The use of wastes as substitute to traditional materials from non-renewable sources has recently increased as a result of growing interest in environmental preservation and solid wastes management. In this work, the effect of waste glasses on the physico-mechanical properties of soft porcelain is investigated. Soft porcelain bodies containing milled waste soda-lime and borosilicate glasses at varied percentage were composed and were uniaxially dry pressed under 40kN load using hydraulic pressing machine. The pressed bodies were oven dried at 110°C and were later subjected to firing at temperatures of 1000°C and 1200°C respectively. Tests such as water absorption, porosity, bulk density and firing shrinkage were used for physical characterization while flexural strength and wear loss was used for mechanical characterization under standard approach to investigate the behavior of the developed soft porcelain bodies at temperatures of 1000°C and 1200°C respectively. The results showed that higher densification was observed at 1200°C while water absorption drastically reduced below the required maximum 1% and porosity fall below 4% for both samples containing waste soda-lime and borosilicate glasses respectively. Improvements in terms of wear loss and strength properties were also observed for samples containing waste soda-lime and borosilicate glasses respectively subjected to temperature of 1200°C. This better behavior observed at temperature of 1200°C can be attributed to better glassy phase formation and mullite growth at that temperature.
El uso de desechos como sustitutos de los materiales tradicionales de fuentes no renovables ha aumentado recientemente como resultado del creciente interés en la preservación del medio ambiente y el tratamiento de desechos sólidos. En este artículo se analiza el efecto de los vidrios de desecho en las propiedades físico-mecánicas de la porcelana blanda. Los cuerpos de porcelana blanda que contenían residuos molidos de vidrio cálico-sódico y vidrio borosilicatado en un porcentaje variable se compusieron y se prensaron uniaxialmente en seco bajo una carga de 40kN con una máquina de prensado hidráulico. Los cuerpos prensados se secaron al horno a 110°C y luego se sometieron a cocción a temperaturas de 1.000 y 1.200°C, respectivamente. Se utilizaron pruebas como absorción de agua, porosidad, densidad de masa y contracción por cocción para la caracterización física mientras que la resistencia a la flexión y la pérdida por desgaste se utilizaron para la caracterización mecánica con un enfoque estándar para analizar el comportamiento de los cuerpos de porcelana blanda elaborados a temperaturas de 1.000 y 1.200°C, respectivamente. Los resultados mostraron que se observó mayor densificación a 1.200°C mientras que la absorción de agua se redujo drásticamente por debajo del máximo requerido del 1% y la porosidad cayó por debajo del 4% en ambas muestras que contenían residuos de vidrio cálico-sódico y vidrio borosilicatado, respectivamente. También se observaron mejoras en términos de pérdida por desgaste y propiedades de resistencia en muestras que contenían residuos de vidrio de soda-lima y borosilicato, respectivamente, sometidas a una temperatura de 1.200°C. Este mejor comportamiento, que se observó a una temperatura de 1.200°C, puede atribuirse a una mejor formación de fase vítrea y crecimiento de mullita a esa temperatura.
The most important development in the history of ceramics centuries ago was the production of a vitrified and translucent porcelain body in China [1]. In present times, the production of porcelain has widely spread out in many countries and its technical know-how is well known and discussed in literatures. The term porcelain if often referred to a variety of vitreous and almost vitreous wares, but it is more limited to translucent vitreous wares [2]. Traditional porcelain formulations usually comprise of 25wt.% plastic component (clay), 25wt.% filler (silica) and 50wt.% flux (sodium feldspar) for soft porcelain and 50wt.% clay, 25wt.% silica and 25wt.% flux (potassium feldspar) for hard porcelain [3,4]. Fired bodies comprising of these three components give a high strength performing porcelain with low porosity, chemical resistant and grain bond microstructure, containing large grain or filler which is usually quartz held together by a finer matrix of mullite crystals and a glassy phase [5,6]. Today, triaxial porcelain is one of the widely studied ceramic systems with several applications in insulators, stoneware and whitewares. Extensive work on porcelain showed its complicated nature, with significant challenges in understanding porcelain in connection to raw materials, processing technology, phase and microstructure evolution [7–9]. As a result, the raw materials normally used for the porcelain body formulation is divided into three major mineral groups with each performing its respective function: the cay materials enhance body plasticity, while the complementary non-plastic materials enhance melting characteristic (flux) and impart structure (filler) [2].
Demands of ceramics wares are increasing on daily basis and the researchers are becoming keen in developing ceramics products with high mechanical strength for household uses as well as for decorating purposes. Usually ceramic decorative ware are manufactured by using clay, quartz and feldspar but presently waste materials such as waste glass powder are used to make them. This waste glass is able to replace the traditional fluxing agents like feldspar without changing the process and quality of the final products. Ceramic products are manufactured using high amount of fluxing agents like sodium and potassium, feldspar, nepheline, talc and ceramics frits [10].
In Nigeria, over 32 million tons of solid wastes are annually generated out of which 20–30% is collected while waste glasses and agro-wastes account for over 70% of these solid wastes. Hence, importance has now been placed on the recycling and valorization of by-products and all manner of wastes emanating from industrial and urban areas to harness them into useful and marketable products at lower cost of production.
Recently, as a result of growing concern in environmental preservation the utilization of waste as an alternative to conventional materials from non-renewable sources has greatly increased and these include wastes from mineral processing, industrial wastes, recycling of foundry sands and waste glasses [11–13]. Several researchers have studied and reported on the utilization of waste glasses in industrial products and have proven that such wastes can serve as potential alternative to the conventional raw materials [14]. Evaluated the introduction of recycled glass from discarded bottles as substitute to part of sand associated with the clay-like material in the production of ceramic pieces. They observed excellent results due to the positive effects of recycled glass introduced into the ceramic pieces in terms of apparent porosity, water absorption and apparent shrinkage particularly when a 10% recycled glass powder was incorporated into the ceramic mass. Suitability of crushed waste glass powder as a partial cement replacement in mortar has also been studied by [15]; it was observed that the introduction of waste glass powder improves the resistance to sulphate attack far higher than silica fume without undermining the strength. Investigation has also been conducted on the effects of soda-lime waste glass from the recovery of container glass cullet as partial alternative to Na-feldspar for the production of ceramic sanitary ware [16]; the results showed that mullite growth reaction was accelerated for the glass substituted feldspar from 30 to 50wt.% while energy consumption was reduced [17]. Also investigated on the feasibility of incorporating soda-lime glass sourced from the glass lapidary process in the production of red ceramic products. They reported that the use of residue glass contributed to the acceleration of densification process during firing while glassy phase formation was facilitated and the production of red ceramic with appropriate technological properties was enhanced. The utilization of waste glass in porcelain has also been investigated [12]; it was observed that glass powder porcelain showed improvement in bulk density, lower shrinkage and water absorption while strength was improved as compared with traditional porcelain.
In this present work, the influence of waste glasses (soda-lime and borosilicate glasses) on the physico-mechanical behavior of soft porcelain ceramics was investigated. Technical parameters like water absorption, porosity, shrinkage, flexural strength, wear loss, bulk density and microstructural evaluation were determined.
Material and methodsMaterialThe raw materials used in this present investigation for the porcelain preparation are Kaolin, Ball clay, Flint and Whitening while soda-lime and borosilicate waste glasses were introduced as sole replacement for feldspar, part of quartz sand and clay. All these materials were locally sourced from different deposits in Nigeria while soda-lime and borosilicate waste glasses were obtained from a Glass Pilot Plant. All the above materials were first crushed, ground and further milled using ball mill and finally sieved to obtain an average size particles <150μm for each material prior to further use. The elemental analysis of the waste soda-lime and borosilicate glasses was based on their industrial typical compositions while the mineralogical composition of the clays were examined by using the BRUKER AXS with D8 Advance diffractometer Cu Kα radiation XRD in the range of 2theta 10–90.
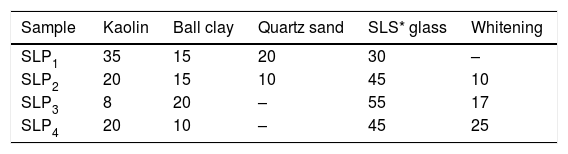
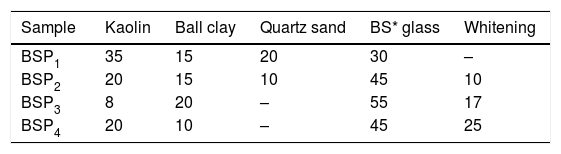
Batch formulation/sample designationEight different batch compositions were formulated from the raw materials in this work. Four batch compositions for porcelain body containing waste soda-lime glass while the other four batch compositions is for porcelain body containing waste borosilicate glass. The blends percentage composition and their sample designations are shown in Tables 1 and 2.
Various batches for each body formulated were dry mixed and moistened with 5% H2O of the total weight mix to enhance bindability during pressing. Samples were then prepared using the uniaxial pressing technique in a 50mm×50mm×10mm rectangular die at 40kN. The compressed samples were first air-dried for 24h followed by complete drying in oven at 110°C for 12h. The samples were then placed in the kiln for sintering. The samples were sintered at two different temperatures of 1000°C and 1200°C respectively. The samples were left till the following day in order to soak and cool before offloading them and the soaking time was from 1h to 2h.
Characterization of the samplesIn order to characterize the fired samples, different tests were carried out using standard procedures. Water absorption was measured according to ASTM D570 in which the dry weight of the sample is subtracted from the saturated weight divided by the dry weight; while percentage porosity of the fired samples was determined according to ASTM C373 as stated by [18] using boiling method. The bulk density was also determined in accordance with [18] in which samples of dimension 50mm×50mm×10mm was prepared from the fired samples, oven dried at 110°C, and transferred into a beaker containing distilled water to boil for 2h in order to assist in releasing entrapped air. It was then allowed to soak and the saturated weight void of excess water was taken. The firing shrinkage of the fired samples was carried out by measuring the dimensional changes that occurred between the dried samples at 110°C and the fired samples at 1000°C and 1200°C respectively according to [19]. The flexural strength was carried out using a Universal Testing Machine (Instron 3369, 50kN load capacity) with strain/load rate of 5mm/min. Each sample was placed on the two supporting rods of the machine, in such a way that the projecting ribs of the sample had equal distance from the two supporting rods. Load was applied on the sample in such a way as to obtain a rate of increase of stress of 0.2N/mm per sec. Load was applied on the sample until fractured and the force at the point of fracture was noted. The rate of wear was studied on the developed soft porcelain samples in accordance with [20] as stated by ASTM G-65 guideline inside a ball mill with grinding balls acting as grinding media at a speed of 60 revolutions per minute for 3min. The effects of collision forces with the introduced weight help in bringing about abrasive/wear effects. The microstructure characteristics were examined on the sintered samples using Scanning Electron Microscopy (FEGSEM). The samples were initially coated with gold particle prior to SEM examination.
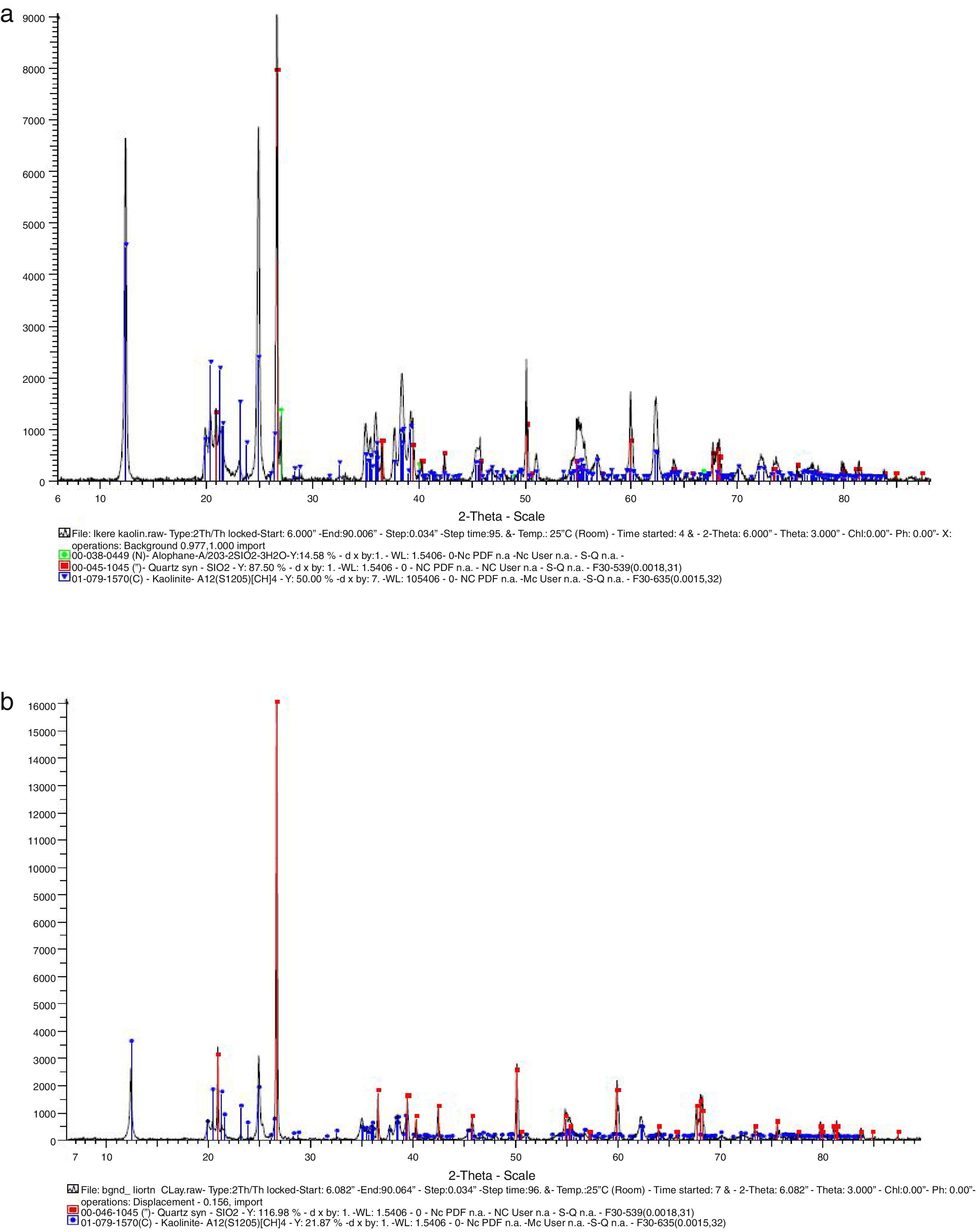
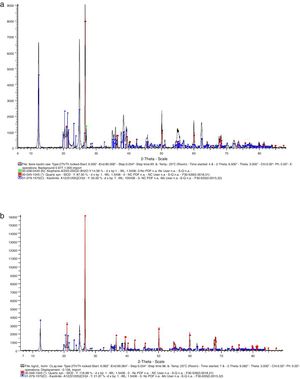
Results and discussionMaterials characterizationThe chemical composition of the glasses used based on the standard typical composition shows that soda-lime glass consists of 73% SiO2, 13.5% Na2O, 8.5% CaO, 1.5% Al2O3 and 3.5% MgO while borosilicate consists of 80% SiO2, 13% B2O3, 4% Na2O and 3% Al2O3. The results of the mineralogical compositions of the kaolin and ball clay used are presented in Fig. 1(a) and (b). Fig. 1(a) represents the XRD pattern of the Kaolin; it shows that the Kaolin consist of 14.58% of Allophane (Al2O3·2SiO2·3H2O) indicated by the green spectrum, 50% Kaolinite – Al2 (Si2O5)(OH)4 indicated by the blue spectrum and Quartz (SiO2) indicated by the red spectrum. Fig. 1(b) represents the XRD pattern of the Ball clay; it shows that the Ball clay also consists of 21.87% Kaolinite – Al2 (SiO2)(OH)4 indicated by the blue spectrum while highest portion is Quartz (SiO2) indicated by the red spectrum.
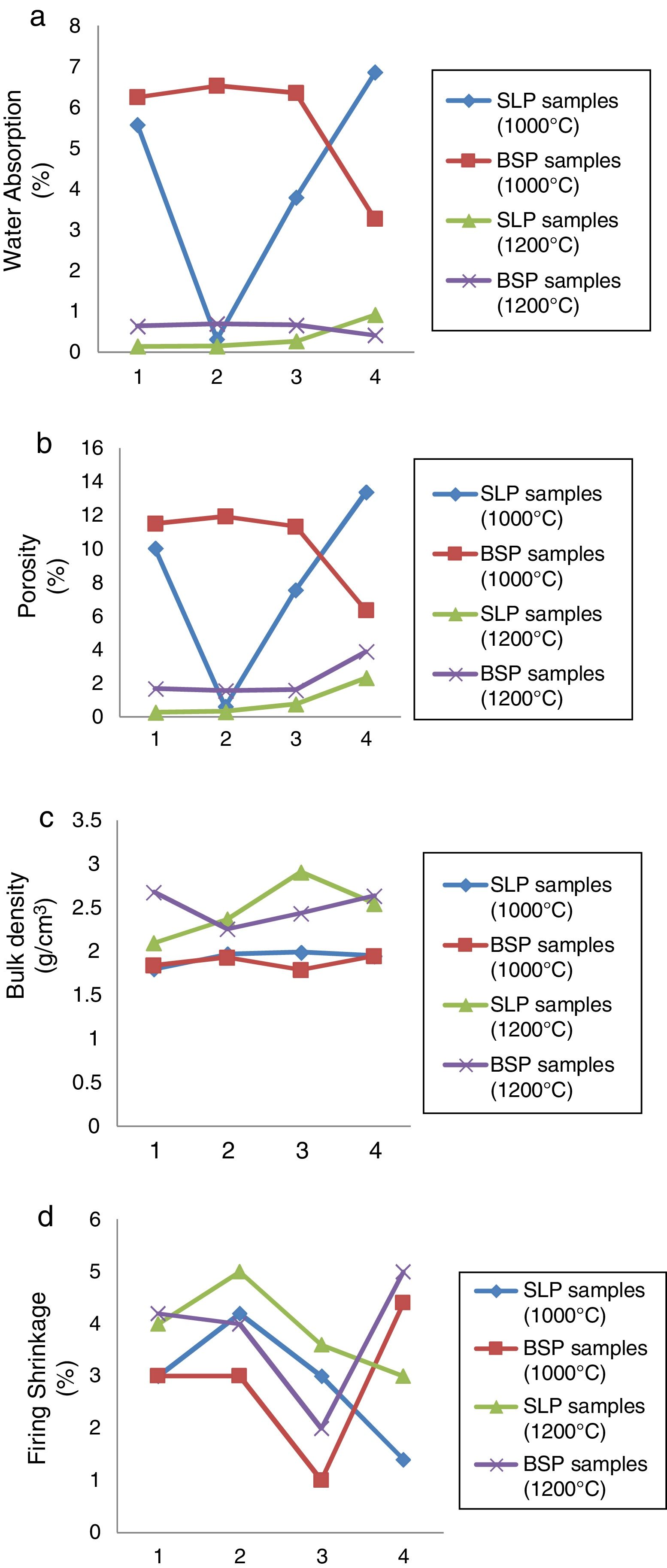
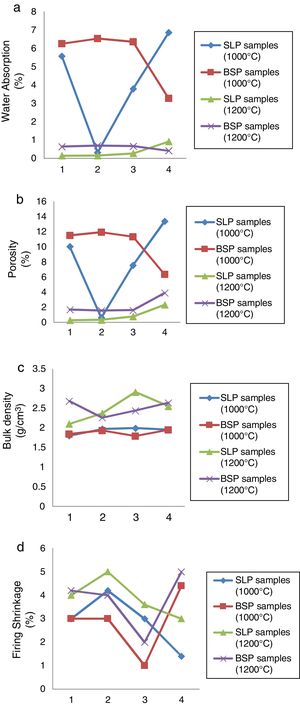
Physical characterizationThe results of the water absorption, estimated percentage porosity, bulk density and firing shrinkage are presented in Fig. 2(a)–(d) respectively. From Fig. 2(a), at 1000°C, for soda-lime containing porcelain (SLP series); a high water absorption >5% is observed in SLP1 and SLP4 while SLP2 and SLP3 has absorption <2% and <4% respectively indicating better closed pores. However, for BSP series, only BSP4 showed water absorption <4%. The high water absorption observed in most samples might be attributed to higher number of pores still present at this particular temperature as less glassy phase is formed. At 1200°C, a significant fall <1% which conform with the standard value (0–1%) is observed for all the samples of SLP series and BSP series which might be as a result of more formation of glassy phase and mullite growth to seal these pores, thereby increasing consolidation at higher temperature as stated by [21]. However, SLP series displayed the least water absorption at 1200°C as the amount of soda-lime content increases indicating the ability of soda-lime glass to form better glassy phase. The estimated % porosity in Fig. 2(b) shows similar trend as observed in Fig. 1(a), in which the SLP and BSP samples fired at 1000°C exhibited higher porosity while drastic fall in porosity <4% was observed at 1200°C which might also be attributed to increasing glassy phase formation at higher temperature according to [2]. Porosity has also been known to be related with property such as water absorption. In terms of the bulk density shown in Fig. 2(c), at 1000°C: it can be observed that both SLP and BSP series show similar behavior as there is no significant difference as their bulk densities are within the range 1.5–2g/cm3. However, at 1200°C, there is a general increment of 16.7%, 20.3% 46.2%, 30.8% for SLP series over their counterparts at 1000°C with SLP3 showing the highest densification due to higher content (55%) of soda-lime while increment of 45.6%, 17.1%, 36.3% and 35.4% in bulk densities are observed in BSP series over their counterparts at 1000°C. This behavior might be attributed to decrease in porosity arising from glassy phase formation sealing the pores, thereby increasing densification as stated by [21]. The results of the linear firing shrinkage shown in Fig. 2(d) shows that samples fired at temperature of 1000°C exhibited lower shrinkage value for both SLP series and BSP series respectively compare with SLP and BSP series fired at 1200°C which is actually normal for ceramic wares generally to shrink as firing temperature progresses. However, shrinkage of about 5% can still be considered as vitrification increases [5].
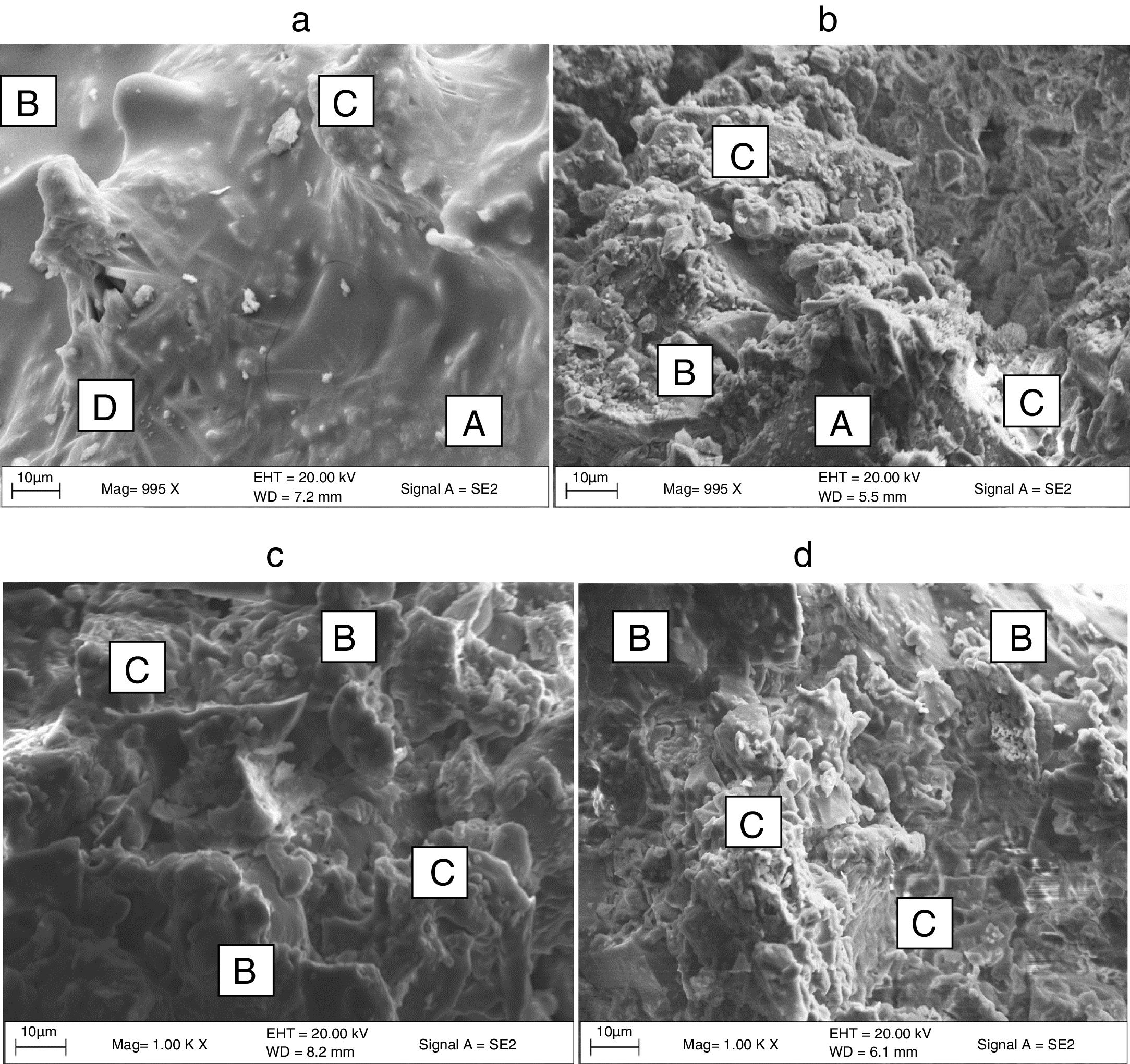
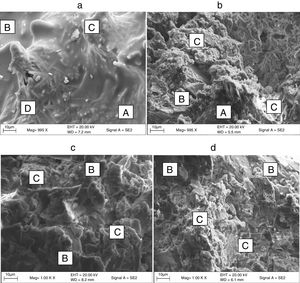
Microstructure characterizationRepresentative microstructure of the developed soft porcelain bodies containing soda-lime and borosilicate glasses sintered at 1000°C and 1200°C respectively are presented in Fig. 3(a)–(d). Fig. 3(a) and (b) represents porcelain containing soda-lime and borosilicate glasses sintered at 1000°C respectively while Fig. 3(c) and (d) represents porcelain containing soda-lime and borosilicate glasses sintered at 1200°C respectively. From Fig. 2(a) and (b), at 1000°C, it will be observed that the porcelain containing soda-lime glass consist of quartz, glassy phase, primary mullite (Si, Al, O) and secondary mullite (Si, Al, O, K, Na) indicated by a spindle-like structure while the porcelain containing borosilicate glass consist mainly of quartz, less glassy phase, primary mullite and the existence of Boric oxide in the structure being a network former with silica makes the structure partially coarse in nature. However, at 1200°C, porcelain containing soda-lime glass (Fig. 3(c)) consists mainly primary mullite and a large portion of glassy phase as more glassy phase and mullite growth are more pronounced at elevated temperature while the porcelain containing borosilicate glass (Fig. 3(d)) consists some glassy phase formation due to elevated temperature and a large portion of primary mullite. The better properties observed for samples sintered at 1200°C can be attributed to the structure displayed at that temperature.
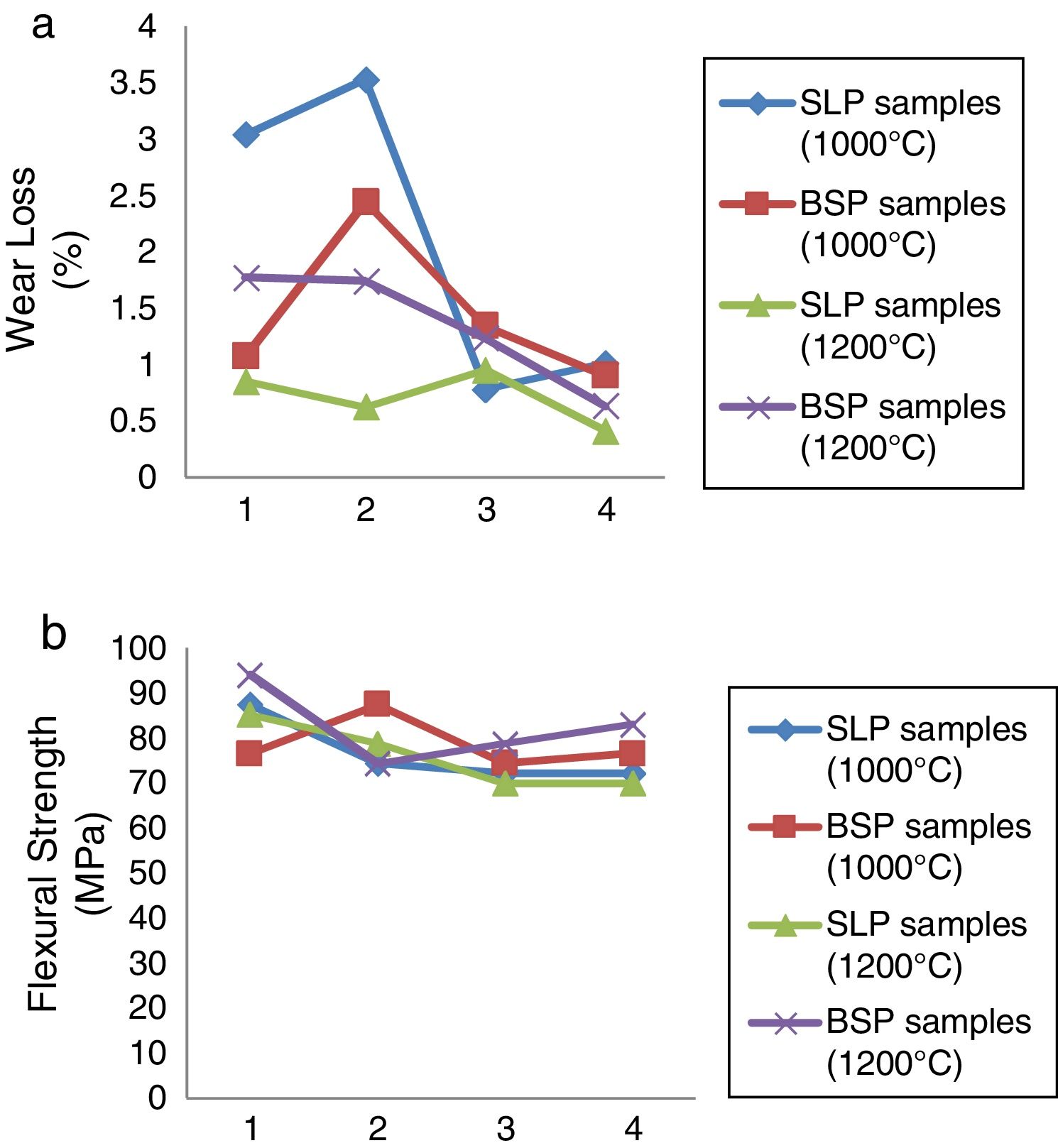
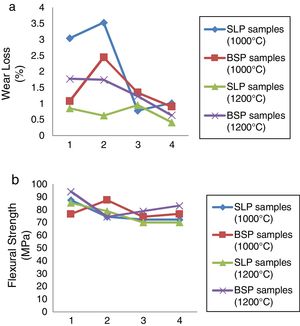
Mechanical characterizationThe results of the mechanical behavior of the developed soft porcelain bodies sintered at 1000°C and 1200°C respectively in terms of wear loss and flexural strength are presented in Fig. 4(a) and (b). From Fig. 4(a), at 1000°C: SLP1 and SLP2 showed high wear loss >3% while significant fall in wear loss is observed for SLP3 and SLP4 while wear loss falls below 2.5% for BSP samples showing better performance. At 1200°C, the % wear loss decreases across the BSP series as borosilicate glass content increases while for SLP series, their wear loss falls <1% across the series. This better wear loss performance observed at 1200°C can be attributed to the growth of hard mullite phase, more glass formation and closed pores at this temperature resulting into better strength and invariably wear property has reported by [2]. However, porcelain samples containing waste soda-lime glass (SLP series) displayed better wear behavior compared with samples containing borosilicate glass (BSP series) which indicates that soda-lime glass enhances more mullite growth (known to be hard phase) than borosilicate glass. Fig. 4(b) shows the results of the flexural strength values for both samples fired at 1000°C and 1200°C respectively. However, this result is not as consistent as we observed in other tested parameters discussed so far in the course of this research. Nonetheless, samples fired at 1200°C show promising results on the average while the highest strength was observed in sample BSP1 at 1200°C.
ConclusionThe effect of waste glasses (waste soda-lime and borosilicate glasses) on the physic-mechanical properties of soft porcelain has been investigated. The results show that:
- •
Water absorption reduces drastically below 1% while porosity falls below 4% for samples fired at 1200°C conforming to the standard requirement.
- •
Bulk density increases with respect to sintering temperature and increase in the amount of waste glasses as more glass phase is obtained while shrinkage increases to about 5% at 1200°C though still tolerable.
- •
Minimum wear loss was observed for samples subjected to 1200°C as compared with samples fired at 1000°C.
- •
The flexural strength does not show consistency as compared with other tested parameters.
- •
The sintering temperature to obtain better behavior of soft porcelain is 1200°C which has also been supported by literature.
- •
Porcelain containing waste soda-lime glasses showed overall better promising results indicating that soda-lime glass enhances better mullite growth and increase glassy phase formation.