as: superparamagnetism on ZnFe2O4[22], electrical properties of calcium phosphates [23], optical properties of green spinel of ZnCr2O4[24], and TiO2:Pt for photocatalytic application [25]. SCS employs a self-sustaining exothermic reaction of oxidation-reduction between a fuel and an oxidant that allows obtaining high temperatures in short reaction times with a simple experimental assembly [26]. The fuel is one the most important parameters of the combustion reaction because plays an important role in controlling the temperature of reaction and therefore to obtain the phase in one-step. Urea, citric acid, and glycine are the fuels most used in the synthesis of chromites, therefore, it is interesting to use other alternative fuels with the high aim to obtain chromites in one step.
In the present paper, we report the preparation of ceramic pigments of Co1−xMgxCr2O4 (x=0.0, 0.2, 0.5, 0.7 and 1.0) for the first time a single-step using 6-aminohexanoic acid as an alternative fuel to traditional and study the effect of the substitution of Co2+ by Mg2+ on the color of CoCr2O4.
Experimental procedureSynthesis of ceramic pigmentsThe ceramic pigments Co1−xMgxCr2O4 (x=0.0, 0.2, 0.5, 0.8 and 1.0) with spinel structures were synthesized by gel-combustion in a single-step using 6-aminohexanoic acid (H2N(CH2)5CO2H, Sigma–Aldrich, 98.5% of purity) as fuel while Chromium (III) nitrate nonahydrate (Cr(NO3)3·9H2O, Panreac, 98% of purity), Cobalt (II) nitrate hexahydrate (Co(NO3)2·6H2O, Panreac, 98% of purity), and Magnesium nitrate hexahydrate (Mg(NO3)2·6H2O, Aldrich, 98% of purity) are used as oxidants. The stoichiometric equation (1) of the combustion reaction in the synthesis of CoCr2O4 and MgCr2O4 is as follows:
where M can be Co2+ or Mg2+.For obtaining 2g of CoCr2O4. First, stoichiometric amounts of Co(NO3)2·6H2O and Cr(NO3)3·9H2O were taken in a glass beaker of 1000mL and dissolved in deionized water. Then, 1.21 mole of the fuel 6-aminohexanoic acid was added to the above solution and dissolved with magnetic stirring. Afterward, the solution was slowly evaporated at 90°C until a gel was formed. Finally, the gel was slowly heated until a reaction with flame and with gases was completed in less of 60s. Similarly, the previous procedure was also used for the spinel structures of Co0.8Mg0.2Cr2O4, Co0.5Mg0.5Cr2O4, Co0.2Mg0.8Cr2O4, and MgCr2O4.
CharacterizationCrystalline structure was determined by X-ray diffraction (XRD) with a diffractometer (Model D8Controller, Bruker) equipped with a graphite monochromator using Cu Kα radiation (λ=1.54184Å), between 10° and 70° (2θ). The experiments were run with 1 and 6mm divergence and antiscattering slits, respectively, with a step size of 0.02° (2θ) and an accumulated counting time of 0.2s.
Fourier transform infrared spectrometer (FTIR, Agilent Cary 630) with attenuated total reflection (ATR) was used between 400cm−1 and 4000cm−1. On the other hand, the microstructure of the as-prepared powders was analyzed by Field Emission Scanning Electron Microscopy FESEM at 20kV (Model S-4100, Hitachi Ltd., Tokyo, Japan). The optical properties of the inorganic pigments were studied using a Jasco V-670 UV-Vis-NIR spectrophotometer with diffuse reflectance and measurement range between 200 and 1800nm with UV–vis bandwidth of 5nm and NIR bandwidth of 20nm, scan speed of 400nm/min with light source of deuterium arc lamp and Tungsten Halogen Lamp with change of source at 340nm and change of grating at 340nm. The color coordinates of the pigments were obtained in CIE 1976 L*,a*,b* color space using the CIE standard illuminant D65 and the CIE Standard observer 10°, where L* coordinate represents darkness (0) and brightness (100), while the a* (red (+)–green (−) axis) and b* (yellow(+)–blue (−)) using the ASTM E308 [27]. The color difference of the powders was calculated using Eq. (2) for chroma C*ab, Eq. (3) for hue hab, and Eq. (4) for the color difference which is defined in the standard practice for calculating of color differences D2244 [28].
CIE 1976 chroma:
CIE 1976 hue angles:
whereif astandard*bB*>aB*bstandard* then
s=1
else
s=−1
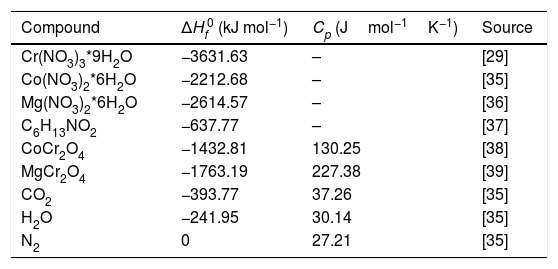
Results and discussionAdiabatic temperatures for the combustion reactions on the synthesis of CoCr2O4 and MgCr2O4Fig. 1 shows digital images of the combustion reactions of all the reactions as can be seen there is the presence of a flame permits where the least intense is for the synthesis of MgCr2O4. The heats of combustion ΔH0 for the synthesis of CoCr2O4 and MgCr2O4 were calculated using the thermodynamic data in Table 1 which can be expressed using Eq. (5)
where n is the number of moles and ΔHf0 is the standard enthalpy of formation for the reactants and products.The adiabatic flame temperatures were estimated using Eq. (6) where m is the number of moles of each product, Tad is the adiabatic flame temperature and Cp represents the specific heat of the products.
In this process, the combustion reaction occurs when the precursor mixture is dehydrated into gel above the room temperature until its ignition temperature.
The heat of the reactions are ΔH=−1761kJ and ΔH=−1689kJ for CoCr2O4 and MgCr2O4, respectively, and the adiabatic flame temperatures are 1482K and 1347K for CoCr2O4 and MgCr2O4, respectively. These results show that the high temperature during the reactions can be sufficient for the formation of the spinel phase in a single state where the temperature of the reaction when MgCr2O4 was synthesized is lower than CoCr2O4. Recently, Chamyani et al. [29] estimated adiabatic flame temperatures of the combustion reactions in the synthesis of CoCr2O4 using different fuels, and they obtained a Tad=1266K when the citric acid was used as fuel, but if the mixture of fuels citric acid and ethylenediamine was used the Tad=1412K. However, in our case when the 6-aminohexanoic acid as single fuel, the adiabatic flame temperature estimated is higher than the mixture of fuels used by Chamyani et al. On the other hand, De Andrade et al. [11] calculated the adiabatic flame temperature in the synthesis of MgCr2O4 using glycine and urea as fuels, Tad=1300K and Tad=1200K, respectively. In our case using a 6-aminohexanoic acid as single fuel, the adiabatic flame temperature is also higher than the mixture of fuel and extra-oxidant used by De Andrade et al.
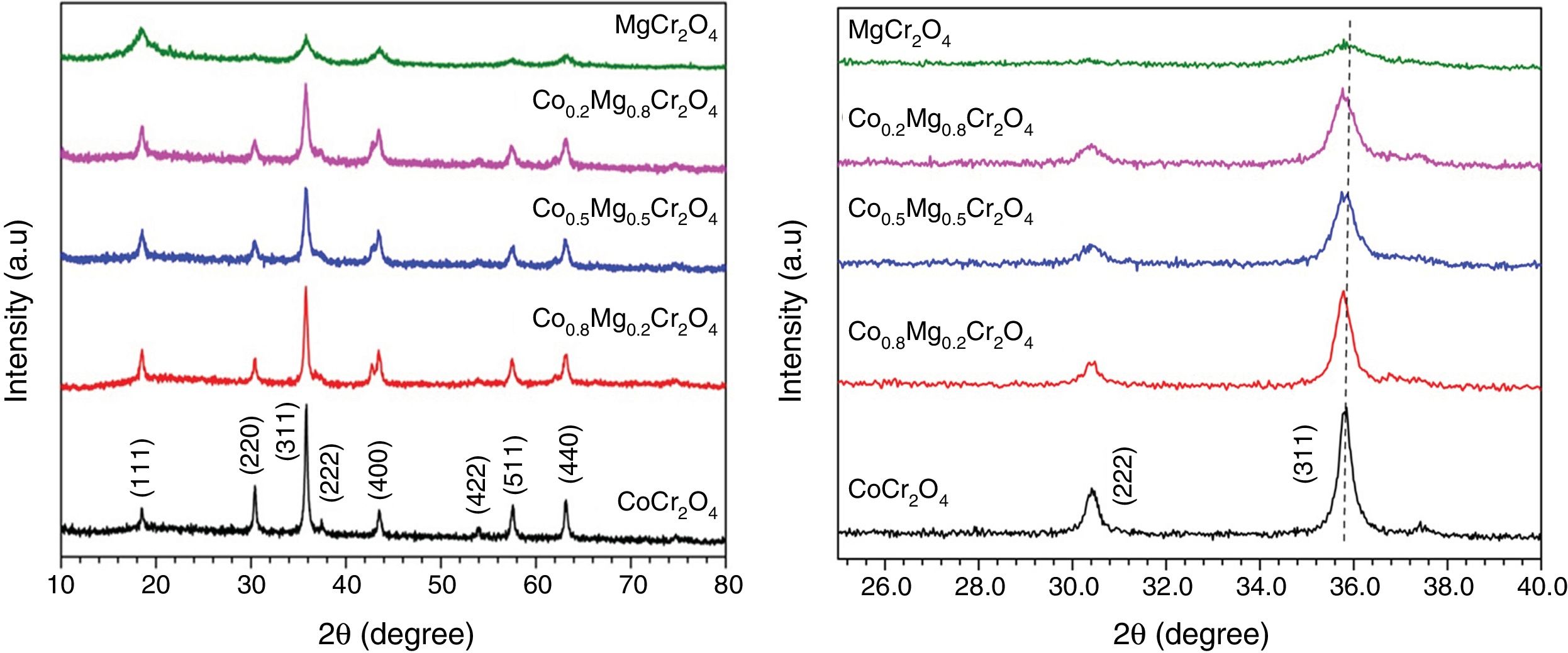
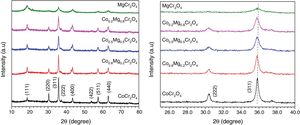
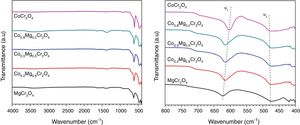
X-ray diffraction patterns and IR spectra of the ceramic pigmentsFig. 2 shows the XRD patterns of the as-prepared powders with compositions CoCr2O4, Co0.8Mg0.2Cr2O4, Co0.5Mg0.5Cr2O4, Co0.2Mg0.8Cr2O4, and MgCr2O4. The patterns obtained confirm the formation of the cubic spinel structure with Space Group Fd3m (227) and with well-defined peaks around 18.5°, 30.4°, 35.8°, 37.4°, 43.5°, 53.9°, 57.6° and 63.2° (2θ) which are respectively ascribed to the (111), (220), (311), (222), (400), (422), (511) and (440) planes of CoCr2O4 (JCPDS 22-1084) and MgCr2O4 (JCPDS 10-0351). The intensity of the main peak (311) of the as-prepared powder decreases with the increase of the concentration of Mg this is due at the different temperatures during the combustion reaction as it was shown in the calculation of flame temperature. Therefore, in the pattern of CoCr2O4 is observed that there is a higher crystallization than MgCr2O4. It is interesting to observe the slight shift to right in the peak associated with the main plane (331) in the XRD patterns with respect to the standard, this is due to differences in ionic size, The cristal radii of Co2+ in four coordination and High Spin is 72 pm, while for the Mg2+ cation in four coordination is 71 pm [30]. Using the Bragg's law, the lattice parameter was determined for CoCr2O4 and its value is 0.8314nm, whereas for MgCr2O4 was 0.8312nm which shows a slight variation when Co2+ is replaced by Mg2+, this observation is in agreement with the small difference of their crystal radii. These results agree with those reported by Hu et al. [31]. Moreover, the different ionic sizes and chemical behavior of Mg2+ and Co2+ could originate different crystallization rates for each chromite. For example, Gilabert et al. [21] found that there is a huge change in the crystallinity of the spinel as one cation is substituted by another.
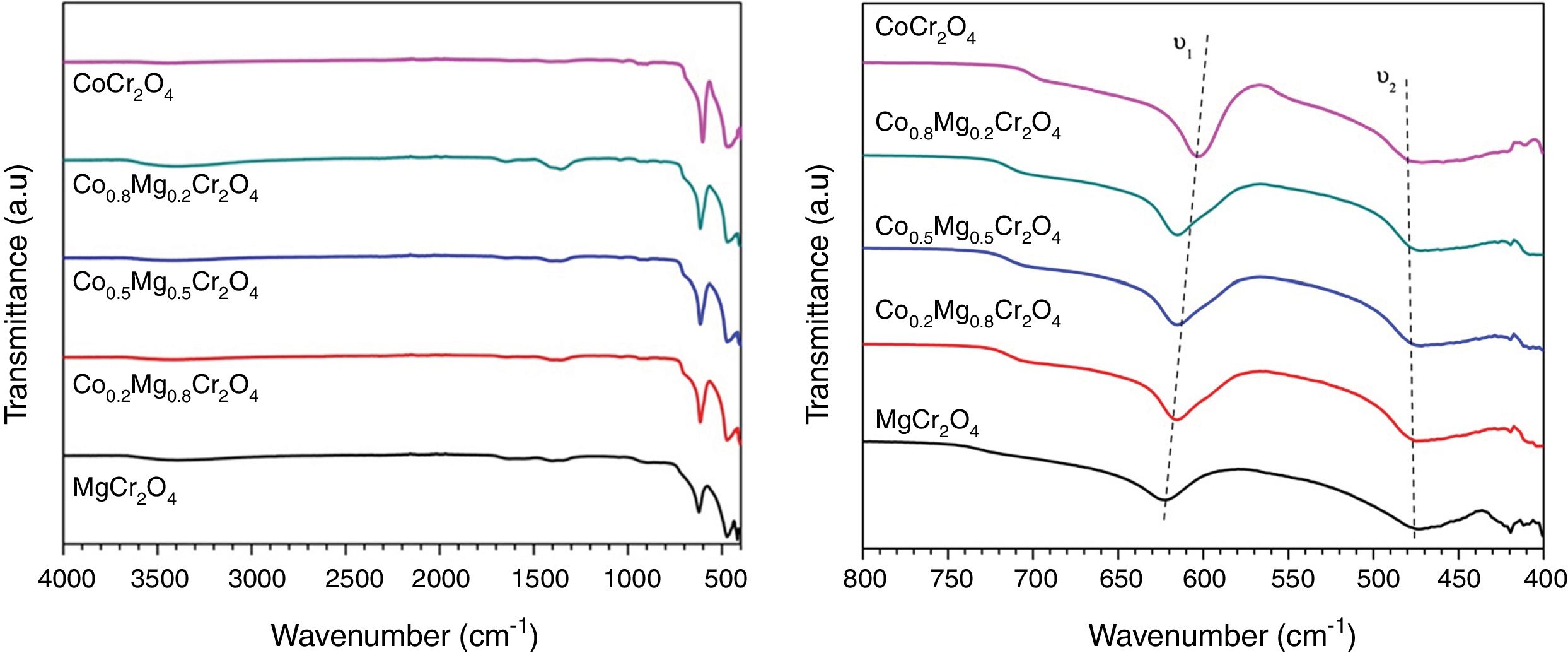
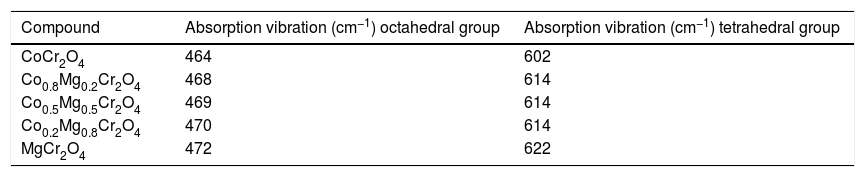
Fig. 3 shows the FTIR spectra of the as-prepared powders where the characteristic absorption bands of the structure are shown in Table 2. Kumar et al. [16] report the FTIR spectrum of MgCr2O4 and observed absorption bands at υ1=632 and υ2=430cm−1 arising from the vibration absorption of octahedral for Cr–O and tetrahedral for Co-O, respectively. On the other hand, Maczka et al. [5] obtained the FTIR spectrum of CoCr2O4 and found absorption bands at υ1=631 and υ2=493cm−1 which can be attributed to the vibration absorption of tetrahedral group CoO4 and octahedral group CrO6, respectively. All the observed vibrations have an increase in wavenumber when cobalt is replaced by magnesium which is due to differences in ionic sizes while the shift in band positions not is observed for the octahedral group CrO6, this can be associated with normal spinels synthesized. Therefore, these results confirm the formation of the spinel structure.
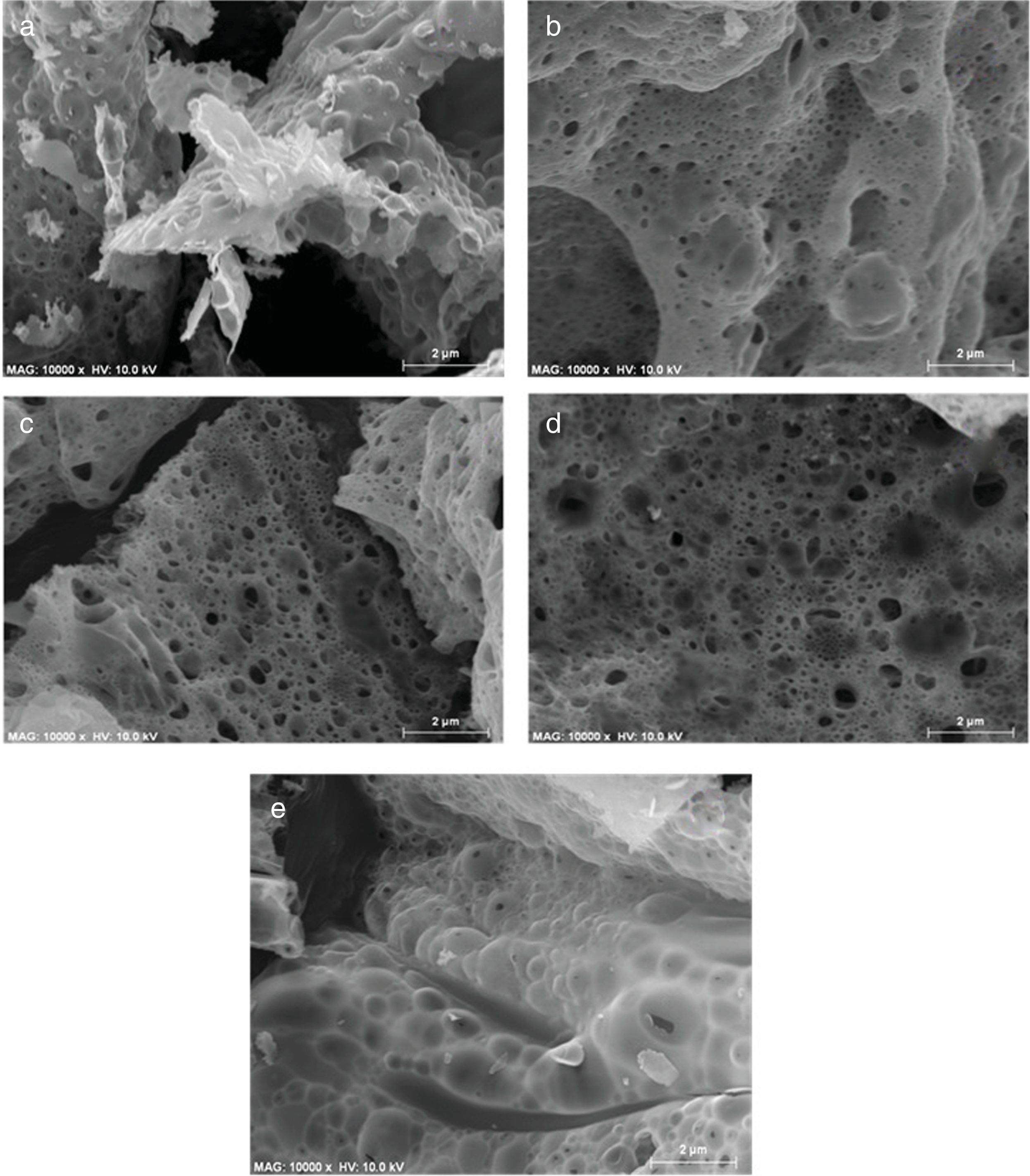
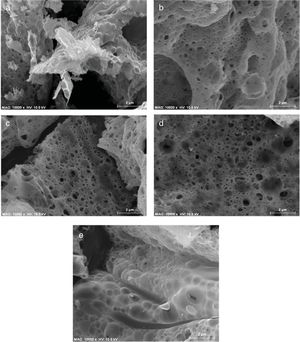
Fig. 4 shows the SEM micrographs of the as-prepared powders and is evident the formation of porous powders, which is due to the gases released during the reaction of combustion as has occurred in other combustion syntheses [26], there is also the formation of agglomerates of primary particles with irregular shape, this is a consequence of the high temperatures during the combustions. Images of SEM show that it is not possible to find an effect on the morphology with the increase of the concentration of Mg2+ in the structure.
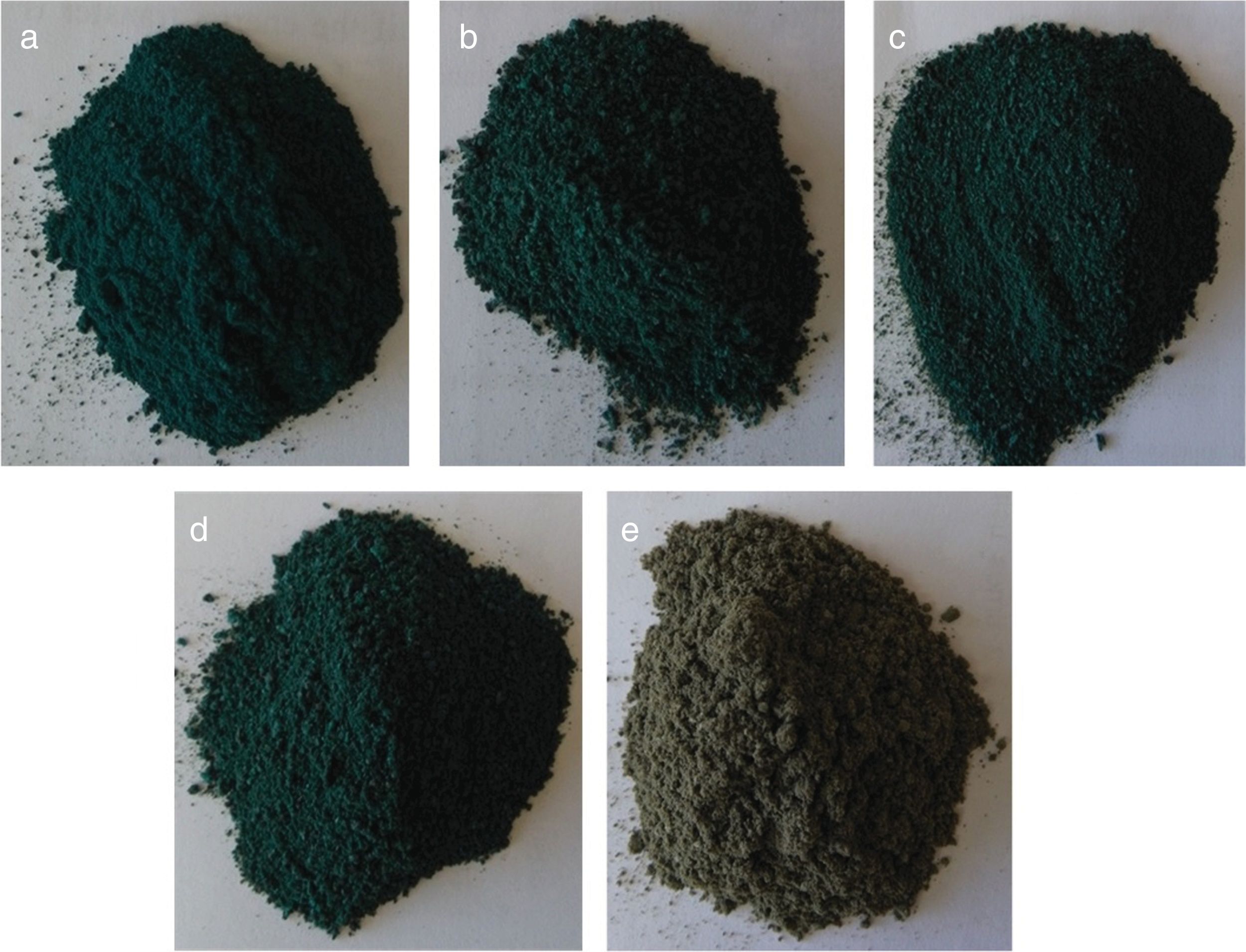
Optical absorption and color coordinates L*a*b*Fig. 5 shows the digital images of the as-prepared powders where is evident the change in the color in the powders between CoCr2O4 and MgCr2O4 due to the absorption of the visible light, because when Co2+ is present the green color is darker than with Mg2+.
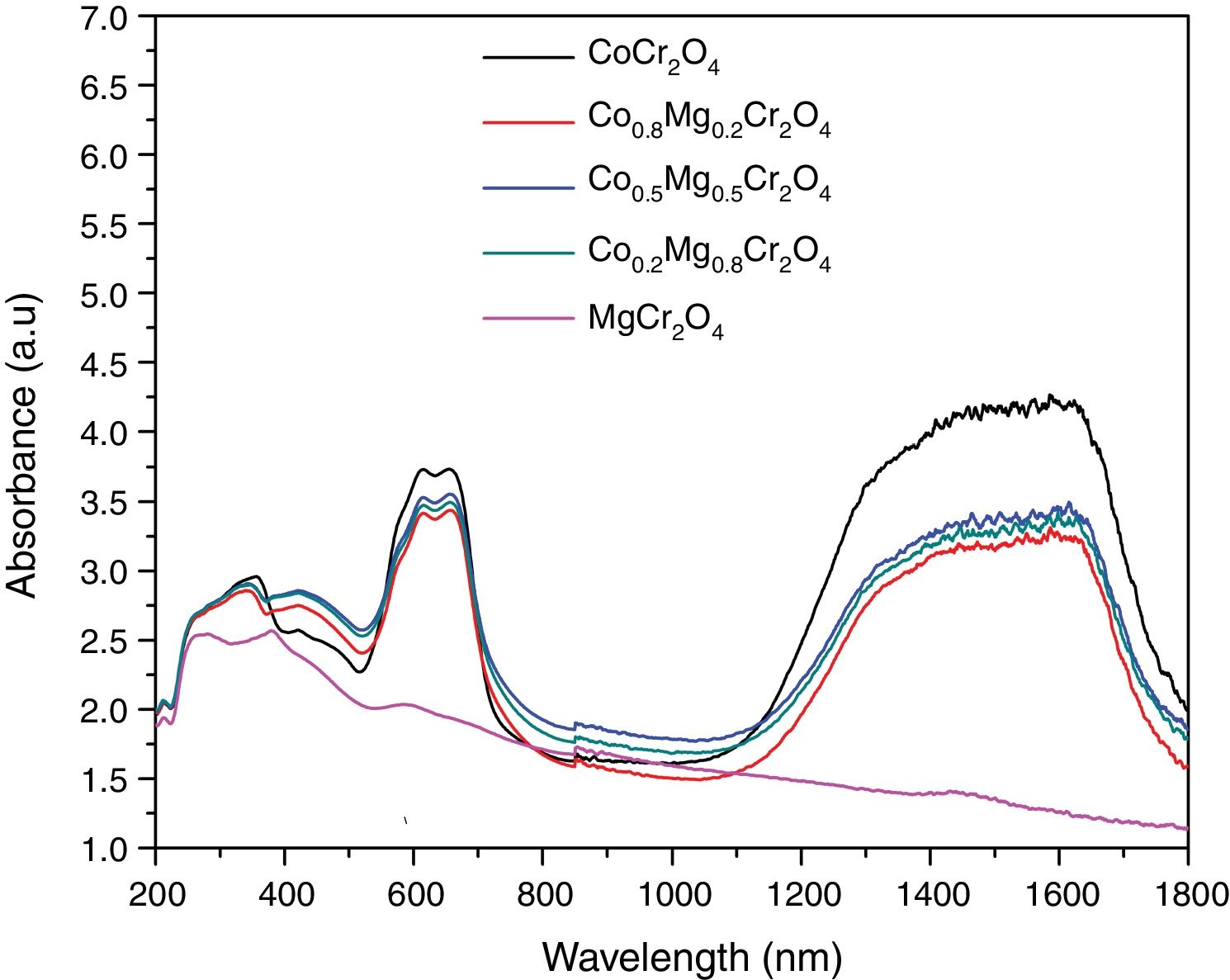
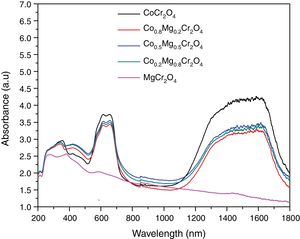
Fig. 6 shows the spectra of the as-prepared powders with compositions Co1−xMgxCr2O4 (x=0.0, 0.2, 0.5, 0.7 and 1.0) which have bands around 272, 345, 423, 609, 657 and 1560nm. These spectra are very similar to the spectra of MgCr2O4 and CoCr2O4 reported in the literature [32–34]. Therefore, the band around 272nm can be attributed to the charge-transfer transition between O2− and Cr3+[32–34]. Bands in the range 350–500nm are associated to 4A2g→4T1g and 4A2g→2T2g transitions of the octahedral coordinated Cr3+ ion [32–34]. The bands around 609 and 657nm can be assigned with 4A2(4F)→4T1(4P) transitions of the Co2+ ion in tetrahedral coordination as well as 4A2g→4T2g and 4A2g→2T1g transitions of Cr3+[32–34]. Finally, bands in the 1300–1620nm range are due to 4A2(4F)→4T1(4F) transitions of Co2+[32–34]. Spectra of the powders are very similar, but the spectrum of MgCr2O4 is different due to the absence of the electronic transitions of Co2+. Therefore, our absorption spectra show very clearly the effect of substituting the chromophore on the optical properties of the powders.
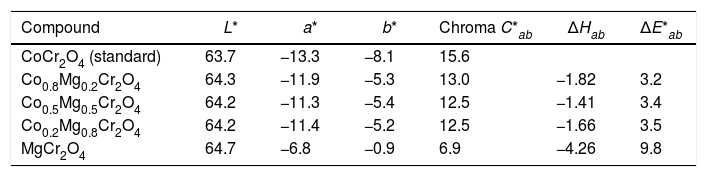
Table 3 shows the change in the color coordinates CIEL*a*b* of the as-prepared powders are green-blue where the a* and b* coordinates decrease when the Co2+ is substituted by Mg2+ which is due to the disappearance of electronic d-d transitions in Co2+. Therefore, the chroma decreases when the substitution by Mg2+ increases. On the other hand, the hue shows a change of color toward a green which is due to a decrease in the blue coordinate. However, all the powders have not changed in the L* values, which can be associated with a few influences of the morphology in the scattering of the light. The highest color change (ΔE*ab) using the as-prepared powder of CoCr2O4 as reference was observed when there is a substitution complete between Co2+ and Mg2+. Moreover, the change in the color (ΔE*ab) for the substitutions of Mg between x=0.2 and x=0.8 was 3.2 which shows that the dilution of CoCr2O4 permits to obtain green pigments without a change in the color when the cobalt is in the structure.
Color coordinates CIEL*a*b* for the ceramic pigments.
| Compound | L* | a* | b* | Chroma C*ab | ΔHab | ΔE*ab |
|---|---|---|---|---|---|---|
| CoCr2O4 (standard) | 63.7 | −13.3 | −8.1 | 15.6 | ||
| Co0.8Mg0.2Cr2O4 | 64.3 | −11.9 | −5.3 | 13.0 | −1.82 | 3.2 |
| Co0.5Mg0.5Cr2O4 | 64.2 | −11.3 | −5.4 | 12.5 | −1.41 | 3.4 |
| Co0.2Mg0.8Cr2O4 | 64.2 | −11.4 | −5.2 | 12.5 | −1.66 | 3.5 |
| MgCr2O4 | 64.7 | −6.8 | −0.9 | 6.9 | −4.26 | 9.8 |
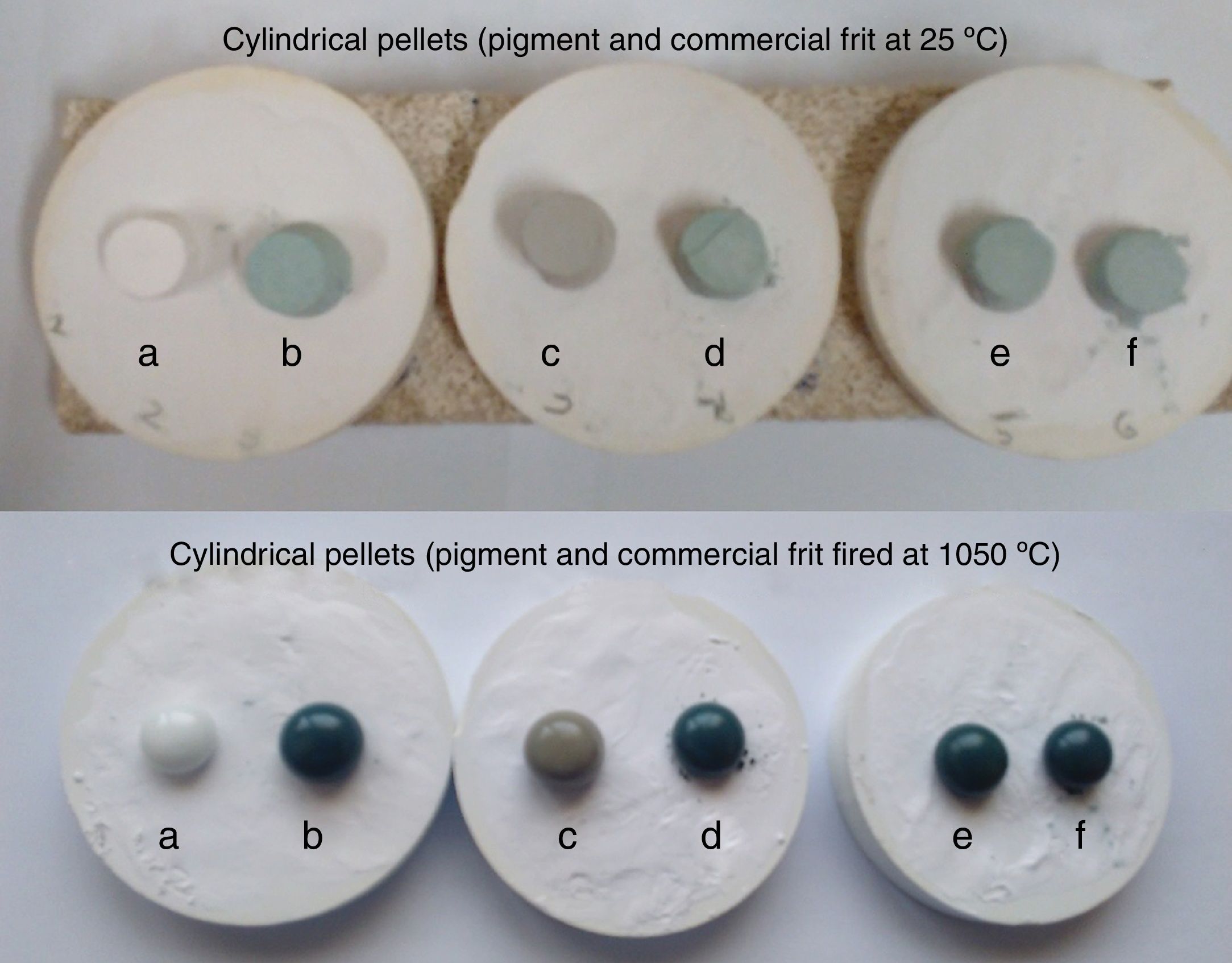
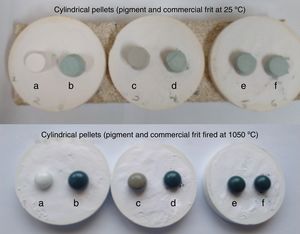
The thermal stability of the pigments prepared by solution combustion was evaluated in a commercial frit as is shown in Fig. 7. The pigments were mixed with an industrial CaO–ZnO–SiO2 frit (5wt% of the pigment) and cylindrical pellets have been made and after drying the samples were fired at 1050°C for 5min. The heating rate up to 1050°C has been 10°Cmin−1. As can be seen good thermal and chemical stability into the frit was obtained in all samples and the pigments had green color after glazing. Therefore, the pigments can be classified in the category A of CPMA which is important in the production of inorganic pigments with a lower concentration of cobalt for applications in ceramic decoration.
ConclusionsCeramic pigments with compositions Co1−xMgxCr2O4 (x=0.0, 0.2, 0.5, 0.7 and 1.0) were synthesized by gel combustion in one-step using 6-aminohexanoic acid. The adiabatic flame temperatures are 1482K and 1347K for CoCr2O4 and MgCr2O4, respectively, which is important for the control of crystallization during the reaction. Moreover, the micrographs in SEM showed agglomerated particles and porosity in the powders. On the other hand, the effect of substituting Co2+ by Mg2+ change of the color on the powders with the highest color change (ΔE*ab) using the as-prepared powder of CoCr2O4 as reference was observed when there is a substitution complete between Co2+ and Mg2+. Furthermore, these results show the possibility of using gel-combustion in the synthesis of ceramic pigments. The results of thermal stability of the green pigments using a commercial frit showed that they could be used in ceramic decoration at 1050°C.
Authors are very grateful to Colciencias - Colombia. Project “Fellowship for National Doctorates calls 647” for Doctoral Training Award to E. A. Chavarriaga. Fellowship Jóvenes Investigadores 2017 - Universidad de Valencia, Spain.